Product Selectivity in Homogeneous Artificial Photosynthesis Using [(bpy)Rh(Cp*)X]n+-Based Catalysts
Abstract
:1. Introduction
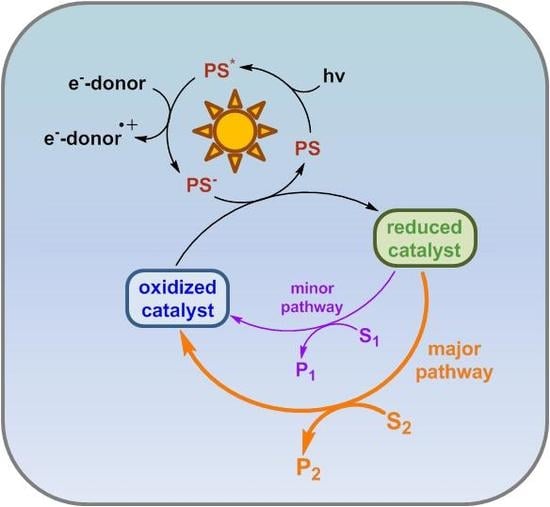
2. General Considerations on Product Selectivity within Artificial Photosynthetic Systems and Selected Aspects of the Homogeneous Photocatalytic CO2 Reduction
3. Application of [(bpy)Rh(Cp*)X]n+-Like Coordination Compounds as Multifunctional Redox Catalysts
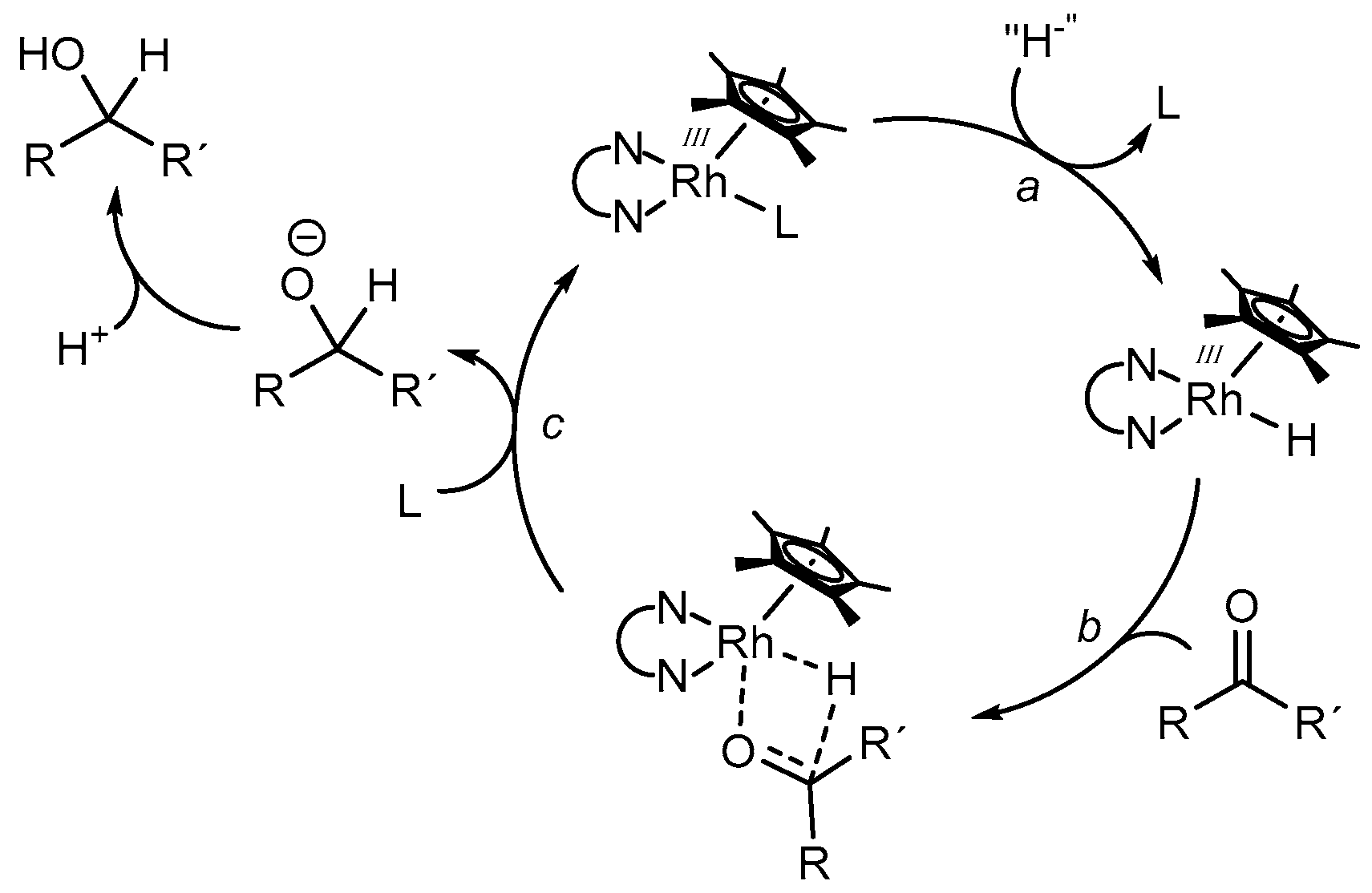
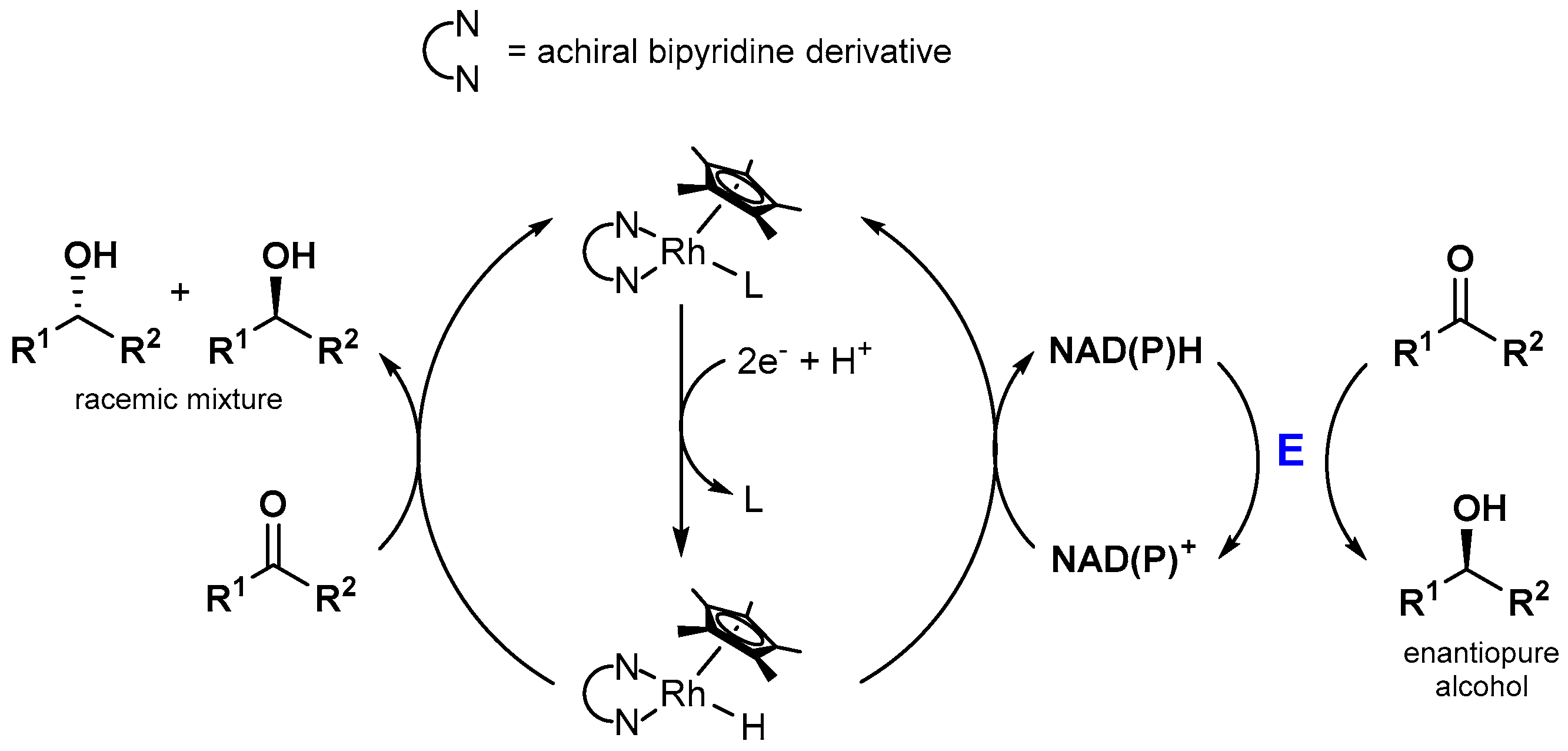
3.1. Reduction of Carbonyl Compounds to Primary or Secondary Alcohols
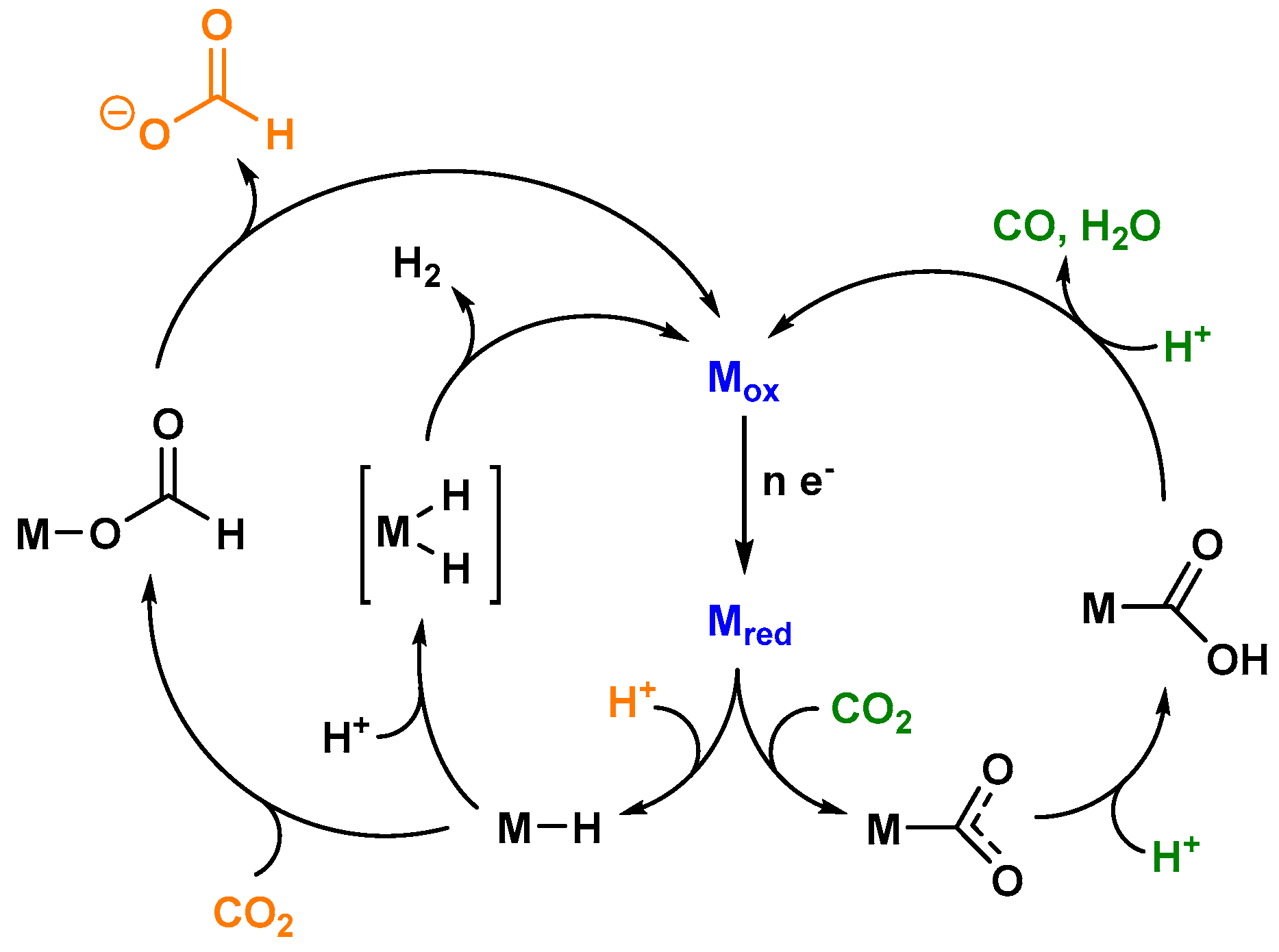
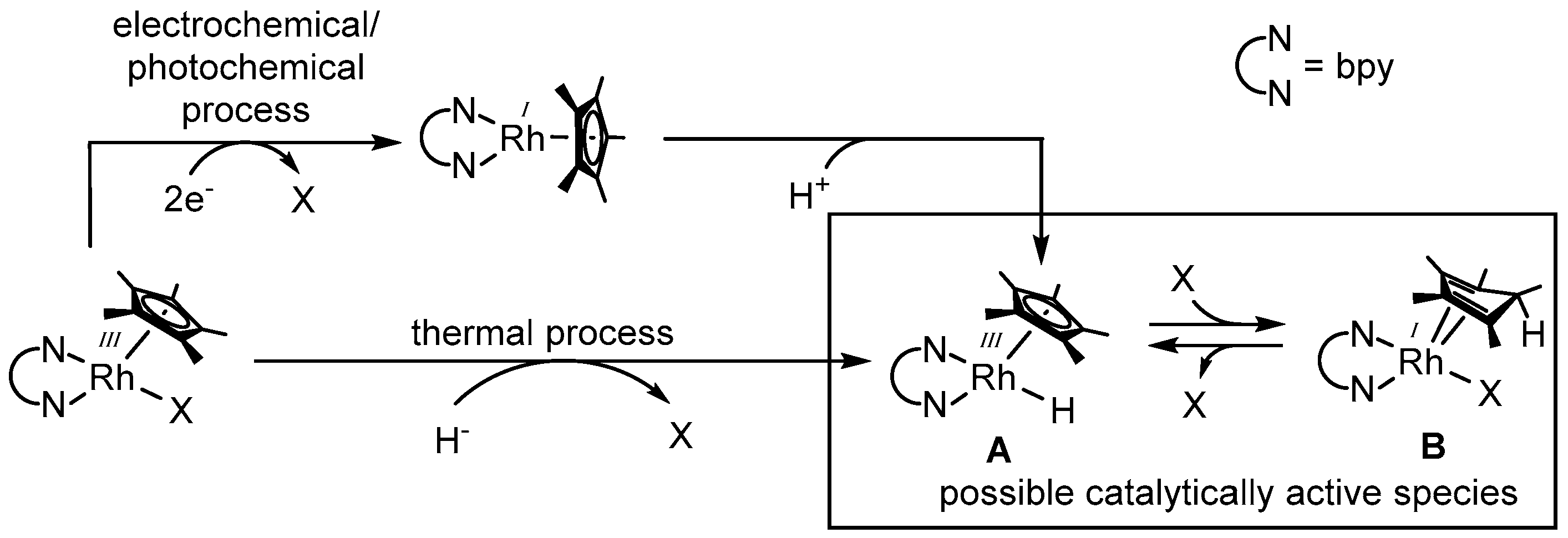
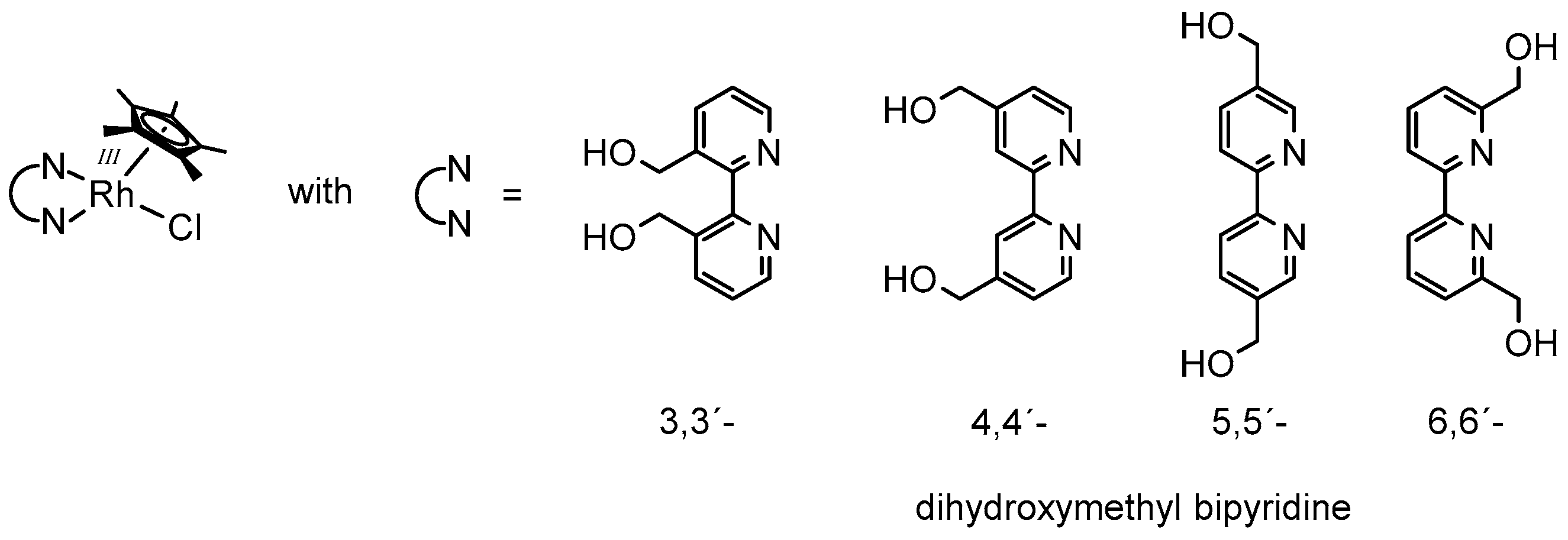
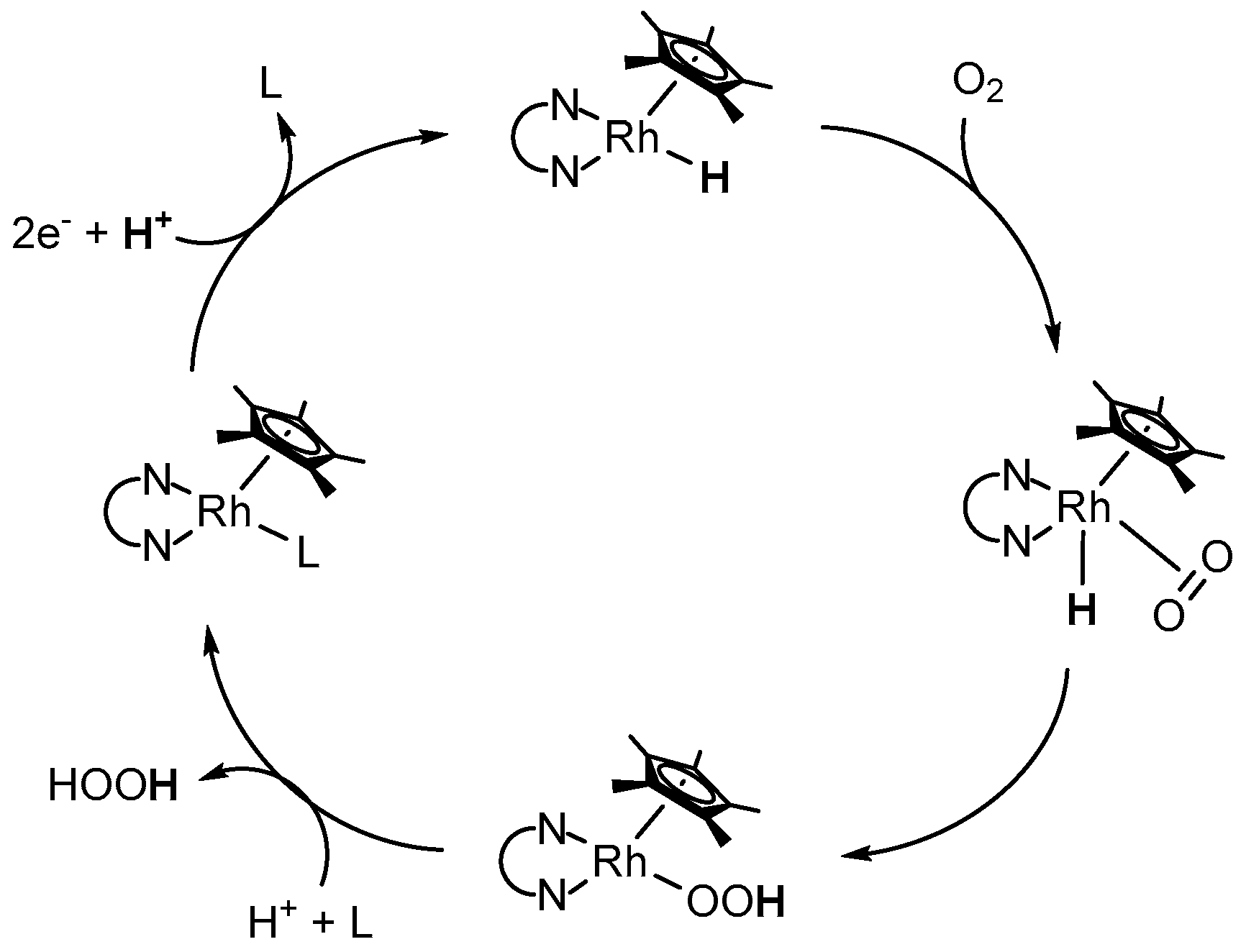
3.2. Use of [(bpy)Rh(Cp*)X]n+ Catalysts for Hydrogen Evolution and CO2 Reduction
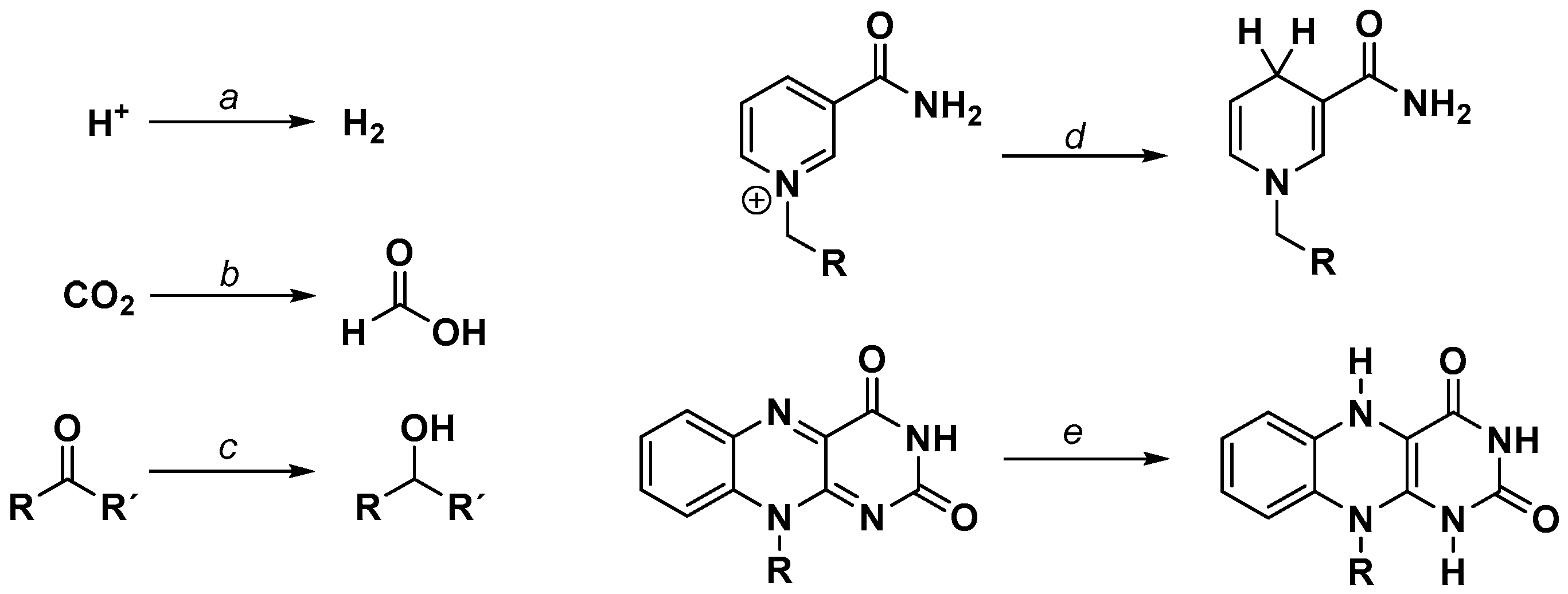
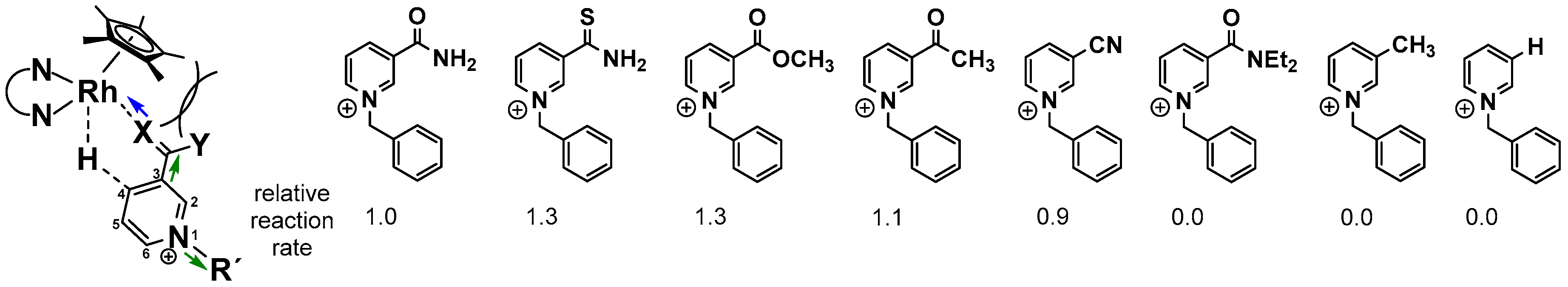
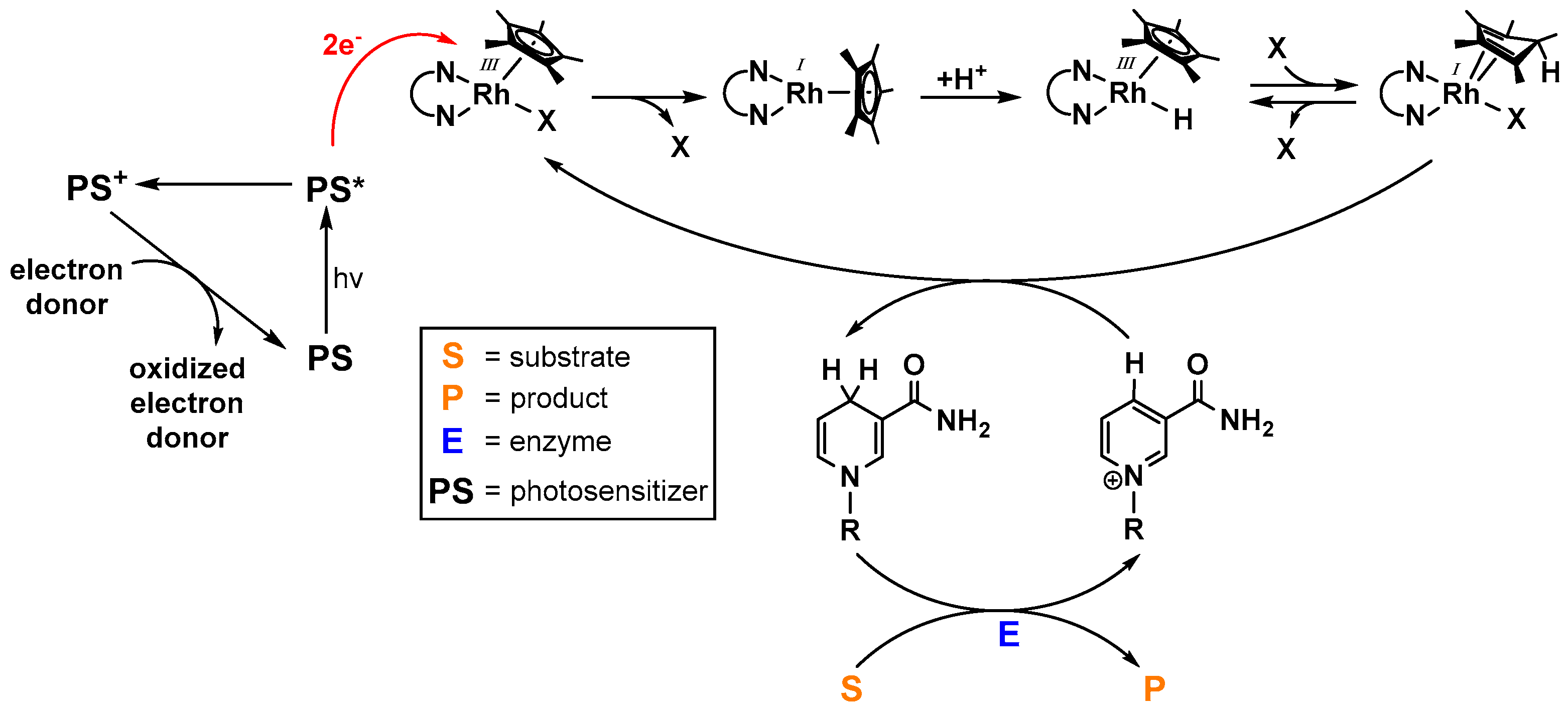
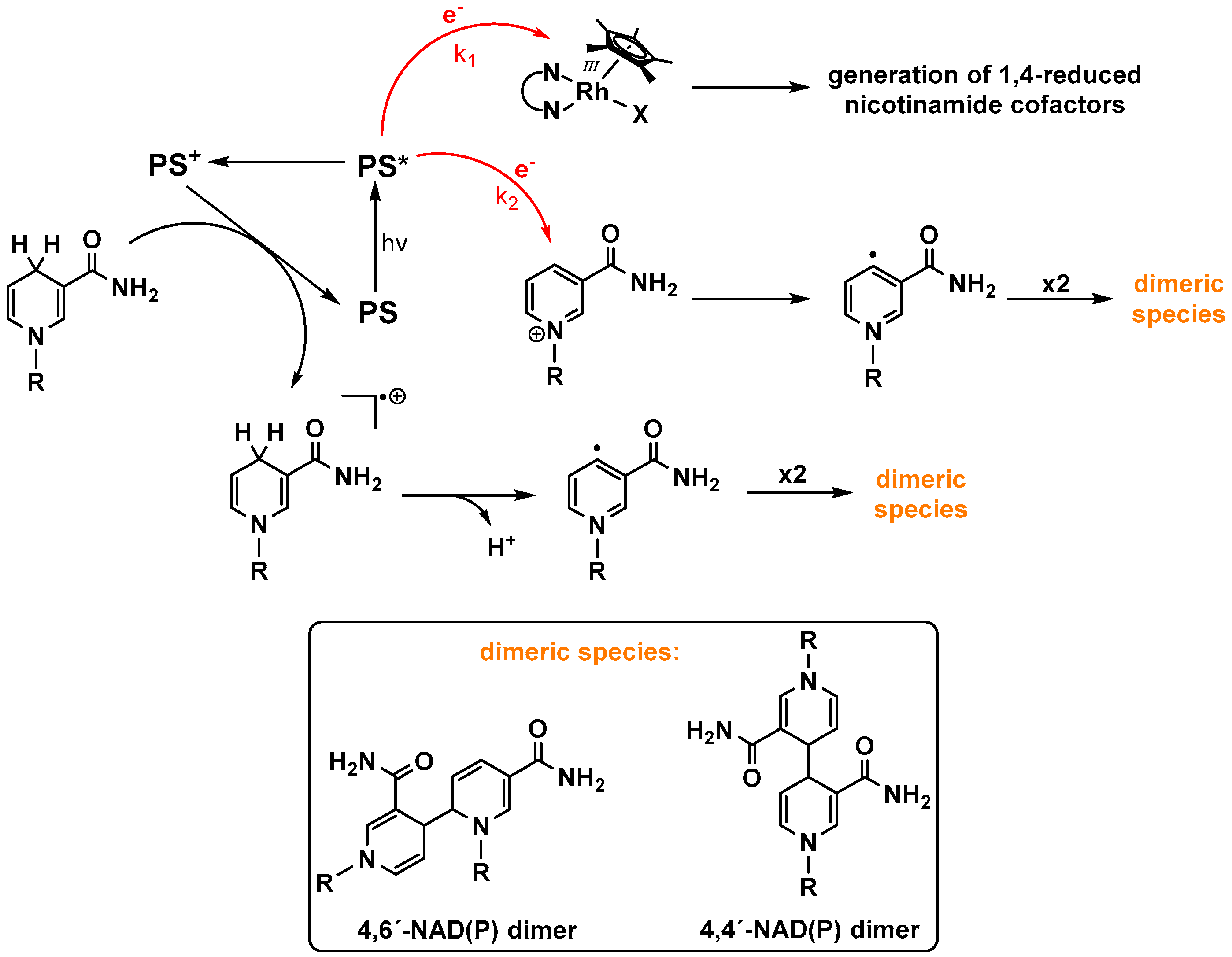
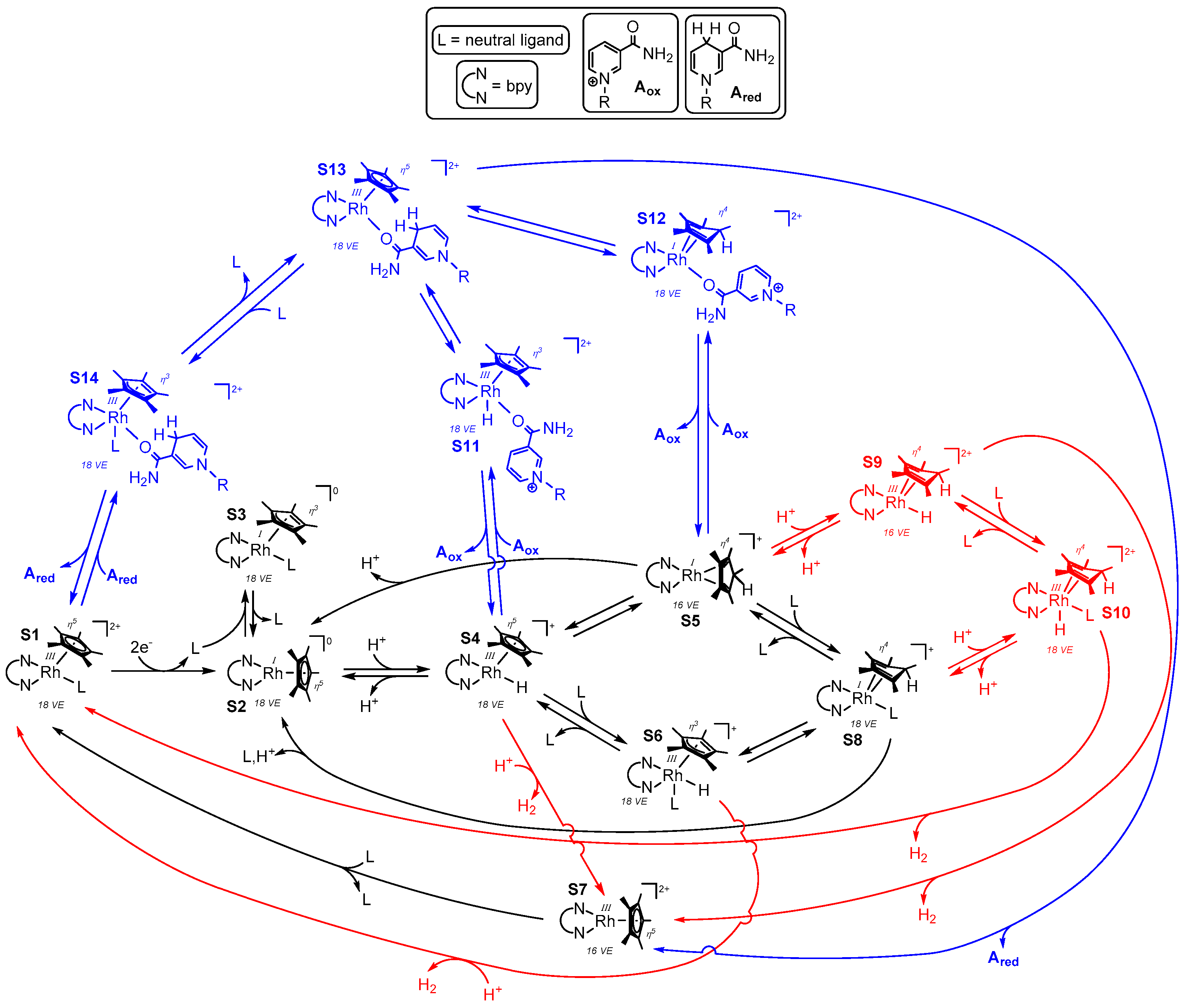
3.3. NAD(P)H Formation Using the [(bpy)Rh(Cp*)X]n+ Motive
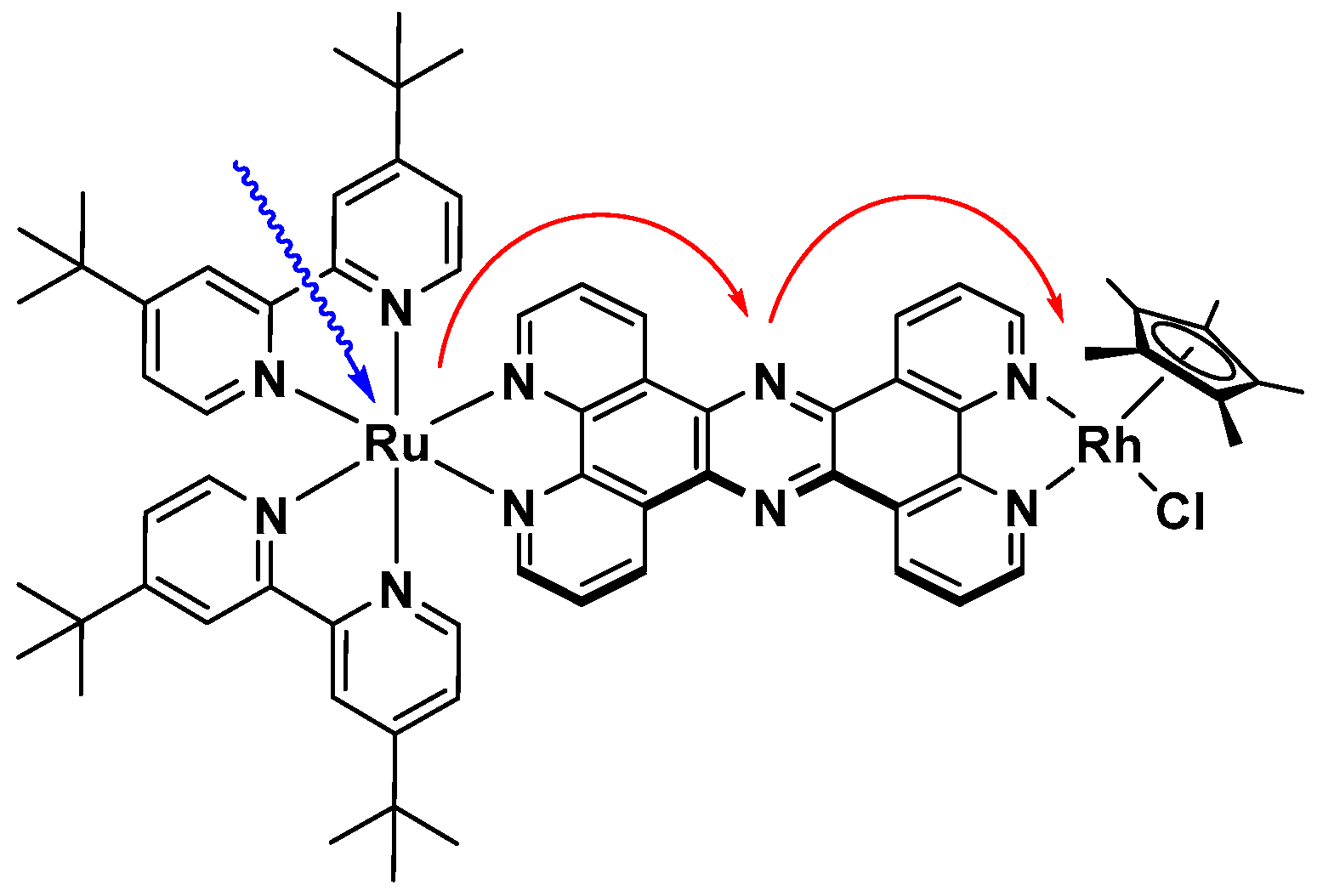
3.4. Hypothesis of an Overall Reaction Scheme for the Competing Generation of Molecular Hydrogen and Reduced Nicotinamide Cofactors: Application of [(bpy)Rh(Cp*)X]n+ as a Catalytic Center for Selective Homogeneous Photocatalysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monastersky, R. The human age. Nature 2015, 519, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Armor, J.N. A history of industrial catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Tang, W. Transition-metal catalyzed asymmetric carbon–carbon cross-coupling with chiral ligands. Tetrahedron 2016, 72, 6143–6174. [Google Scholar] [CrossRef]
- Renger, G. The light reactions of photosynthesis. Curr. Sci. 2010, 98, 1305–1319. [Google Scholar]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic reduction of CO2 using metal complexes. J. Photochem. Photobiol. C 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Sutin, N.; Creutz, C.; Fujita, E. Photo-Induced Generation of Dihydrogen and Reduction of Carbon Dioxide Using Transition Metal Complexes. Comments Inorg. Chem. 1997, 19, 67–92. [Google Scholar] [CrossRef]
- Walther, D.; Ruben, M.; Rau, S. Carbon dioxide and metal centres: From reactions inspired by nature to reactions in compressed carbon dioxide as solvent. Coord. Chem. Rev. 1999, 182, 67–100. [Google Scholar] [CrossRef]
- Fujita, E. Photochemical carbon dioxide reduction with metal complexes. Coord. Chem. Rev. 1999, 185–186, 373–384. [Google Scholar] [CrossRef]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Acc. Chem. Res. 2009, 43, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Ishitani, O. Development of efficient photocatalytic systems for CO2 reduction using mononuclear and multinuclear metal complexes based on mechanistic studies. Coord. Chem. Rev. 2010, 254, 346–354. [Google Scholar] [CrossRef]
- Doherty, M.D.; Grills, D.C.; Muckerman, J.T.; Polyansky, D.E.; Fujita, E. Toward more efficient photochemical CO2 reduction: Use of scCO2 or photogenerated hydrides. Coord. Chem. Rev. 2010, 254, 2472–2482. [Google Scholar] [CrossRef]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Ishitani, O. Substantial improvement in the efficiency and durability of a photocatalyst for carbon dioxide reduction using a benzoimidazole derivative as an electron donor. J. Catal. 2013, 304, 22–28. [Google Scholar] [CrossRef]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Yamazaki, Y.; Ishitani, O. Red-Light-Driven Photocatalytic Reduction of CO2 using Os(II)–Re(I) Supramolecular Complexes. Inorg. Chem. 2013, 52, 11902–11909. