Single Crystal Growth and Anisotropic Magnetic Properties of Li2Sr[Li1 − xFexN]2
Abstract
:1. Introduction
2. Results
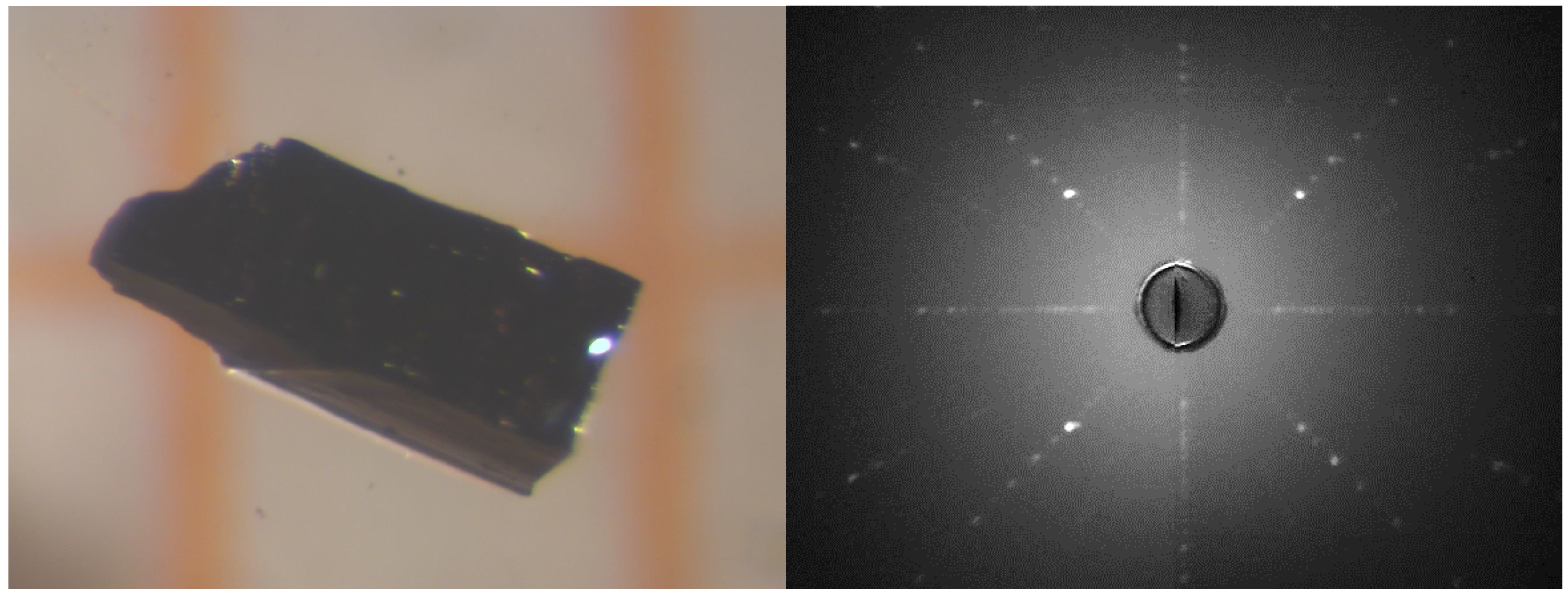
2.1. Single Crystal Growth
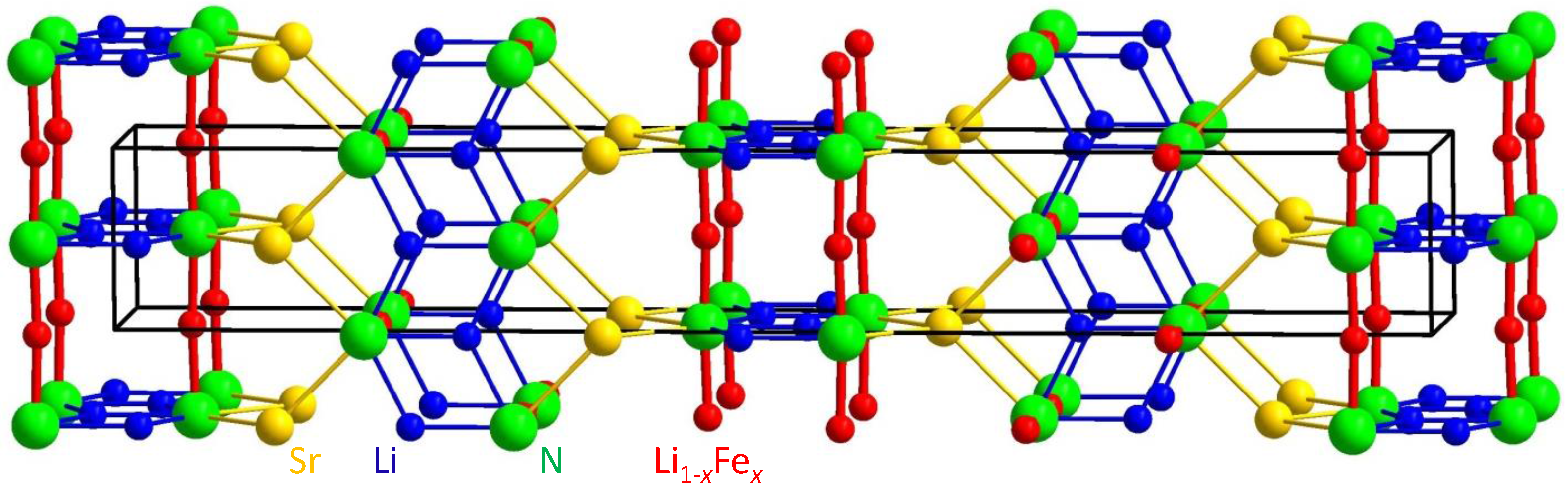
2.2. Crystal Structure
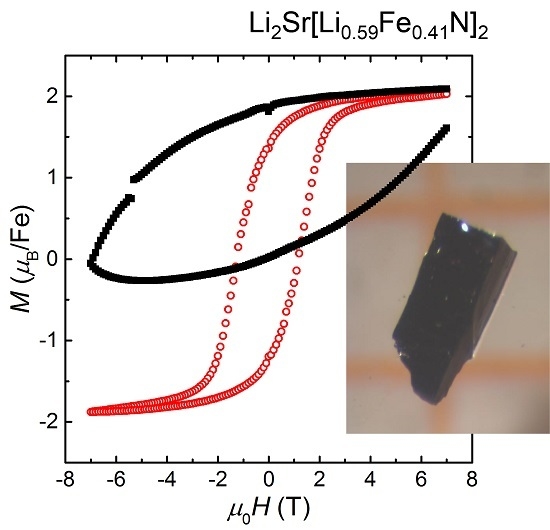
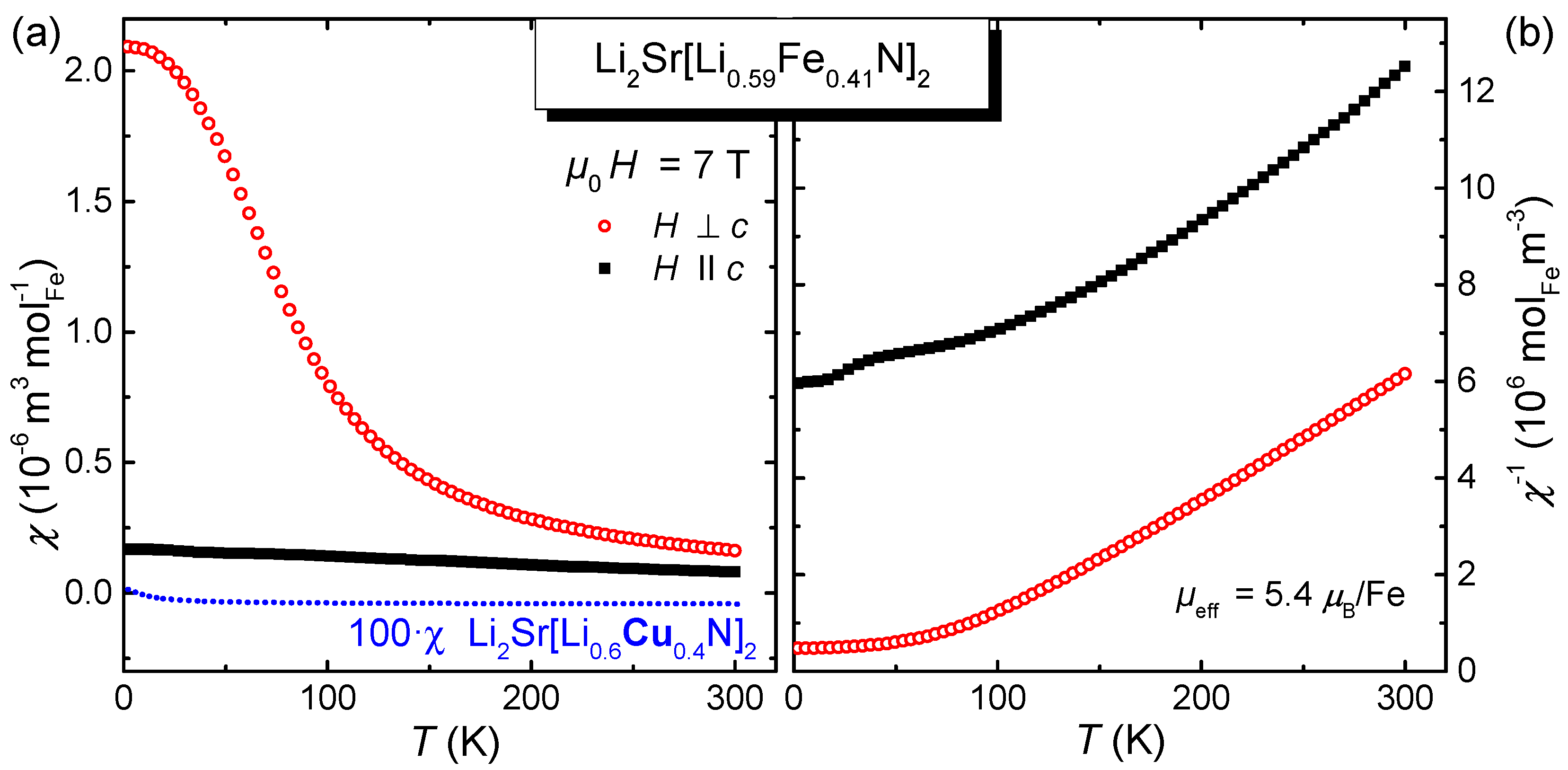
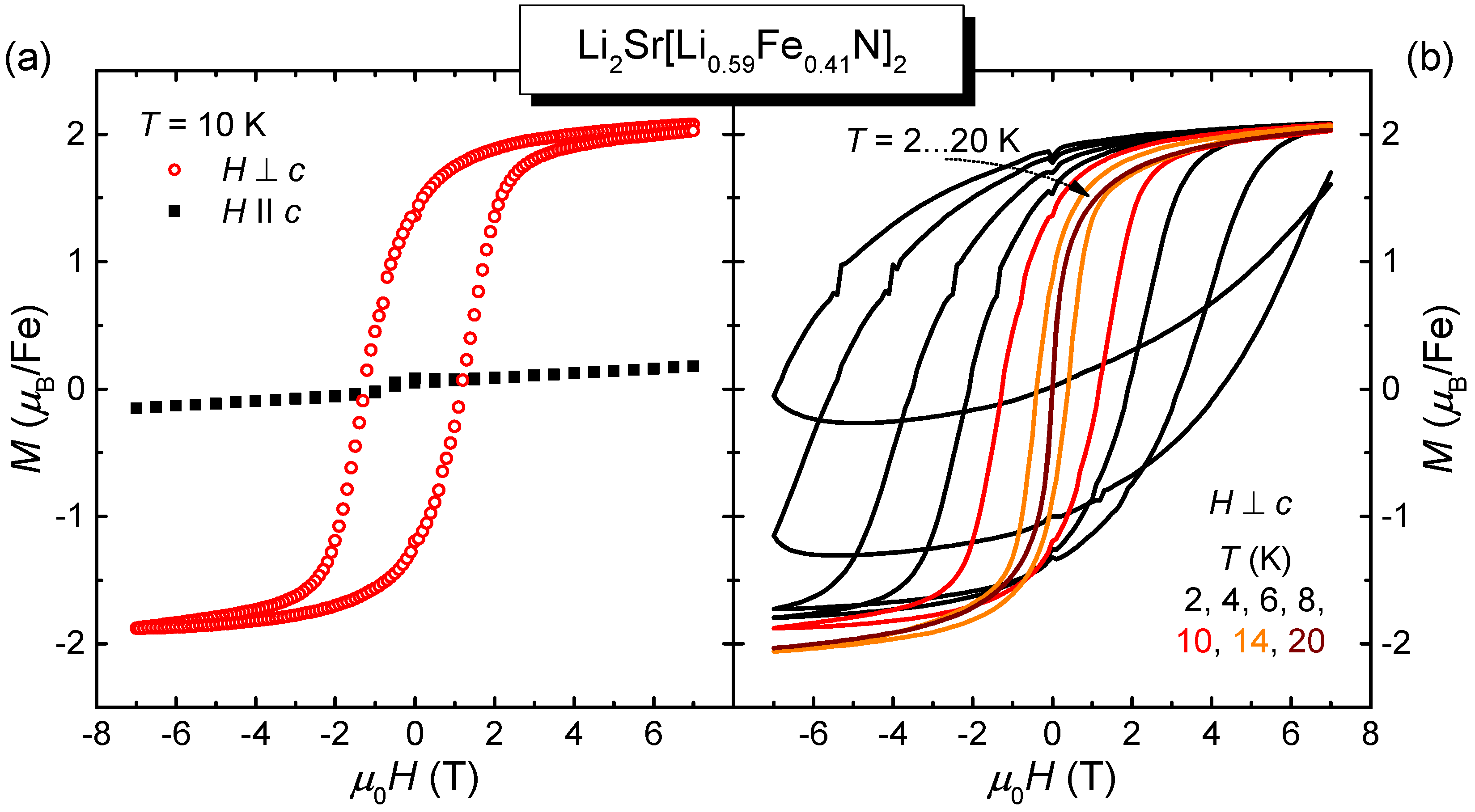
2.3. Magnetic Properties
3. Discussion
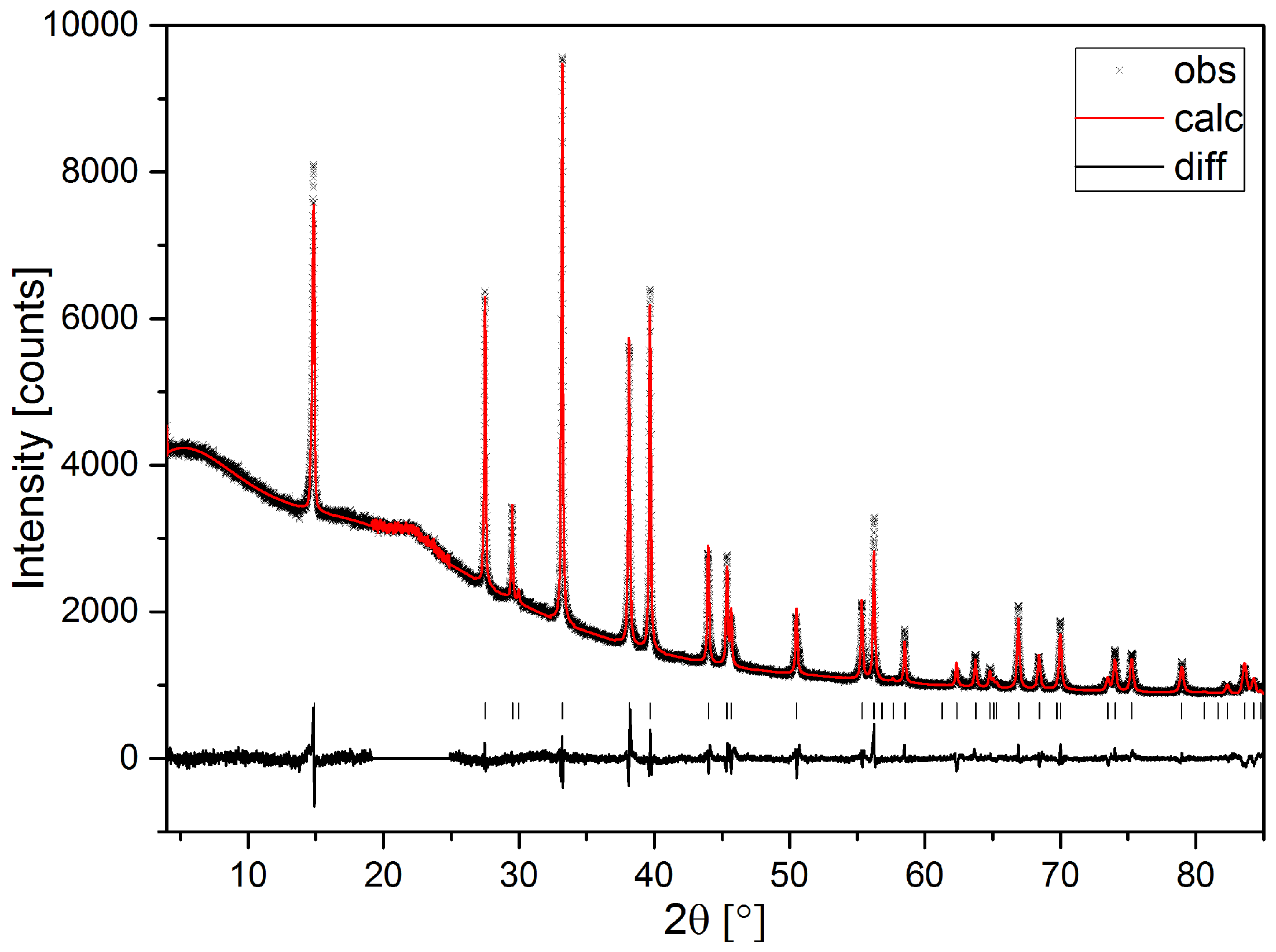
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kniep, R.; Höhn, P. Low-Valency Nitridometalates. In Comprehensive Inorganic Chemistry {II}, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 137–160. [Google Scholar]
- Gudat, A.; Kniep, R.; Rabenau, A.; Bronger, W.; Ruschewitz, U. Li3FeN2, a ternary nitride with chains: Crystal structure and magnetic properties. J. Less Common Met. 1990, 161, 31–36. [Google Scholar] [CrossRef]
