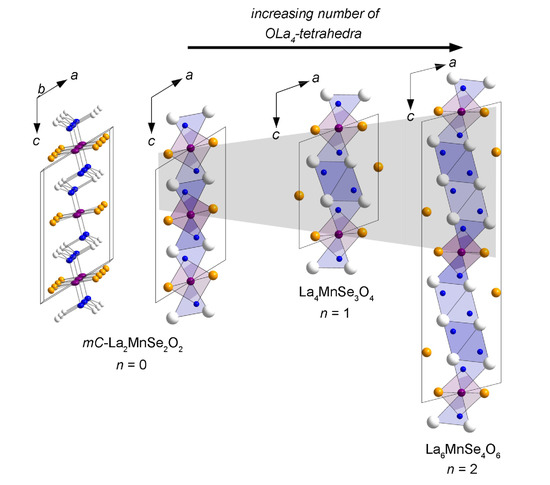
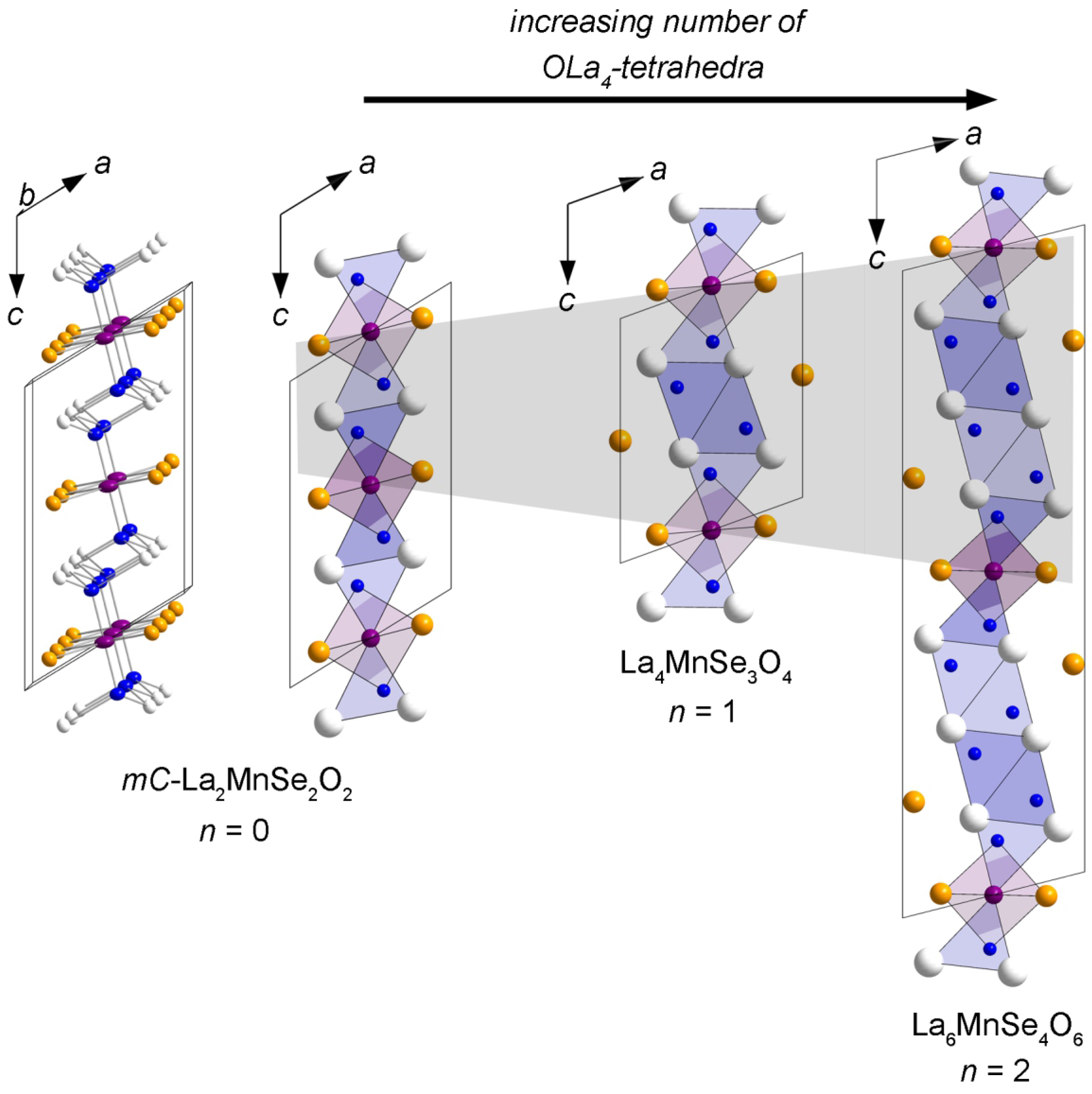
Flux Synthesis, Crystal Structures, and Magnetism of the Series La2n+2MnSen+2O2n+2 (n = 0–2)
Abstract
:1. Introduction
2. Results
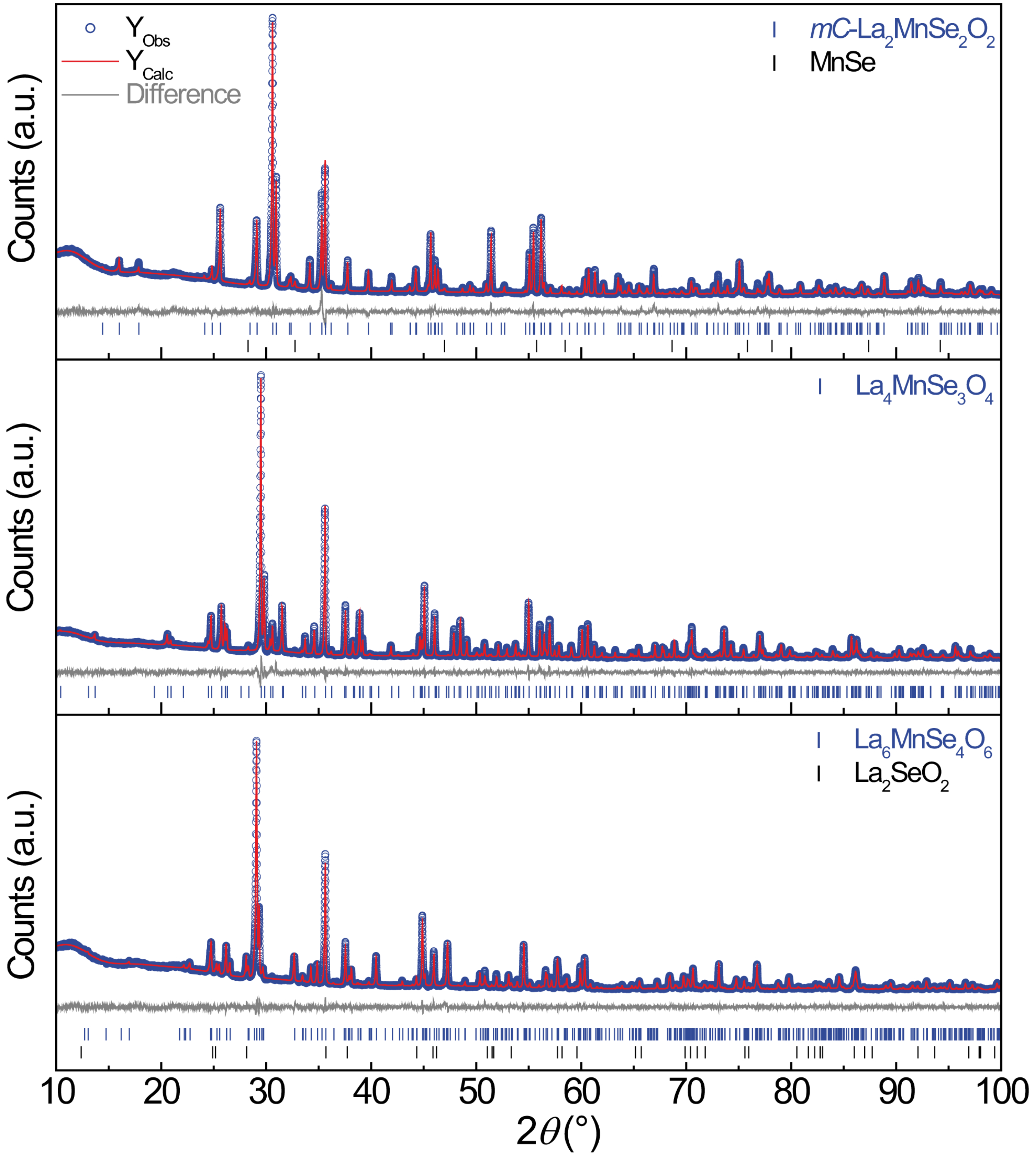
2.1. -La2MnSe2O2
2.2. La4MnSe3O4 and La6MnSe4O6
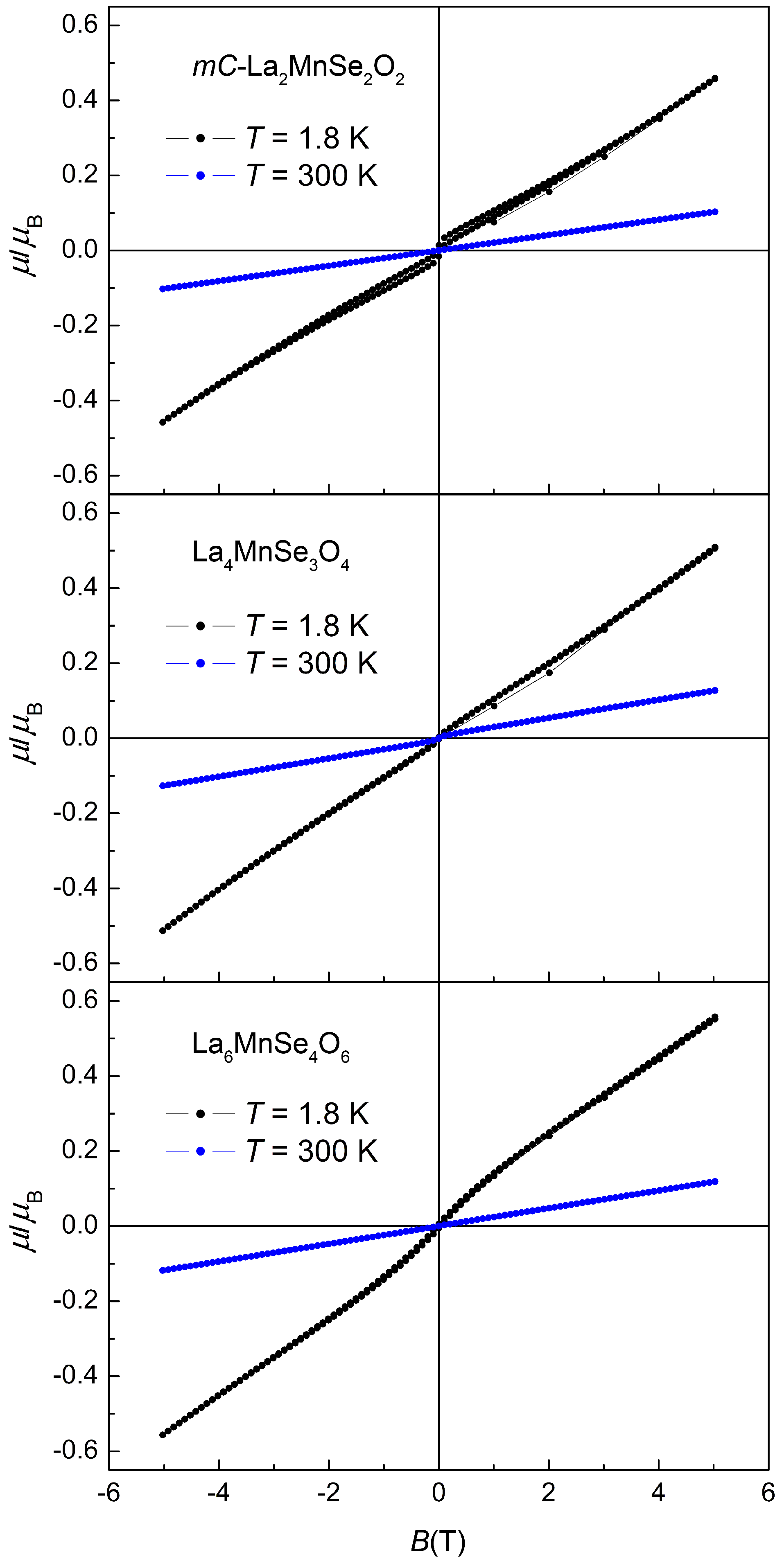
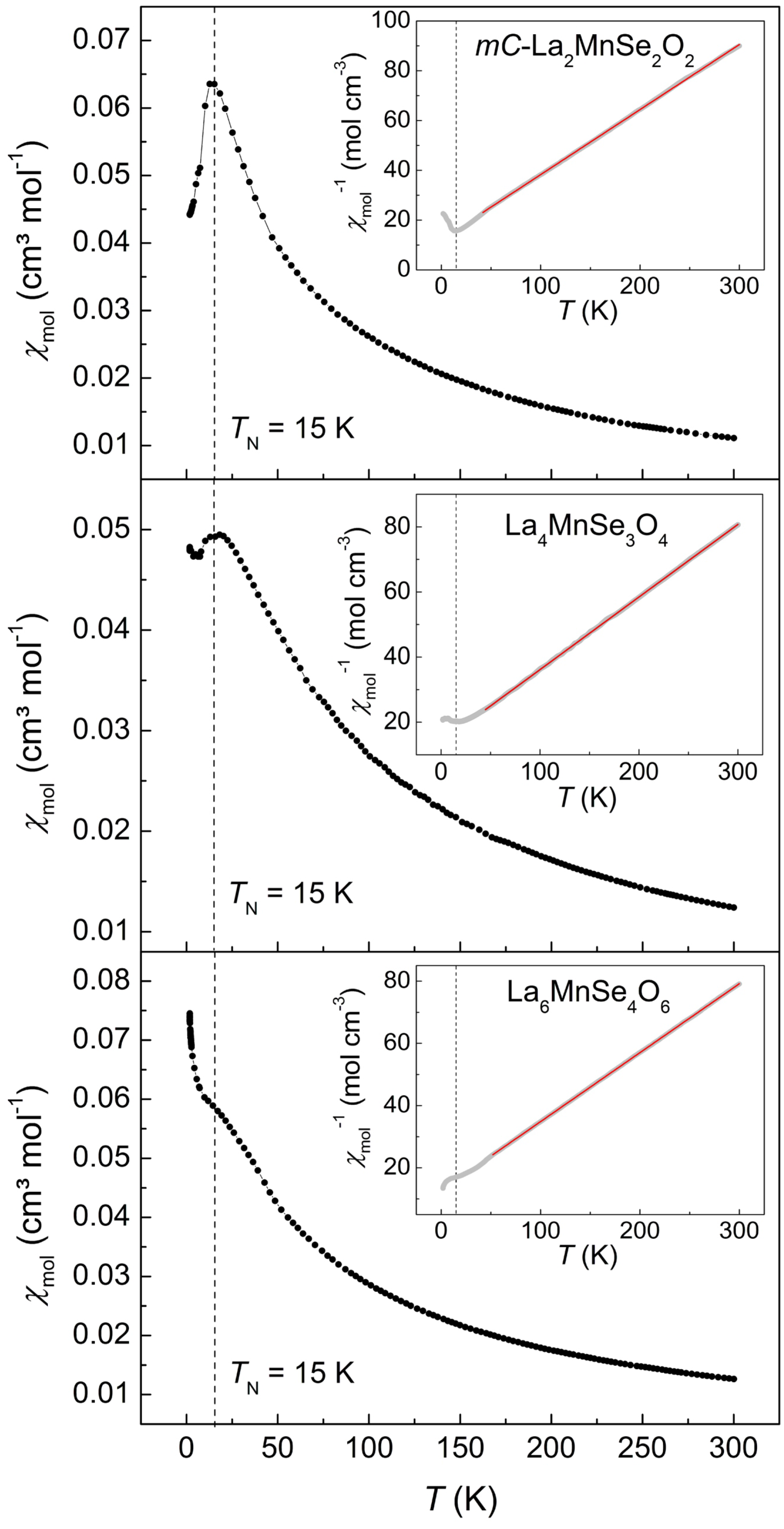
2.3. Magnetism
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Formula weight (g·mol−1) | 522.7 | |||||
| Space group, Z | C2/m, 2 | |||||
| a, b, c (Å) | 11.662(1), 3.972(1), 7.205(1) | |||||
| β () | 121.7(1) | |||||
| Volume (Å3), ρX-ray (g·cm−3) | 284.1(1), 6.11 | |||||
| Crystal size (mm3) | 0.06 × 0.02 × 0.01 | |||||
| Diffractometer | Bruker D8 QUEST | |||||
| Radiation λ (pm) | 71.073 | |||||
| Absorption coeff. μ (mm−1) | 29.6 | |||||
| 2θ range () | 6.64–107.76 | |||||
| Index range (hkl) | −19 ≤ h ≤ 25, k ± 8, −16 ≤ l ≤ 14 | |||||
| No. reflections collected | 5770 | |||||
| No. unique data, Rint, Rσ | 1731, 0.02, 0.03 | |||||
| No. data with I > 3σ(I) | 1441 | |||||
| No. parameters | 23 | |||||
| R1(obs/all) | 0.024/0.036 | |||||
| wR2(obs/all) | 0.048/0.051 | |||||
| 1.92/−2.53 | ||||||
| Atomic and Displacement Parameters | ||||||
| Site | x, y, z | U11 | U22 | U33 | Occ. | |
| La | 4i | 0.6923(1), 0, 0.2430(1) | 0.0073(1) | 0.0058(1) | 0.0059(1) | 1 |