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Imori, D.; Morimoto, T.; Koike, K.; Ishitani, O. High catalytic abilities of binuclear rhenium(I) complexes in the photochemical reduction of CO2 with a ruthenium(II) photosensitiser. Dalton Trans. 2016, 45, 14668–14677. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Ishitani, O. Iridium(III) 1-Phenylisoquinoline Complexes as a Photosensitizer for Photocatalytic CO2 Reduction: A Mixed System with a Re(I) Catalyst and a Supramolecular Photocatalyst. Inorg. Chem. 2016, 55, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Tamaki, Y.; Ueno, K.; Kato, E.; Nishikawa, T.; Ohkubo, K.; Yamazaki, Y.; Morimoto, T.; Ishitani, O. Photocatalytic Reduction of Low Concentration of CO2. J. Am. Chem. Soc. 2016, 138, 13818–13821. [Google Scholar] [CrossRef] [PubMed]
- Rohacova, J.; Ishitani, O. Rhenium(I) trinuclear rings as highly efficient redox photosensitizers for photocatalytic CO2 reduction. Chem. Sci. 2016, 7, 6728–6739. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Koike, K.; Nakashima, T.; Morimoto, T.; Ishitani, O. Photocatalytic CO2 Reduction to Formic Acid Using a Ru(II)–Re(I) Supramolecular Complex in an Aqueous Solution. Inorg. Chem. 2015, 54, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Huang, H.-H.; Wang, J.-W.; Zhong, D.-C.; Lu, T.-B. A Dinuclear Cobalt Cryptate as a Homogeneous Photocatalyst for Highly Selective and Efficient Visible-Light Driven CO2 Reduction to CO in CH3CN/H2O Solution. Angew. Chem. Int. Ed. 2017, 56, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Morimoto, T.; Koike, K.; Ishitani, O. Photocatalytic CO2 reduction with high turnover frequency and selectivity of formic acid formation using Ru(II) multinuclear complexes. Proc. Natl. Acad. Sci. USA 2012, 109, 15673–15678. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Koike, K.; Ishitani, O. Highly efficient, selective, and durable photocatalytic system for CO2 reduction to formic acid. Chem. Sci. 2015, 6, 7213–7221. [Google Scholar] [CrossRef]
- Kuriki, R.; Matsunaga, H.; Nakashima, T.; Wada, K.; Yamakata, A.; Ishitani, O.; Maeda, K. Nature-Inspired, Highly Durable CO2 Reduction System Consisting of a Binuclear Ruthenium(II) Complex and an Organic Semiconductor Using Visible Light. J. Am. Chem. Soc. 2016, 138, 5159–5170. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Slanina, T.; König, B. Visible light photocatalytic reduction of aldehydes by Rh(III)–H: A detailed mechanistic study. Chem. Sci. 2015, 6, 2027–2034. [Google Scholar] [CrossRef]
- Blacker, A.J.; Clot, E.; Duckett, S.B.; Eisenstein, O.; Grace, J.; Nova, A.; Perutz, R.N.; Taylor, D.J.; Whitwood, A.C. Synthesis and structure of ‘‘16-electron’’ rhodium(III) catalysts for transfer hydrogenation of a cyclic imine: Mechanistic implications. Chem. Commun. 2009, 44, 6801–6803. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Han, J.; Lee, E.S.; Choi, E.B.; Lee, H.-K. Enantioselective Synthesis of Cyclic Sulfamidates by Using Chiral Rhodium-Catalyzed Asymmetric Transfer Hydrogenation. Org. Lett. 2010, 12, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lin, Z.; Wang, Q.; Wang, X.; Cun, L.; Yuan, W.; Zhu, J.; Deng, J. Rh(II)-Cp*-TsDPEN catalyzed aqueous asymmetric transfer hydrogenation of chromenones into saturated alcohol: C=C and C=O reduction in one step. Tetrahedron Lett. 2012, 53, 3828–3830. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Wu, X.; Pettman, A.; Xiao, J. pH-Regulated Asymmetric Transfer Hydrogenation of Quinolines in Water. Angew. Chem. Int. Ed. 2009, 48, 6524–6528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiu, R.; Xue, X.; Pan, Y.; Xu, C.; Li, H.; Xu, L. Versatile (Pentamethylcyclopentadienyl) rhodium-2,2’-Bipyridine (Cp*Rh-bpy) Catalyst for Transfer Hydrogenation of N-Heterocycles in Water. Adv. Synth. Catal. 2015, 357, 3529–3537. [Google Scholar] [CrossRef]