- Nishijima, M.; Takeda, Y.; Imanishi, N.; Yamamoto, O.; Takano, M. Li Deintercalation and Structural Change in the Lithium Transition Metal Nitride Li3FeN2. J. Solid State Chem. 1994, 113, 205–210. [Google Scholar] [CrossRef]
- Cordier, G.; Kniep, R.; Höhn, P.; Rabenau, A. Ca6GaN5 und Ca6FeN5. Verbindungen mit [CO3]2− -isosteren Anionen [GaN3]6− und [GaN3]6−. Z. Anorg. Allg. Chem. 1990, 591, 58–66. [Google Scholar] [CrossRef]
- Bendyna, J.K.; Höhn, P.; Kniep, R. Crystal structure of tristrontium trinitridoferrate(III), Sr3[FeN3]. Z. Kristallogr. New Cryst. Struct. 2008, 223, 109–110. [Google Scholar]
- Höhn, P.; Kniep, R.; Rabenau, A. Ba3[FeN3], new nitridoferrate(III) with [CO3]2− isosteric anions [FeN3]6−. Z. Kristallogr. 1991, 196, 153–158. [Google Scholar] [CrossRef]
- Höhn, P. Ternäre und quaternäre Nitridometallate: Verbindungen in den Systemen Li-Erdalkalimetall-Übergangsmetall-Stickstoff (Übergangsmetall = Ta, Mo, W, Fe, Co). Ph.D. Thesis, TH Darmstadt, Darmstadt, Germany, 1992; pp. 1–284. [Google Scholar]