| Mn | 2d | 0, , | 0.0063(2) | 0.0141(2) | 0.0137(2) | 1 |
| Se | 4i | 0.9405(1), 0, 0.1797(1) | 0.0087(1) | 0.0088(1) | 0.0080(1) | 1 |
| O | 4i | 0.8028(2), , 0.4187(1) | 0.0059(1) | 0.0077(7) | 0.0081(6) | 1 |
| Formula weight (g·mol−1) | 911.4 | |||||
| Space group, Z | P2/m, 1 | |||||
| a, b, c (Å) | 9.006(1), 4.019(1), 7.195(1) | |||||
| β () | 109.7(1) | |||||
| Volume (Å3), ρX-ray (g·cm−3) | 245.1(1), 6.17 | |||||
| Crystal size (mm3) | 0.04 × 0.02 × 0.01 | |||||
| Diffractometer | Bruker D8 QUEST | |||||
| Radiation λ (pm) | 71.073 | |||||
| Absorption coeff. μ (mm−1) | 29.4 | |||||
| 2θ range () | 4.80–69.96 | |||||
| Index range (hkl) | h ± 14, −6 ≤ k ≤ 5, l ± 11 | |||||
| No. reflections collected | 6887 | |||||
| No. unique data, Rint, Rσ | 1210, 0.03, 0.02 | |||||
| No. data with I > 3σ(I) | 1017 | |||||
| No. parameters | 39 | |||||
| R1(obs/all) | 0.023/0.032 | |||||
| wR2(obs/all) | 0.050/0.056 | |||||
| 3.85/−1.71 | ||||||
| Atomic and Displacement Parameters | ||||||
| Site | x, y, z | U11 | U22 | U33 | Occ. | |
| La1 | 2n | 0.2245(1), , 0.1732(1) | 0.0053(1) | 0.0056(1) | 0.0046(1) | 1 |
| La2 | 2m | 0.6430(1), 0, 0.3496(1) | 0.0053(1) | 0.0044(1) | 0.0048(1) | 1 |
| Mn1 | 1f | 0, , | 0.0120(6) | 0.0113(5) | 0.0115(5) | 1 |
| Se1 | 1e | , , 0 | 0.0072(3) | 0.0062(3) | 0.0048(3) | 1 |