- Ryu, J.; Nam, D.H.; Lee, S.H.; Park, C.B. Biocatalytic Photosynthesis with Water as an Electron Donor. Chem. Eur. J. 2014, 20, 12020–12025. [Google Scholar] [CrossRef] [PubMed]
- Kölle, U.; Grätzel, M. Organometallic Rhodium(III) Complexes as Catalysts for the Photoreduction of Protons to Hydrogen on Colloidal TiO2. Angew. Chem. Int. Ed. Engl. 1987, 26, 567–570. [Google Scholar] [CrossRef]
- Cosnier, S.; Deronzier, A.; Vlachopoulos, N. Carbon/Poly{pyrrole-[(C5Me5)RhIII(bpy)Cl]+} Modified Electrodes; a Molecularly-based Material for Hydrogen Evolution (bpy = 2,2′-bipyridine). J. Chem. Soc. Chem. Commun. 1989, 1259–1261. [Google Scholar] [CrossRef]
- Chardon-Noblat, S.; Cosnier, S.; Deronzier, A.; Vlachopoulos, N. Electrochemical properties of [(C5Me5)RhIII(L)Cl]+ complexes (L = 2,2′-bipyridine or 1,10-phenanthroline derivatives) in solution and in related polypyrrolic films. Application to electrocatalytic hydrogen generation. J. Electroanal. Chem. 1993, 352, 213–228. [Google Scholar] [CrossRef]
- Caix, C.; Chardon-Noblat, S.; Deronzier, A.; Moutet, J.-C.; Tingry, S. (Pentamethylcyclopentadienyl) (polypyridyl) rhodium and iridium complexes as electrocatalysts for the reduction of protons to dihydrogen and the hydrogenation of organics. J. Organomet. Chem. 1997, 540, 105–111. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. Photocatalytic Production of Hydrogen by Disproportionation of One-Electron-Reduced Rhodium and Iridium–Ruthenium Complexes in Water. Angew. Chem. Int. Ed. 2011, 50, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.D.; Gupto, A.; Warren, J.J.; Brunschwig, B.S.; Gray, H.B. Noncovalent Immobilization of Electrocatalysts on Carbon Electrodes for Fuel Production. J. Am. Chem. Soc. 2013, 135, 18288–18291. [Google Scholar] [CrossRef] [PubMed]
- Caix, C.; Chardon-Noblat, S.; Deronzier, A. Electrocatalytic reduction of CO2 into formate with [(η5-Me5C5)M(L)CI]+ complexes (L = 2,2′-bipyridine ligands; M = Rh(III) and Ir(III)). J. Electroanal. Chem. 1997, 434, 163–170. [Google Scholar] [CrossRef]
- Chambers, M.B.; Wang, X.; Elgrishi, N.; Hendon, H.C.; Walsh, A.; Bonnefoy, J.; Canivet, J.; Quadrelli, E.A.; Farrusseng, D.; Mellot-Draznieks, C.; et al. Photocatalytic Carbon Dioxide Reduction with Rhodium-based Catalysts in Solution and Heterogenized within Metal–Organic Frameworks. ChemSusChem 2015, 8, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Leiva, C.; Lo, H.C.; Fish, R.H. Aqueous organometallic chemistry. 3. Catalytic hydride transfer reactions with ketones and aldehydes using [Cp*Rh(bpy)(H2O)](OTf)2 as the precatalyst and sodium formate as the hydride source: Kinetic and activation parameters, and the significance of steric and electronic effects. J. Organomet. Chem. 2010, 695, 145–150. [Google Scholar]
- Montalvo-González, R.; Chávez, D.; Aguirre, G.; Parra-Hake, M.; Samanathan, R. Asymmetric Transfer Hydrogenation of Ketones in Aqueous Solution Catalyzed by Rhodium(III) Complexes with C3-Symmetric Fluorene-Ligands Containing Chiral (1R,2R)-Cyclohexane-1,2-diamine. J. Braz. Chem. Soc. 2010, 21, 431–435. [Google Scholar] [CrossRef]
- Himeda, Y.; Onozawa-Komatsuzaki, N.; Sugihara, H.; Arakawa, H.; Kasuga, K. Transfer hydrogenation of a variety of ketones catalyzed by rhodium complexes in aqueous solution and their application to asymmetric reduction using chiral Schiff base ligands. J. Mol. Catal. A Chem. 2003, 195, 95–100. [Google Scholar] [CrossRef]