- Bendyna, J.K.; Höhn, P.; Kniep, R. Crystal structure of octastrontium bistrinitridoferrate(III) dinitridoferrate(II), Sr8[FeN3]2[FeN2]. Z. Kristallogr. New Cryst. Struct. 2008, 223, 181–182. [Google Scholar] [CrossRef]
- Bendyna, J.K. New Developments in Nitridometalates and Cyanamides: Chemical, Structural and Physical Properties. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2009; pp. 1–227. [Google Scholar]
- Höhn, P.; Kniep, R. Ca2[FeN2] and Sr2[FeN2]: Nitridoferrate(II) mit isolierten Anionen [Fe2N4]8− und [FeN2]4−. Z. Naturforsch. B 1992, 47, 477–481. [Google Scholar] [CrossRef]
- Höhn, P.; Haag, S.; Milius, W.; Kniep, R. Sr2Li[Fe2N3] und Ba2Li[Fe2N3]: Nitridoferrate(II) Isotypes with Anions. Angew. Chem. Int. Ed. 1991, 30, 831–832. [Google Scholar] [CrossRef]
- Bendyna, J.K.; Höhn, P.; Kniep, R. Crystal structure of octastrontium bistrinitridomanganate(III) dinitridoferrate(II), Sr8[MnN3]2[FeN2]. Z. Kristallogr. New Cryst. Struct. 2008, 223, 183–184. [Google Scholar] [CrossRef]