| Se2 | 2m | 0.0627(1), 0, 0.7971(1) | 0.0078(2) | 0.0095(2) | 0.0079(2) | 1 |
| O1 | 2m | 0.3651(4), 0, 0.3114(5) | 0.0048(16) | 0.0069(16) | 0.0082(16) | 1 |
| O2 | 2n | 0.2317(4), , 0.5070(5) | 0.0046(16) | 0.0057(15) | 0.0079(16) | 1 |
| Formula weight (g·mol−1) | 1300.2 | |||||
| Space group, Z | C2/m, 2 | |||||
| a, b, c (Å) | 24.760(1), 4.036(1), 7.185(1) | |||||
| β () | 104.2(1) | |||||
| Volume (Å3), ρX-ray (g·cm−3) | 696.2(1), 6.20 | |||||
| Crystal size (mm3) | 0.03 × 0.02 × 0.01 | |||||
| Diffractometer | Bruker D8 QUEST | |||||
| Radiation λ (pm) | 71.073 | |||||
| Absorption coeff. μ (mm−1) | 29.3 | |||||
| 2θ range () | 5.84–70.20 | |||||
| Index range (hkl) | −40 ≤ h ≤ 38, k ± 6, l ± 11 | |||||
| No. reflections collected | 8892 | |||||
| No. unique data, Rint, Rσ | 1375, 0.05, 0.05 | |||||
| No. data with I > 3σ(I) | 957 | |||||
| No. parameters | 53 | |||||
| R1(obs/all) | 0.023/0.049 | |||||
| wR2(obs/all) | 0.041/0.049 | |||||
| 1.65/−1.70 | ||||||
| Atomic and Displacement Parameters | ||||||
| Site | x, y, z | U11 | U22 | U33 | Occ. | |
| La1 | 4i | 0.0788(1), , 0.1453(1) | 0.0050(2) | 0.0060(2) | 0.0049(2) | 1 |
| La2 | 4i | 0.2263(1), 0, 0.2665(1) | 0.0045(2) | 0.0044(2) | 0.0043(2) | 1 |
| La3 | 4i | 0.1261(1), 0, 0.6051(61) | 0.0056(2) | 0.0045(2) | 0.0052(2) | 1 |
| Mn1 | 2d | 0, , | 0.0110(9) | 0.0110(7) | 0.0100(8) | 1 |
