(18-Crown-6)potassium(I) Trichlorido[28-acetyl-3-(tris-(hydroxylmethyl)amino-ethane)betulinic ester-κN]platinum(II): Synthesis and In Vitro Antitumor Activity
Abstract
:1. Introduction
2. Results and Discussion
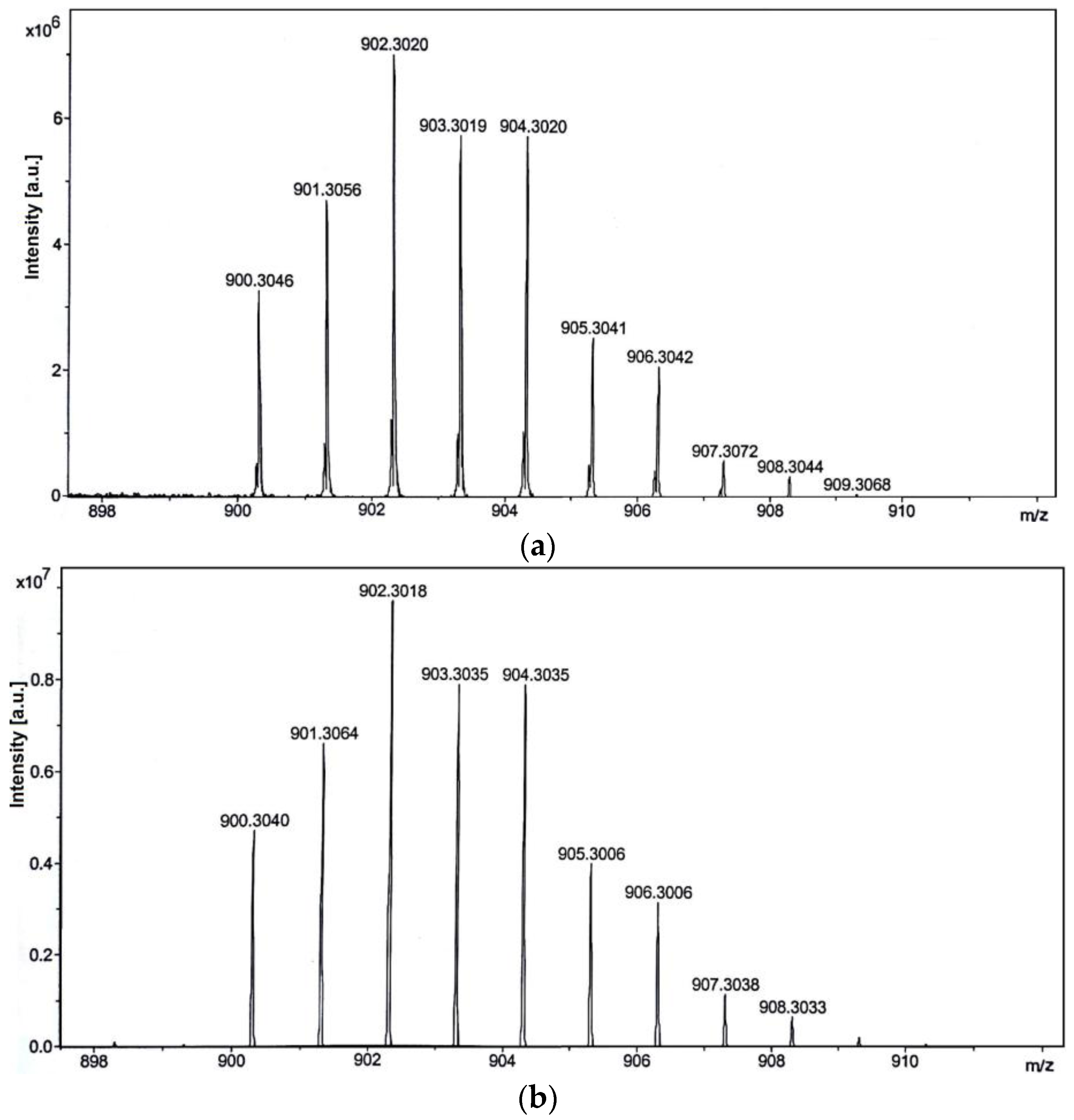
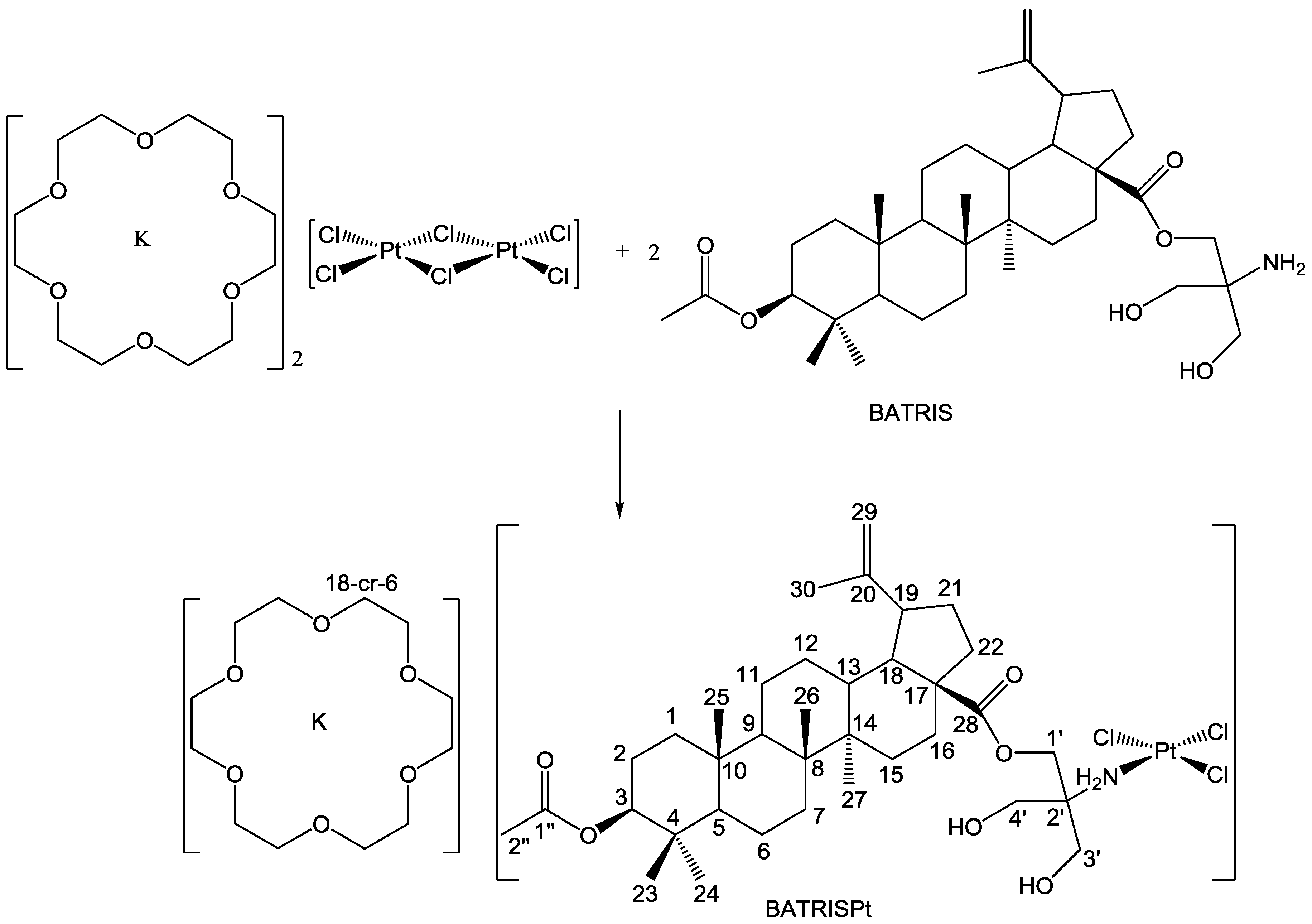
2.1. Synthesis and Characterization
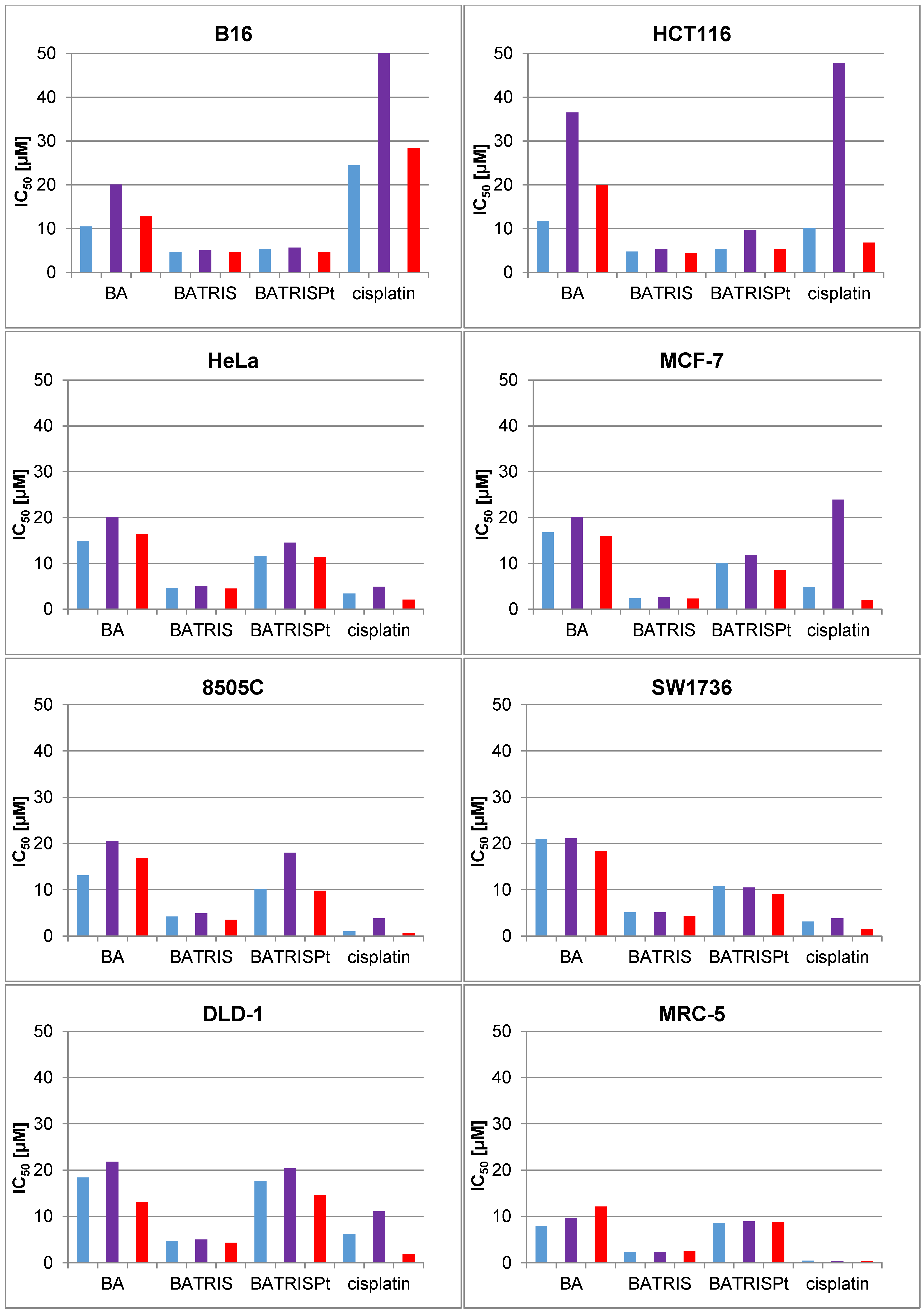
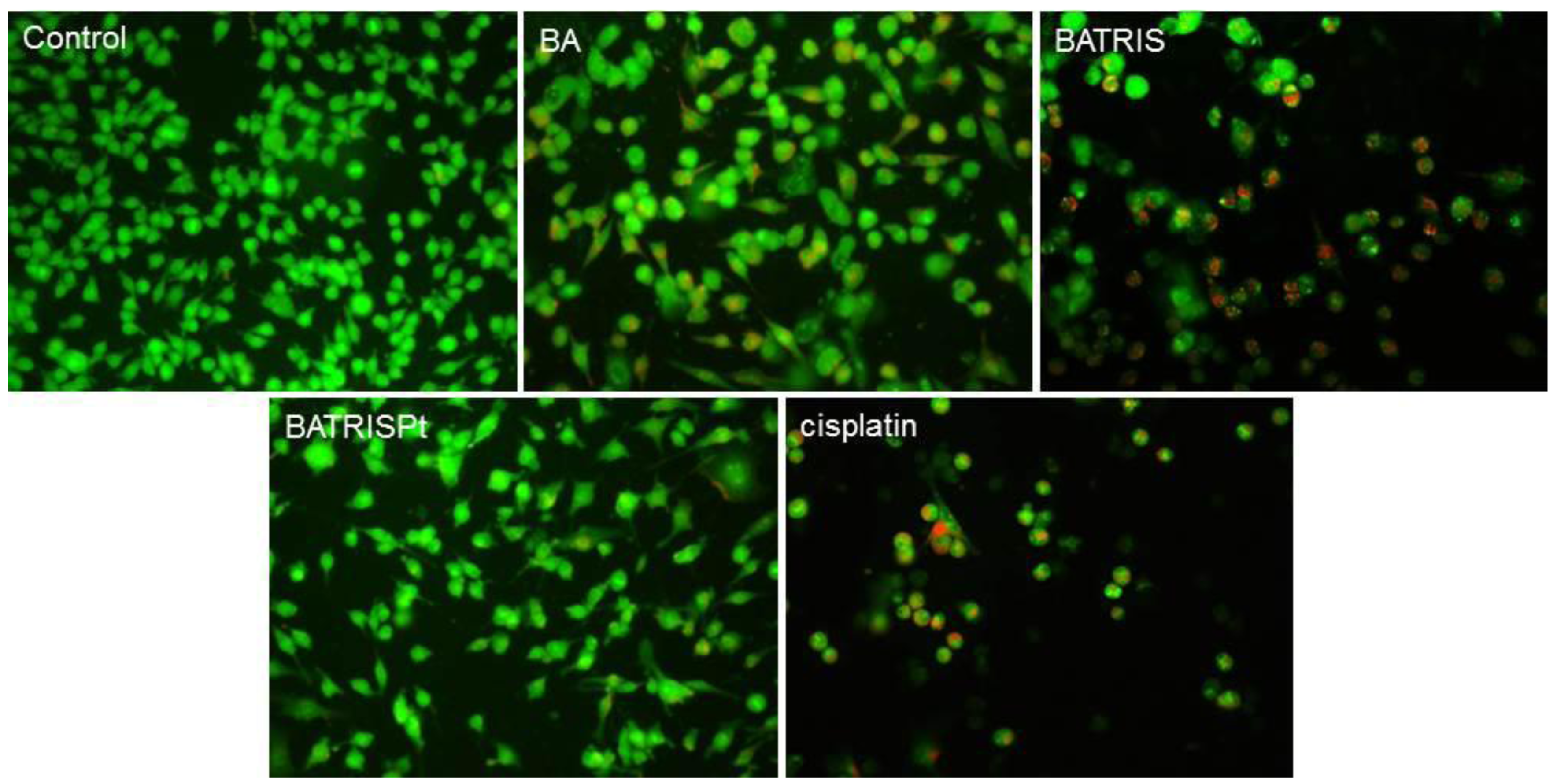
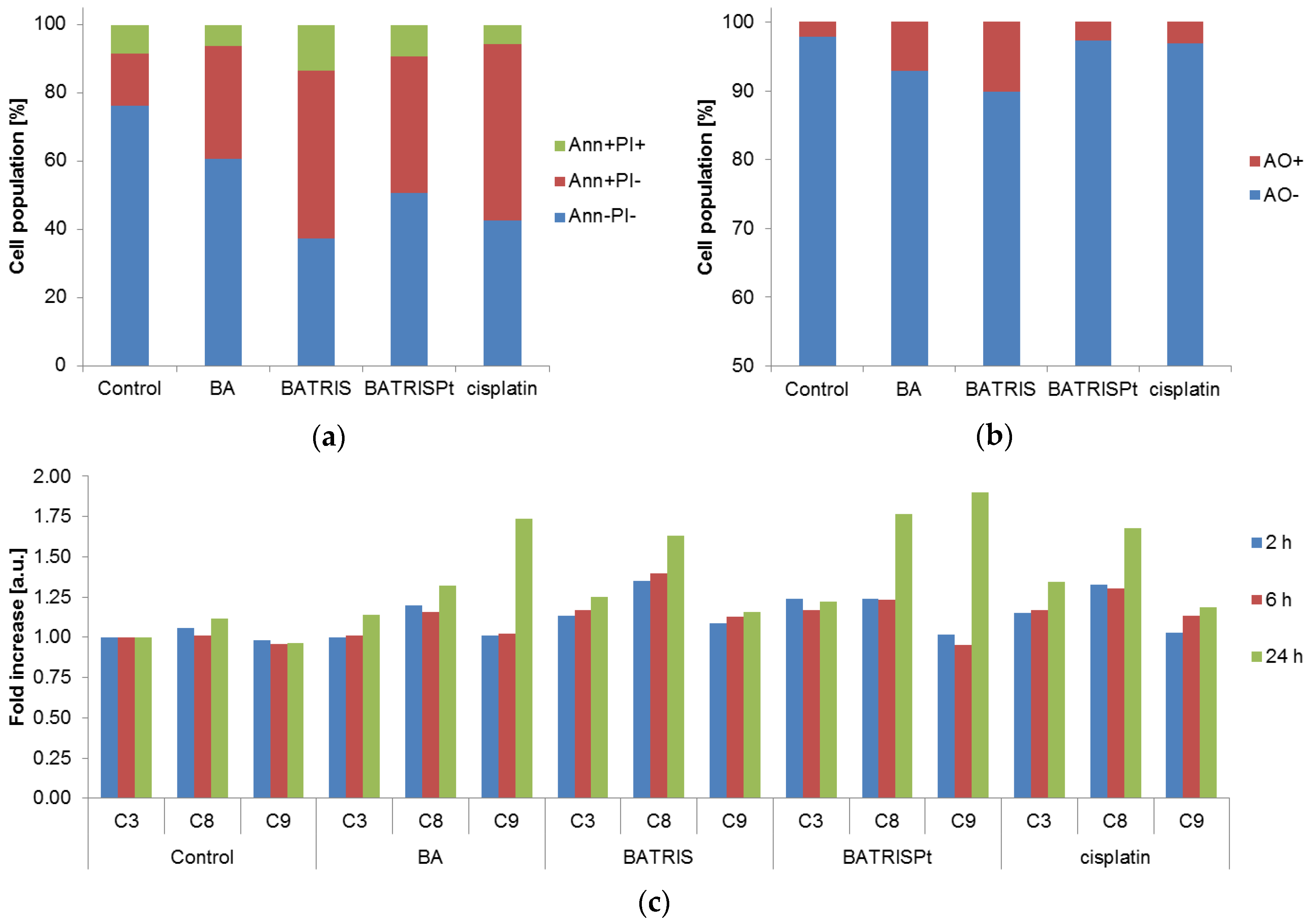
2.2. Cytotoxicity of BATRISPt
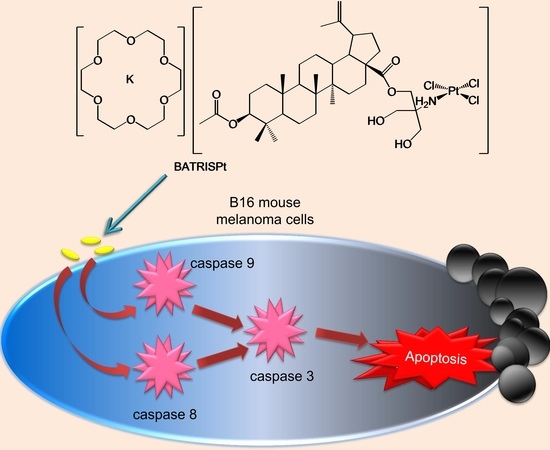
2.3. Mechanism of Action
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of the Platinum(II) Complex, BATRISPt
3.3. Preparation of Drug Solution
3.4. Cell Culture and Conditions
3.5. MTT and CV Assays for Cellular Viability
3.6. SRB Assay
3.7. Annexin V-FITC/PI and Acridin Orange Assays
3.8. Caspase 3, 8, and 9 Activation Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Paschke, R. Anticancer metallotherapeutics in preclinical development. Curr. Med. Chem. 2011, 18, 4738–4752. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Cervera, P.; Svrcek, M.; Pellat, A.; Dreyer, C.; de Gramont, A.; André, T. BRAF-Mutated Colorectal Cancer: What Is the Optimal Strategy for Treatment? Curr. Treat. Options Oncol. 2017, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Morgensztern, D. Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.-L.; Hsieh, C.-W.; Tai, M.-H.; Weng, C.-H.; Wu, D.-C.; Wu, W.-J.; Yeh, B.-W.; Hsieh, S.-L.; Kuo, C.-H.; Hung, H.-S.; et al. Nanoscale characterization illustrates the cisplatin-mediated biomechanical changes of B16-F10 melanoma cells. Phys. Chem. Chem. Phys. PCCP 2016, 18, 7124–7131. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zervoudakis, A.; Boucher, T.; Kemeny, N.E. Treatment Options in Colorectal Liver Metastases: Hepatic Arterial Infusion. Visc. Med. 2017, 33, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Gajek, A.; Marczak, A. Two drugs are better than one. A short history of combined therapy of ovarian cancer. Contemp. Oncol. Pozn. Pol. 2015, 19, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Intini, F.P.; Zajac, J.; Novohradsky, V.; Saltarella, T.; Pacifico, C.; Brabec, V.; Natile, G.; Kasparkova, J. Novel Antitumor Platinum(II) Conjugates Containing the Nonsteroidal Anti-inflammatory Agent Diclofenac: Synthesis and Dual Mechanisms of Antiproliferative Effects. Inorg. Chem. 2017, 56, 1483–1497. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, X.; Hao, W.; Huang, Z.; Wang, X.; Wang, P.G. Mono-functionalized glycosylated platinum(IV) complexes possessed both pH and redox dual-responsive properties: Exhibited enhanced safety and preferentially accumulated in cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2017, 128, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, Z.; Zhao, M.; Gao, X.; Li, H.; Mi, Q.; Liu, P.; Yang, J.; Yao, Z.; Gao, Q. GLUT1-mediated selective tumor targeting with fluorine containing platinum(II) glycoconjugates. Oncotarget 2017, 8, 39476–39496. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Fang, L.; Chen, F.; Gou, S. Conjugation of platinum(IV) complexes with chlorambucil to overcome cisplatin resistance via a “joint action” mode toward DNA. Eur. J. Med. Chem. 2017, 137, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Yuan, H.; Weissleder, R.; Cantley, L.C.; Josephson, L. A Wortmannin−Cetuximab as a Double Drug. Bioconjug. Chem. 2009, 20, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Denel-Bobrowska, M.; Marczak, A. Structural modifications in the sugar moiety as a key to improving the anticancer effectiveness of doxorubicin. Life Sci. 2017, 178, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Onrubia, M.; Cusidó, R.M.; Ramirez, K.; Hernández-Vázquez, L.; Moyano, E.; Bonfill, M.; Palazon, J. Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: Paclitaxel and its derivatives. Curr. Med. Chem. 2013, 20, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.; Karagöz, A.Ç.; Ghoochani, A.; Buchfelder, M.; Eyüpoglu, I.; Tsogoeva, S.B.; Savaskan, N. Cytotoxic profiling of artesunic and betulinic acids and their synthetic hybrid compound on neurons and gliomas. Oncotarget 2017. [Google Scholar] [CrossRef]
- Gupta, N.; Rath, S.K.; Singh, J.; Qayum, A.; Singh, S.; Sangwan, P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017, 135, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Chanda, J.; Kar, A.; Mukherjee, P.K. Tyrosinase inhibitory mechanism of betulinic acid from Dillenia indica. Food Chem. 2017, 232, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Navanesan, S.; Abdul Wahab, N.; Manickam, S.; Cheow, Y.L.; Sim, K.S. Intrinsic capabilities of Leptospermum javanicum in inducing apoptosis and suppressing the metastatic potential of human lung carcinoma cells. Chem. Biol. Interact. 2017, 273, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Fang, D.; Chu, P.; Wu, H.; Zhang, Z.; Tang, Z. Multiple molecular targets in breast cancer therapy by betulinic acid. Biomed. Pharmacother. 