- Thorpe, T.; Blacker, J.; Brown, S.M.; Bubert, C.; Crosby, J.; Fitzjohn, S.; Muxworthy, J.P.; Williams, J.M.J. Efficient rhodium and iridium-catalysed asymmetric transfer hydrogenation using water-soluble aminosulfonamide ligands. Tetrahedron Lett. 2001, 42, 4041–4043. [Google Scholar] [CrossRef]
- Ruppert, R.; Herrmann, S.; Steckhan, E. Very Efficient Reduction of NAD(P)+ with Formate catalysed by Cationic Rhodium Complexes. J. Chem. Soc. Chem. Commun. 1988, 1150–1151. [Google Scholar] [CrossRef]
- Steckhan, E.; Herrmann, S.; Ruppert, R.; Thömmes, J.; Wandrey, C. Continuous Generation of NADH from NAD+ and Formate Using a Homogeneous Catalyst with Enhanced Molecular Weight in a Membrane Reactor. Angew. Chem. Int. Ed. Engl. 1990, 29, 388–390. [Google Scholar] [CrossRef]
- Westerhausen, D.; Herrmann, S.; Hummel, W.; Steckhan, E. Formate-Driven, Non-Enzymatic NAD(P)H Regeneration for the Alcohol Dehydrogenase Catalyzed Stereoselective Reduction of 4-Phenyl-2-butanone. Angew. Chem. Int. Ed. Engl. 1992, 31, 1529–1531. [Google Scholar] [CrossRef]
- Hollmann, F.; Kleeb, A.; Otto, K.; Schmid, A. Coupled chemoenzymatic transfer hydrogenation catalysis for enantioselective reduction and oxidation reactions. Tetrahedron Asymmetry 2005, 16, 3512–3519. [Google Scholar] [CrossRef]
- Canivet, J.; Süss-Fink, G.; Stepnicka, P. Water-Soluble Phenanthroline Complexes of Rhodium, Iridium and Ruthenium for the Regeneration of NADH in the Enzymatic Reduction of Ketones. Eur. J. Inorg. Chem. 2007, 4736–4742. [Google Scholar] [CrossRef]
- Ruppert, R.; Herrmann, S.; Steckhan, E. Efficient Indirect Electrochemical in-situ Regeneration of NADH: Electrochemically Driven Enzymatic Reduction of Pyruvate Catalyzed by D-LDH. Tetrahedron Lett. 1987, 28, 6583–6586. [Google Scholar] [CrossRef]
- Steckhan, E.; Herrmann, S.; Ruppert, R.; Dietz, E.; Frede, M.; Spika, E. Analytical Study of a Series of Substituted (2,2′-Bipyridyl) (pentamethylcyclopentadienyl)rhodium and -iridium Complexes with Regard to Their Effectiveness as Redox Catalysts for the Indirect Electrochemical and Chemical Reduction of NAD(P)+. Organometallics 1991, 10, 1568–1577. [Google Scholar] [CrossRef]
- Cosnier, S.; Gunther, H. A polypyrrole [RhIII(C5Me5)(bpy)Cl]+ modified electrode for the reduction of NAD+ cofactor. J. Electroanal. Chem. 1991, 315, 307–312. [Google Scholar] [CrossRef]
- Hollmann, F.; Schmid, A.; Steckhan, E. The First Synthetic Application of a Monooxygenase Employing Indirect Electrochemical NADH Regeneration. Angew. Chem. Int. Ed. 2001, 40, 169–171. [Google Scholar] [CrossRef]
- Hildebrand, F.; Kohlmann, C.; Franz, A.; Lütz, S. Synthesis, Characterization and Application of New Rhodium Complexes for Indirect Electrochemical Cofactor Regeneration. Adv. Synth. Catal. 2008, 350, 909–918. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.H.; Park, C.B. Coupling Photocatalysis and Redox Biocatalysis toward Biocatalyzed Artificial Photosynthesis. Chem. Eur. J. 2013, 19, 4392–4406. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Witholt, B.; Schmid, A. [Cp*Rh(bpy)(H2O)]2+: A versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes. J. Mol. Catal. B Enzym. 2003, 19–20, 167–176. [Google Scholar] [CrossRef]
- Grau, M.M.; Poizat, M.; Arends, I.W.C.E.; Hollmann, F. Phosphite-driven, [Cp*Rh(bpy)(H2O)]2+-catalyzed reduction of nicotinamide and flavin cofactors: Characterization and application to promote chemoenzymatic reduction reactions. Appl. Organomet. Chem. 2010, 24, 380–385. [Google Scholar] [CrossRef]