- Gudat, A.; Kniep, R.; Rabenau, A. Li4[FeN2]: A Nitridoferrate(II) with Anions Isosteric with CO2. A Defect Variant of the Li3N Type Structure. Angew. Chem. Int. Ed. 1991, 30, 199–200. [Google Scholar] [CrossRef]
- Niewa, R.; Huang, Z.L.; Schnelle, W.; Hu, Z.; Kniep, R. Preparation, Crystallographic, Spectroscopic and Magnetic Characterization of Low-Valency Nitridometalates Li2[(Li1 − xMx)N] with M = Cu, Ni. Z. Anorg. Allg. Chem. 2003, 629, 1778–1786. [Google Scholar] [CrossRef]
- Niewa, R.; Hu, Z.; Kniep, R. Mn and Fe K-edge XAS Spectra of Manganese and Iron Nitrido Compounds. Eur. J. Inorg. Chem. 2003, 2003, 1632–1634. [Google Scholar] [CrossRef]
- Klatyk, J.; Schnelle, W.; Wagner, F.R.; Niewa, R.; Novák, P.; Kniep, R.; Waldeck, M.; Ksenofontov, V.; Gütlich, P. Large Orbital Moments and Internal Magnetic Fields in Lithium Nitridoferrate(I). Phys. Rev. Lett. 2002, 88, 207202. [Google Scholar] [CrossRef] [PubMed]