| Se1 | 4i | 0.8252(1), , 0.0613(1) | 0.0069(3) | 0.0067(3) | 0.0048(3) | 1 |
| Se2 | 4i | 0.4781(1), , 0.2116(1) | 0.0076(4) | 0.0095(3) | 0.0075(4) | 1 |
| O1 | 4i | 0.2752(2), , 0.3975(6) | 0.004(2) | 0.009(2) | 0.006(2) | 1 |
| O2 | 4i | 0.1288(2), 0, 0.2662(7) | 0.007(2) | 0.007(2) | 0.007(2) | 1 |
| O3 | 4i | 0.0823(2), , 0.4801(6) | 0.003(2) | 0.005(2) | 0.010(2) | 1 |
References
- Peschke, S.; Nitsche, F.; Johrendt, D. Flux Synthesis, Modulated Crystal Structures, and Physical Properties of REMn0.5SeO (RE = La, Ce). Z. Anorg. Allg. Chem. 2015, 641, 529–536. [Google Scholar] [CrossRef]
- Nitsche, F.; Niklaus, R.; Johrendt, D. New Polymorphs of RE2FeSe2O2 (RE = La, Ce). Z. Anorg. Allg. Chem. 2014, 640, 2897–2902. [Google Scholar] [CrossRef]
- Wang, C.H.; Ainsworth, C.M.; Gui, D.Y.; McCabe, E.E.; Tucker, M.G.; Evans, I.R.; Evans, J.S.O. Infinitely Adaptive Transition Metal Oxychalcogenides: The Modulated Structures of Ce2O2MnSe2 and (Ce0.78La0.22)2O2MnSe2. Chem. Mater. 2015, 27, 3121–3134. [Google Scholar] [CrossRef] [Green Version]
- McCabe, E.E.; Free, D.G.; Evans, J.S.O. A new iron oxyselenide Ce2O2FeSe2: Synthesis and characterisation. Chem. Commun. 