- Van Esch, J.H.; Hoffmann, M.A.M.; Nolte, R.J.M. Reduction of Nicotinamides, Flavins, and Manganese Porphyrins by Formate, Catalyzed by Membrane-Bound Rhodium Complexes. J. Org. Chem. 1995, 60, 1599–1610. [Google Scholar] [CrossRef]
- Hollmann, F.; Lin, P.-C.; Witholt, B.; Schmid, A. Stereospecific Biocatalytic Epoxidation: The First Example of Direct Regeneration of a FAD-Dependent Monooxygenase for Catalysis. J. Am. Chem. Soc. 2003, 125, 8209–8217. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. Efficient Catalytic Decomposition of Formic Acid for the Selective Generation of H2 and H/D Exchange with a Water-Soluble Rhodium Complex in Aqueous Solution. ChemSusChem 2008, 1, 827–834. [Google Scholar] [PubMed]
- Pitman, C.L.; Finster, O.N.L.; Miller, A.J.M. Cyclopentadiene-mediated hydride transfer from rhodium complexes. Chem. Commun. 2016, 52, 9105–9108. [Google Scholar] [CrossRef] [PubMed]
- Aguirre Quintana, L.M.; Johnson, S.I.; Corona, S.L.; Villatoro, W.; Goddard, W.A., III; Takase, M.K.; VanderVelde, D.G.; Winkler, J.R.; Gray, H.B.; Blakemore, J.D. Proton–hydride tautomerism in hydrogen evolution catalysis. Proc. Natl. Acad. Sci. USA 2016, 113, 6409–6414. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Sivanesan, D.; Yoon, S. Correlation between the Structure and Catalytic Activity of [Cp*Rh(Substituted Bipyridine)] Complexes for NADH Regeneration. Inorg. Chem. 2017, 56, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Fiedler, J.; Reinhardt, R.; Kaim, W. Metal vs. Ligand Reduction in Complexes of Dipyrido[3,2-a:2′,3′-c]phenazine and Related Ligands with [(C5Me5)ClM]+ (M = Rh or Ir): Evidence for Potential Rather Than Orbital Control in the Reductive Cleavage of the Metal-Chloride Bond. Inorg. Chem. 2004, 43, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Kölle, U.; Kang, B.-S.; Infelta, P.; Comte, P.; Grätzel, M. Elektrochemische und pulsradiolytische Reduktion von (Pentamethylcyclopentadienyl)(polypyridyl)rhodium-Komplexen. Chem. Ber. 1989, 122, 1869–1880. [Google Scholar] [CrossRef]
- Nakai, H.; Jeong, K.; Matsumoto, T.; Ogo, S. Catalytic C–F Bond Hydrogenolysis of Fluoroaromatics by [(η5-C5Me5)RhI(2,2′-bipyridine)]. Organometallics 2014, 33, 4349–4352. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Joó, F. Breakthroughs in Hydrogen Storage—Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Kölle, U.; Fränzl, H. Oxidation of Alcohols by [Cp*Rh(ppy)(OH)]+. Monatsh. Chem. 2000, 131, 1321–1326. [Google Scholar] [CrossRef]
- Kim, S.; Lee, G.Y.; Baeg, J.-O.; Kim, Y.; Kim, S.-J.; Kim, J. Visible-Light-Driven Photoproduction of Hydrogen Using Rhodium Catalysts and Platinum Nanoparticles with Formate. J. Phys. Chem. C 2014, 118, 25844–25852. [Google Scholar] [CrossRef]
- Himeda, Y. Conversion of CO2 into Formate by Homogeneously Catalyzed Hydrogenation in Water: Tuning Catalytic Activity and Water Solubility through the Acid–Base Equilibrium of the Ligand. Eur. J. Inorg. Chem. 2007, 22, 3927–3941. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E.; Buehler, K. Biocatalytic Redox Reactions for Organic Synthesis: Nonconventional Regeneration Methods. ChemCatChem 2010, 2, 762–782. [Google Scholar] [CrossRef]
- Kohlmann, C.; Märkle, W.; Lütz, S. Electroenzymatic synthesis. J. Mol. Catal. B Enzym. 2008, 51, 57–72. [Google Scholar] [CrossRef]
- Quinto, T.; Köhler, V.; Ward, T.R. Recent Trends in Biomimetic NADH Regeneration. Top. Catal. 2014, 57, 321–331. [Google Scholar] [CrossRef]
- Wienkamp, R.; Steckhan, E. Indirect Electrochemical Regeneration of NADH by a Bipyridinerhodium(I) Complex as Electron-Transfer Agent. Angew. Chem. Int. Ed. Engl. 1982, 21, 782–783. [Google Scholar] [CrossRef]