- Klatyk, J.; Kniep, R. Crystal structure of alkaline earth dilithium bis(nitridolithiate/ferrates(I)), Ca{Li2[(Li1 − xFex)N]2}, x = 0.30 and Sr{Li2[(Li1 − xFexN)]2}, x = 0.46. Z. Kristallogr. New Cryst. Struct. 1999, 214, 449–450. [Google Scholar]
- Klatyk, J.; Kniep, R. Crystal structure of dicalcium (dinitridolithiate/ferrate(I)), Ca2{Li[(Li1 − xFex)N2]}, x = 0.82. Z. Kristallogr. New Cryst. Struct. 1999, 214, 451–452. [Google Scholar]
- Höhn, P.; Schnelle, W.; Zechel, K. Book of Abstracts, SCTE-19, Genoa; SCTE: Genoa, Italy, 2014; p. 71. [Google Scholar]
- Jesche, A.; Canfield, P.C. Single crystal growth from light, volatile and reactive materials using lithium and calcium flux. Philos. Mag. 2014, 94, 2372–2402. [Google Scholar] [CrossRef]
- Jesche, A.; Ke, L.; Jacobs, J.L.; Harmon, B.; Houk, R.S.; Canfield, P.C. Alternating magnetic anisotropy of Li2(Li1 − xTx)N with T = Mn, Fe, Co, and Ni. Phys. Rev. B 2015, 91, 180403. [Google Scholar] [CrossRef]