2011, 47, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Tuxworth, A.J.; McCabe, E.E.; Free, D.G.; Clark, S.J.; Evans, J.S.O. Structural Characterization and Physical Properties of the New Transition Metal Oxyselenide La2O2ZnSe2. Inorg. Chem. 2013, 52, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.M.; Wang, C.H.; Tucker, M.G.; Evans, J.S.O. Synthesis, Structural Characterization, and Physical Properties of the New Transition Metal Oxyselenide Ce2O2ZnSe2. Inorg. Chem. 2015, 54, 1563–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiramatsu, H.; Ueda, K.; Kamiya, T.; Ohta, H.; Hirano, M.; Hosono, H. Synthesis of single-phase layered oxychalcogenide La2CdO2Se2: Crystal structure, optical and electrical properties. J. Mater. Chem. 2004, 14, 2946–2950. [Google Scholar] [CrossRef]
- McCabe, E.E.; Free, D.G.; Mendis, B.G.; Higgins, J.S.; Evans, J.S.O. Preparation, Characterization, and Structural Phase Transitions in a New Family of Semiconducting Transition Metal Oxychalcogenides β-La2O2MSe2 (M = Mn, Fe). Chem. Mater. 2010, 22, 6171–6182. [Google Scholar] [CrossRef]
- Ainsworth, C.M.; Wang, C.H.; Johnston, H.E.; McCabe, E.E.; Tucker, M.G.; Brand, H.E.A.; Evans, J.S.O. Infinitely Adaptive Transition-Metal Ordering in Ln2O2MSe2-Type Oxychalcogenides. Inorg. Chem. 2015, 54, 7230–7238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tougait, O.; Ibers, J.A. Synthesis and Characterization of Three New Rare-Earth Titanium Oxyselenides: Ln3.67Ti2O3Se6 (Ln = Ce, Nd, Sm). Chem. Mater. 2000, 12, 2653–2658. [Google Scholar] [CrossRef]