- Wienkamp, R.; Steckhan, E. Selective Generation of NADH by Visible Light. Angew. Chem. Int. Ed. Engl. 1983, 22, 497. [Google Scholar] [CrossRef]
- Umeda, K.; Nakamura, A.; Toda, F. Photochemical Reduction of NAD+ to 1,4-NADH without an Enzyme. J. Chem. Soc. Chem. Commun. 1990, 885–886. [Google Scholar] [CrossRef]
- Umeda, K.; Nakamura, A.; Toda, F. Catalytic Mechanism and Activity of Bis(2,2′:6′,2′′-terpyridine) rhodium(III) for the Reduction of NAD+ into NADH in a Photosensitized Reaction System. Bull. Chem. Soc. Jpn. 1993, 66, 2260–2267. [Google Scholar] [CrossRef]
- Beley, M.; Collin, J.-P. Electrochemical regeneration of nicotinamide cofactor using a polypyrrole rhodium bis-terpyridine modified electrode. J. Mol. Catal. 1997, 79, 133–140. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.; Lee, J.; Baeg, J.-O.; Kim, J. Photochemical Production of NADH Using Cobaloxime Catalysts and Visible-Light Energy. Inorg. Chem. 2012, 51, 8057–8063. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Fish, R.H. Biomimetic NAD+ Models for Tandem Cofactor Regeneration, Horse Liver Alcohol Dehydrogenase Recognition of 1,4-NADH Derivatives, and Chiral Synthesis. Angew. Chem. Int. Ed. 2002, 41, 478–481. [Google Scholar] [CrossRef]
- Lo, H.C.; Buriez, O.; Kerr, J.B.; Fish, R.H. Regioselective Reduction of NAD+ Models with [Cp*Rh(bpy)H]+: Structure—Activity Relationships and Mechanistic Aspects in the Formation of the 1,4-NADH Derivatives. Angew. Chem. Int. Ed. 1999, 38, 1429–1432. [Google Scholar] [CrossRef]
- Lo, H.C.; Leiva, C.; Buriez, O.; Kerr, J.B.; Olmstead, M.M.; Fish, R.H. Bioorganometallic Chemistry. 13. Regioselective Reduction of NAD+ Models, 1-Benzylnicotinamde Triflate and β-Nicotinamide Ribose-5′-methyl Phosphate, with in Situ Generated [Cp*Rh(Bpy)H]+: Structure-Activity Relationships, Kinetics, and Mechanistic Aspects in the Formation of the 1,4-NADH Derivatives. Inorg. Chem. 2001, 40, 6705–6716. [Google Scholar] [PubMed]
- Lee, S.H.; Lee, H.J.; Won, K.; Park, C.B. Artificial Electron Carriers for Photoenzymatic Synthesis under Visible Light. Chem. Eur. J. 2012, 18, 5490–5495. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Nasraoui, R.; Wang, Z.; Qu, F.; Urbanova, V.; Etienne, M.; Göllü, M.; Demir, A.S.; Gajdzik, J.; Hempelmann, R. Factors affecting the electrochemical regeneration of NADH by (2,2′-bipyridyl) (pentamethylcyclopentadienyl)-rhodium complexes: Impact on their immobilization onto electrode surfaces. Bioelectrochemistry 2011, 82, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Hollmann, F.; Ho, T.V.; Schnyder, A.; Fish, R.H.; Schmid, A. Bioorganometallic chemistry: Biocatalytic oxidation reactions with biomimetic NAD+/NADH co-factors and [Cp*Rh(bpy)H]+ for selective organic synthesis. J. Organomet. Chem. 2004, 689, 4783–4790. [Google Scholar] [CrossRef]
- Hildebrand, F.; Lütz, S. Stable Electroenzymatic Processes by Catalyst Separation. Chem. Eur. J. 2009, 15, 4998–5001. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Nam, D.H.; Park, C.B. Screening Xanthene Dyes for Visible Light-Driven Nicotinamide Adenine Dinucleotide Regeneration and Photoenzymatic Synthesis. Adv. Synth. Catal. 2009, 351, 2589–2594. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, D.H.; Kim, J.H.; Baeg, J.-O.; Park, C.B. Eosin Y-Sensitized Artificial Photosynthesis by Highly Efficient Visible-Light-Driven Regeneration of Nicotinamide Cofactor. ChemBioChem 2009, 10, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Park, C.B. Visible Light-Driven NADH Regeneration Sensitized by Proflavine for Biocatalysis. ChemBioChem 2012, 13, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Baeg, J.-O.; Oh, G.H.; Park, N.-J.; Kong, K.