- Fisk, Z.; Remeika, J.P. Growth of single crystals from molten metal fluxes. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 12. [Google Scholar]
- Canfield, P.C.; Fisk, Z. Growth of single crystals from metallic fluxes. Philos. Mag. B 1992, 65, 1117–1123. [Google Scholar] [CrossRef]
- Boström, M.; Hovmöller, S. Preparation and crystal structure of the novel decagonal approximant Mn123Ga137. J. Alloys Compd. 2001, 314, 154–159. [Google Scholar] [CrossRef]
- Pathak, M.; Bobnar, M.; Schnelle, W.; Höhn, P.; Grin, Y. Li16Sr6M6N (M = Ge, Sn, Pb)—Cubic Tetrelide-Nitrides with an Ordered Ir4Sc11 Type Structure. Z. Anorg. Allg. Chem. 2016, 642, 1075. [Google Scholar]
- Ovchinnikov, A.; Schnelle, W.; Bobnar, M.; Borrmann, H.; Sichelschmidt, J.; Grin, Y.; Höhn, P. Extended anionic frameworks in the AE-Mn-N systems. Synthesis, structure and physical properties of new nitridomanganates. In Proceedings of the European Conference on Solid State Chemistry, Vienna, Austria, 23–26 August 2015; p. 288.