- Tougait, O.; Ibers, J.A. Syntheses and Crystal Structures of the Lanthanum Titanium Oxyselenides La4Ti2O4Se5 and La6Ti3O5Se9. J. Solid State Chem. 2001, 157, 289–295. [Google Scholar] [CrossRef]
- Dung, N.H.; Tien, V.V. Synthèse et structure cristalline d’une nouvelle famille d’oxyséléniures de chrome III et de lanthanides légers, de formule générale RCrSe2O (R = La, Ce). C. R. Seances Acad. Sci. 1981, 293, 933–936. [Google Scholar]
- Meerschaut, A.; Lafond, A.; Meignen, V.; Deudon, C. Crystal Structure and Magnetic Properties of a New Oxyselenide of Gadolinium and Titanium: Gd4TiSe4O4. J. Solid State Chem. 2001, 162, 182–187. [Google Scholar] [CrossRef]
- Tuxworth, A.J.; Evans, J. Synthesis, structure and properties of the oxychalcogenide series A4O4TiSe4 (A = Sm, Gd, Tb, Dy, Ho, Er and Y). J. Solid State Chem. 2014, 210, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Strobel, S.; Choudhury, A.; Dorhout, P.K.; Lipp, C.; Schleid, T. Rare-Earth Metal(III) Oxide Selenides M4O4Se[Se2] (M = La, Ce, Pr, Nd, Sm) with Discrete Diselenide Units: Crystal Structures, Magnetic Frustration, and Other Properties. Inorg. Chem. 2008, 47, 4936–4944. [Google Scholar] [CrossRef] [PubMed]