-J.; Kim, J.; Hwang, D.W.; Biswas, S.K. A Photocatalyst–Enzyme Coupled Artificial Photosynthesis System for Solar Energy in Production of Formic Acid from CO2. J. Am. Chem. Soc. 2012, 134, 11455–11461. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baeg, J.-O.; Park, N.-J.; Yadav, R.K. A Photocatalyst/Enzyme Couple That Uses Solar Energy in the Asymmetric Reduction of Acetophenones. Angew. Chem. Int. Ed. 2012, 51, 11624–11628. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baeg, J.-O.; Park, N.-J.; Yadav, R.K. A solar light-driven, eco-friendly protocol for highly enantioselective synthesis of chiral alcohols via photocatalytic/biocatalytic cascades. Green Chem. 2014, 16, 4389–4400. [Google Scholar] [CrossRef]
- Yadav, R.K.; Baeg, J.-O.; Kumar, A.; Kong, K.-J.; Oh, G.H.; Park, N.-J. Graphene–BODIPY as a photocatalyst in the photocatalytic–biocatalytic coupled system for solar fuel production from CO2. J. Mater. Chem. A 2014, 2, 5068–5076. [Google Scholar] [CrossRef]
- Oppelt, K.T.; Wöß, E.; Stiftinger, M.; Schöfberger, W.; Buchberger, W.; Knör, G. Photocatalytic Reduction of Artificial and Natural Nucleotide Cofactors with a Chlorophyll-Like Tin-Dihydroporphyrin Sensitizer. Inorg. Chem. 2013, 52, 11910–11922. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Anjong, T.F.; Jang, H.Y.; Kim, J.-Y.; Lee, C.; Park, S.; Lee, H.J.; Yoon, J.; Kim, J. Artificial Photocatalytic System Using Polydiacetylene-(−NHphen)Ru(bpy)2 for Cofactor Regeneration and CO2 Reduction. J. Phys. Chem. C 2016, 120, 28407–28414. [Google Scholar] [CrossRef]
- Oppelt, K.T.; Gasiorowski, J.; Egbe, D.A.M.; Kollender, J.P.; Himmelsbach, M.; Hassel, A.W.; Sariciftci, N.S.; Knör, G. Rhodium-Coordinated Poly(arylene-ethynylene)-alt-Poly(arylenevinylene) Copolymer Acting as Photocatalyst for Visible-Light-Powered NAD+/NADH Reduction. J. Am. Chem. Soc. 2014, 136, 12721–12729. [Google Scholar] [CrossRef] [PubMed]
- Mengele, A.K.; Seibold, G.M.; Eikmanns, B.J.; Rau, S. Coupling Molecular Photocatalysis to Enzymatic Conversion. 2017; Submitted. [Google Scholar]
- Shi, Q.; Yang, D.; Jiang, Z.; Li, J. Visible-light photocatalytic regeneration of NADH using P-doped TiO2 nanoparticles. J. Mol. Catal. B Enzym. 2006, 43, 44–48. [Google Scholar] [CrossRef]
- Liu, J.; Antonietti, M. Bio-inspired NADH regeneration by carbon nitride photocatalysis using diatom templates. Energy Environ. Sci. 2013, 6, 1486–1493. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, S.H.; Nam, D.H.; Park, C.B. Rational Design and Engineering of Quantum-Dot-Sensitized TiO2 Nanotube Arrays for Artificial Photosynthesis. Adv. Mater. 2011, 23, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Zhou, H.; Antonietti, M. Uniform Graphitic Carbon Nitride Nanorod for Efficient Photocatalytic Hydrogen Evolution and Sustained Photoenzymatic Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 8434–8440. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Yadav, R.K.; Kumar, A.; Park, N.-J.; Baeg, J.-O. Functionalized Graphene Quantum Dots as Efficient Visible-Light Photocatalysts for Selective Solar Fuel Production from CO2. ChemCatChem 2016, 8, 3389–3393. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Yang, Q.; Liu, Y.; Zhu, Y.; Li, T.; Tsang, Y.H.; Zhang, X. Microfluidic chip-based one-step fabrication of an artificial photosystem I for photocatalytic cofactor regeneration. RSC Adv. 2016, 6, 101974–101980. [Google Scholar] [CrossRef]
- Yadav, R.K.; Kumar, A.; Park, N.-J.; Kong, K.-J.; Baeg, J.-O. A highly efficient covalent organic framework film photocatalyst for selective solar fuel production from CO2. J. Mater. Chem. A 2016, 4, 9413–9418. [Google Scholar] [CrossRef]