- Jesche, A.; McCallum, R.W.; Thimmaiah, S.; Jacobs, J.L.; Taufour, V.; Kreyssig, A.; Houk, R.S.; Bud’ko, S.L.; Canfield, P.C. Giant magnetic anisotropy and tunnelling of the magnetization in Li2(Li1 − xFex)N. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Cordier, G.; Gudat, A.; Kniep, R.; Rabenau, A. LiCaN and Li4SrN2, Derivatives of the Fluorite and Lithium Nitride Structures. Angew. Chem. Int. Ed. 1989, 28, 1702–1703. [Google Scholar] [CrossRef]
- Canfield, P.C.; Fisher, I.R. High-temperature solution growth of intermetallic single crystals and quasicrystals. J. Cryst. Growth 2001, 225, 155–161. [Google Scholar] [CrossRef]
- Banhart, J.; Ebert, H.; Voitländer, J.; Winter, H. Diamagnetic susceptibility of pure metals and binary alloys. J. Magn. Magn. Mater. 1986, 61, 221–224. [Google Scholar] [CrossRef]
- Myers, W.R. The Diamagnetism of Ions. Rev. Mod. Phys. 1952, 24, 15–27. [Google Scholar] [CrossRef]
- Höhn, P.; Hoffmann, S.; Hunger, J.; Leoni, S.; Nitsche, F.; Schnelle, W.; Kniep, R. β-Ca3N2, a Metastable Nitride in the System Ca–N. Chem. Eur. J. 2009, 15, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Massalski, T.B. (Ed.) Binary Alloy Phase Diagrams; ASM International: Metals Park, OH, USA, 1996; Volume 3.
- Höhn, P.; Kniep, R. LiSr2[CoN2]: Ein Nitridocobaltat mit gestreckten Anionen [CoN2]5−. Z. Naturforsch. B 1992, 47, 434–436. [Google Scholar] [CrossRef]
- Pinkerton, F. High coercivity in melt-spun Dy–Fe–B and Tb–Fe–B alloys. J. Magn. Magn. Mater. 1986, 54, 579–582. [Google Scholar] [CrossRef]
- Liu, R.M.; Yue, M.; Na, R.; Deng, Y.W.; Zhang, D.T.; Liu, W.Q.; Zhang, J.X. Ultrahigh coercivity in ternary Tb–Fe–B melt-spun ribbons. J. Appl. Phys. 2011, 109, 07A760. [Google Scholar] [CrossRef]
- Wu, W.; Kiryukhin, V.; Noh, H.J.; Ko, K.T.; Park, J.H.; Ratcliff, W.; Sharma, P.A.; Harrison, N.; Choi, Y.J.; Horibe, Y.; et al. Formation of Pancakelike Ising Domains and Giant Magnetic Coercivity in Ferrimagnetic LuFe2O4. Phys. Rev. Lett. 2008, 101, 137203. [Google Scholar] [CrossRef] [PubMed]
- Iida, J.; Tanaka, M.; Nakagawa, Y.; Funahashi, S.; Kimizuka, N.; Takekawa, S. Magnetization and Spin Correlation of Two-Dimensional Triangular Antiferromagnet LuFe2O4. J. Phys. Soc. Jpn. 1993, 62, 1723–1735. [Google Scholar] [CrossRef]
- Ko, K.T.; Noh, H.J.; Kim, J.Y.; Park, B.G.; Park, J.H.; Tanaka, A.; Kim, S.B.; Zhang, C.L.; Cheong, S.W. Electronic Origin of Giant Magnetic Anisotropy in Multiferroic LuFe2O4. Phys. Rev. Lett. 2009, 103, 207202. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Wagner, F.R. Electronic structure of lithium nitridoferrate: Effects of correlation and spin-orbit coupling. Phys. Rev. B 2002, 66, 184434. [Google Scholar] [CrossRef]