- Peschke, S.; Weippert, V.; Senyshyn, A.; Mühlbauer, M.J.; Janka, O.; Pöttgen, R.; Holenstein, S.; Luetkens, H.; Johrendt, D. Flux synthesis, crystal structures, and magnetic ordering of the rare-earth chromium(II) oxyselenides RE2CrSe2O2 (RE = La–Nd). Inorg. Chem. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- APEX2, version 2012.12-0; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Sheldrick, G.M. SADABS, version 2012/1; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Jana2006, version 26/09/2012; Institute of Physics: Praha, Czech Republic, 2006. [Google Scholar]
- Coelho, A. TOPAS-Academic, version 4.1; Coelho Software: Brisbane, Australia, 2007. [Google Scholar]
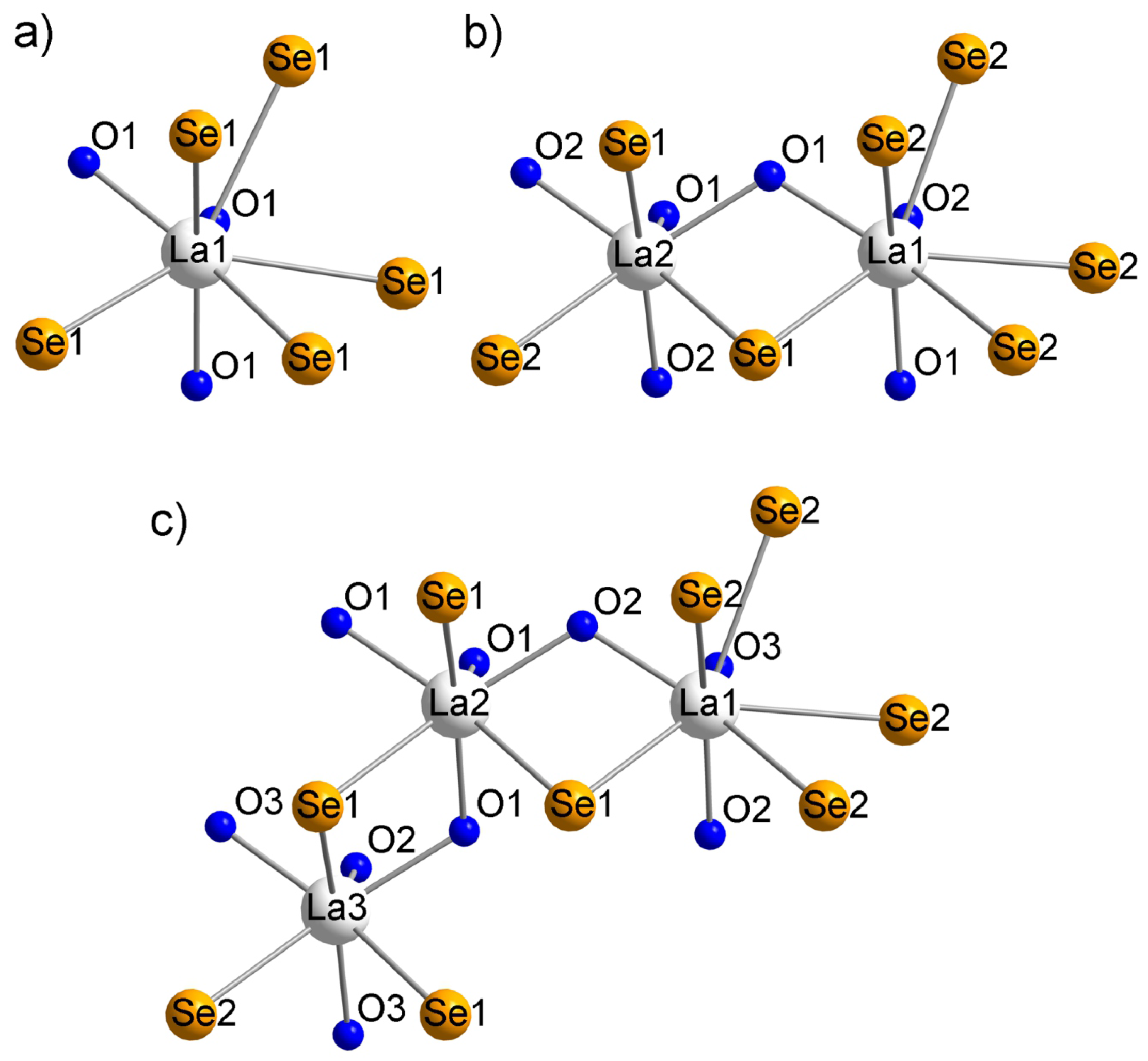
| -La2MnSe2O2 | La4MnSe3O4 | La6MnSe4O6 | |
|---|---|---|---|
| Space group | |||
| a (Å) | 11.6621(5) | 9.0055(4) | 24.760(2) |
| b (Å) | 3.9719(1) | 4.0186(1) | 4.0359(3) |
| c (Å) | 7.2049(3) | 7.1946(3) | 7.1850(6) |
| 121.655(2) | 109.715(2) | 104.162(3) | |
| Volume (Å) | 284.08(2) | 245.11(2) | 696.16(10) |
| Z | 2 | 1 | 2 |
| 0.024 | 0.028 | 0.056 | |
| 0.032 | 0.021 | 0.048 | |
| 53.88 | 34.98 | 35.10 | |
| (obs) | 0.024 | 0.023 | 0.026 |
| (all) | 0.036 | 0.032 | 0.049 |
| (obs) | 0.048 | 0.050 | 0.041 |
| (all) | 0.051 | 0.056 | 0.049 |
| GooF | 1.20 | 1.51 | 1.00 |
| +1.9/−2.5 | +3.9/−1.7 | +1.7/−1.7 |
| n | d | d | d(Mn–O) | d(Mn–Se) | d(La–O) | d(La–Se) |
|---|---|---|---|---|---|---|
| 0 | 397.2(1) | 616.0(1) | 205.4(1) | 284.1(1) | 234.4(1)–240.8(1) | 315.9(1)–337.1(1) |