- Liu, J.; Cazelles, R.; Chen, Z.P.; Zhou, H.; Galarneau, A.; Antonietti, M. The bioinspired construction of an ordered carbon nitride array for photocatalytic mediated enzymatic reduction. Phys. Chem. Chem. Phys. 2014, 16, 14699–14705. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Antonietti, M.; Liu, J. Bio-inspired carbon nitride mesoporous spheres for artificial photosynthesis: Photocatalytic cofactor regeneration for sustainable enzymatic synthesis. J. Mater. Chem. A 2014, 2, 7686–7693. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, J.; Nam, D.H.; Park, C.B. Photoenzymatic synthesis through sustainable NADH regeneration by SiO2-supported quantum dots. Chem. Commun. 2011, 47, 4643–4645. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Baran, T.; Angelini, A.; Labuz, P.; Macyk, W. An integrated photocatalytic/enzymatic system for the reduction of CO2 to methanol in bioglycerol–water. Beilstein J. Org. Chem. 2014, 10, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Land, E.J.; Swallow, A.J. One-Electron Reactions in Biochemical Systems as Studied by Puls Radiolysis. IV. Oxidation of Dihydronicotinamide-adenine dinucleotide. Biochim. Biophys. Acta 1971, 234, 34–42. [Google Scholar] [CrossRef]
- Kiwi, J. Photochemical Generation of Reduced β-Nicotinamide Adenine Dinucleotide (Induced by Light). J. Photochem. 1981, 16, 193–202. [Google Scholar] [CrossRef]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, Y.; Odobel, F. Sacrificial electron donor reagents for solar fuel production. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
- Tamaki, Y.; Watanabe, K.; Koike, K.; Inoue, H.; Morimoto, T.; Ishitani, O. Development of highly efficient supramolecular CO2 reduction photocatalysts with high turnover frequency and durability. Faraday Discuss. 2012, 155, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Suenobu, T.; Patz, M.; Hirasaka, T.; Itoh, S.; Fujitsuka, M.; Ito, O. Selective One-Electron and Two-Electron Reduction of C60 with NADH and NAD Dimer Analogues via Photoinduced Electron Transfer. J. Am. Chem. Soc. 1998, 120, 8060–8068. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Suenobu, T.; Hirasaka, T.; Sakurada, N.; Arakawa, R.; Fujitsuka, M.; Ito, O. Enhanced Reactivity of C70 in the Photochemical Reactions with NADH and NAD Dimer Analogues As Compared to C60 via Photoinduced Electron Transfer. J. Phys. Chem. A 1999, 103, 5935–5941. [Google Scholar] [CrossRef]
- Sastre-Santos, Á.; Parejo, C.; Martín-Gomis, L.; Ohkubo, K.; Fernández-Lázaro, F.; Fukuzumi, S. C60 dimers connected through pleiadene bridges: Fullerenes talking to each other. J. Mater. Chem. 2011, 21, 1509–1515. [Google Scholar] [CrossRef]
- Ali, M.M.; Sato, H.; Haga, M.-A.; Tanaka, K.; Yoshimura, A.; Ohno, T. Two-Electron Reduction of [{(bpy)2Ru(dmbbbpy)}3Ru]8+ from (BNA)2 via Photoinduced Electron Transfer [dmbbbpy = 2,2′-Bis(N-methylbenzimidazole-2-yl)-4,4′-bipyridine]. Inorg. Chem. 1998, 37, 6176–6180. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mengele, A.K.; Rau, S. Product Selectivity in Homogeneous Artificial Photosynthesis Using [(bpy)Rh(Cp*)X]n+-Based Catalysts. Inorganics 2017, 5, 35. https://doi.org/10.3390/inorganics5020035
Mengele AK, Rau S. Product Selectivity in Homogeneous Artificial Photosynthesis Using [(bpy)Rh(Cp*)X]n+-Based Catalysts. Inorganics. 2017; 5(2):35. https://doi.org/10.3390/inorganics5020035
Chicago/Turabian StyleMengele, Alexander K., and Sven Rau. 2017. "Product Selectivity in Homogeneous Artificial Photosynthesis Using [(bpy)Rh(Cp*)X]n+-Based Catalysts" Inorganics 5, no. 2: 35. https://doi.org/10.3390/inorganics5020035