- Antropov, V.P.; Antonov, V.N. Colossal anisotropy of the magnetic properties of doped lithium nitrodometalates. Phys. Rev. B 2014, 90, 094406. [Google Scholar] [CrossRef]
- Ke, L.; van Schilfgaarde, M. Band-filling effect on magnetic anisotropy using a Green’s function method. Phys. Rev. B 2015, 92, 014423. [Google Scholar] [CrossRef]
- Yang, I.K.; Kim, J.; Lee, S.H.; Cheong, S.W.; Jeong, Y.H. Charge ordering, ferroelectric, and magnetic domains in LuFe2O4 observed by scanning probe microscopy. Appl. Phys. Lett. 2015, 106, 152902. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of Polyatomic Molecules in Degenerate Electronic States. I. Orbital Degeneracy. Proc. R. Soc. London, Ser. A 1937, 161, 220–235. [Google Scholar] [CrossRef]
- Kugel’, K.I.; Khomskiĭ, D.I. The Jahn-Teller effect and magnetism: Transition metal compounds. Sov. Phys. Uspekhi 1982, 25, 231. [Google Scholar] [CrossRef]
- WinXPow, STOE & Cie GmbH: Darmstadt, Germany, 2003.
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar]
- Sheldrick, G.M. SHELXS-97-2; Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97-2; Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
| crystal system | tetragonal |
| space group | (No. 141) |
| a (Å) | 3.8011(1) |
| c (Å) | 27.586(3) |
| Fe occupancy (x) | 0.32(1) |
| cell volume (Å3) | 398.57(5) |
| Z | 4 |
| (gcm) | 2.907 |
| crystal color, habit | black, tetragonal column |
| crystal size (mm) | 0.02 × 0.02 × 0.07 |
| μ(Mo, mm) | 15.52 |
| 2θ range () | 5.90–59.60 |
| diffractometer | RIGAKU AFC7 |
| wavelength (Å) | 0.71069 (Mo-) |
| monochromator | graphite |
| temperature | 293 K |
| scan mode | profile data from ϕ scans |
| measured reflections | 3355 |
| observed reflections [F > 4σ(F)] | 2919 |
| independent reflections | 187 |
| R | 0.041 |
| structure solution method | direct |
| number of parameters | 17 |
| goodness-of-fit on F | 1.081 |
| wR2 | 0.078 |
| R1 [F > 4σ(F)] | 0.030 |
| R1 (all data) | 0.035 |
| residual electron density (e pm) | 1.24/−1.70 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höhn, P.; Ballé, T.J.; Fix, M.; Prots, Y.; Jesche, A. Single Crystal Growth and Anisotropic Magnetic Properties of Li2Sr[Li1 − xFexN]2. Inorganics 2016, 4, 42. https://doi.org/10.3390/inorganics4040042
Höhn P, Ballé TJ, Fix M, Prots Y, Jesche A. Single Crystal Growth and Anisotropic Magnetic Properties of Li2Sr[Li1 − xFexN]2. Inorganics. 2016; 4(4):42. https://doi.org/10.3390/inorganics4040042
Chicago/Turabian StyleHöhn, Peter, Tanita J. Ballé, Manuel Fix, Yurii Prots, and Anton Jesche. 2016. "Single Crystal Growth and Anisotropic Magnetic Properties of Li2Sr[Li1 − xFexN]2" Inorganics 4, no. 4: 42. https://doi.org/10.3390/inorganics4040042