| 1 | 401.9(1) | 900.6(1) | 207.0(1) | 284.8(1) | 236.4(1)–246.5(1) | 313.0(1)–333.8(1) |
| 2 | 403.6(1) | 1247.8(2) | 207.7(1) | 284.8(1) | 236.2(1)–245.2(1) | 309.4(1)–333.3(1) |
| n | ∡(La–O–La) | ∡(La–O–Mn) | ∡(Se–Mn–Se) | ∡(Se–Mn–O) |
|---|---|---|---|---|
| 0 | 105.1(1)–115.8(1) | 106.4(1)–111.7(1) | 88.7(1)–91.3(1) | 89.5(1)–90.5(1) |
| 1 | 102.8(1)–116.4(1) | 106.9(1)–109.8(1) | 89.8(1)–90.2(1) | 87.8(1)–92.2(1) |
| 2 | 102.8(1)–117.3(1) | 106.0(1)–109.9(1) | 89.8(1)–90.2(1) | 88.0(1)–92.0(1) |
| Compound | () | θ (K) | C (cm·K·mol) |
|---|---|---|---|
| -La2MnSe2O2 | 5.53(1) | 3.82(1) | |
| La4MnSe3O4 | 5.99(1) | 4.48(1) | |
| La6MnSe4O6 | 6.01(1) | 4.51(1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peschke, S.; Johrendt, D. Flux Synthesis, Crystal Structures, and Magnetism of the Series La2n+2MnSen+2O2n+2 (n = 0–2). Inorganics 2017, 5, 9. https://doi.org/10.3390/inorganics5010009
Peschke S, Johrendt D. Flux Synthesis, Crystal Structures, and Magnetism of the Series La2n+2MnSen+2O2n+2 (n = 0–2). Inorganics. 2017; 5(1):9. https://doi.org/10.3390/inorganics5010009
Chicago/Turabian StylePeschke, Simon, and Dirk Johrendt. 2017. "Flux Synthesis, Crystal Structures, and Magnetism of the Series La2n+2MnSen+2O2n+2 (n = 0–2)" Inorganics 5, no. 1: 9. https://doi.org/10.3390/inorganics5010009