2016, 84, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Bache, M.; Zschornak, M.P.; Passin, S.; Kessler, J.; Wichmann, H.; Kappler, M.; Paschke, R.; Kaluđerović, G.N.; Kommera, H.; Taubert, H.; et al. Increased betulinic acid induced cytotoxicity and radiosensitivity in glioma cells under hypoxic conditions. Radiat. Oncol. 2011, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Blazevski, J.; Petkovic, F.; Momcilovic, M.; Paschke, R.; Kaluđerović, G.N.; Mostarica Stojkovic, M.; Miljkovic, D. Betulinic acid regulates generation of neuroinflammatory mediators responsible for tissue destruction in multiple sclerosis in vitro. Acta Pharmacol. Sin. 2013, 34, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Paschke, R. Lupane Triterpenoids-Betulin and Betulinic acid derivatives induce apoptosis in tumor cells. Investig. New Drugs 2011, 29, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Wacheck, V.; Buckley, J.; Nagy, K.; Thalhammer, J.; Paschke, R.; Triche, T.; Jansen, B.; Selzer, E. Characterization of NVX-207, a novel betulinic acid-derived anti-cancer compound. Eur. J. Clin. Investig. 2009, 39, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Paschke, R. Synthesis and Anticancer Activity of Novel Betulinic acid and Betulin Derivatives. Arch. Pharm. (Weinh.) 2010, 343, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Draeger, B.; Paschke, R. Small structural changes of pentacyclic lupane type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur. J. Med. Chem. 2010, 45, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Dittrich, S.; Kalbitz, J.; Draeger, B.; Mueller, T.; Paschke, R. Carbamate derivatives of betulinic acid and betulin with selective cytotoxic activity. Bioorg. Med. Chem. Lett. 2010, 20, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, D.; Vanchanagiri, K.; Baratto, L.C.; Schmidt, H.; Paschke, R. Synthesis and studies of anticancer properties of lupane-type triterpenoid derivatives containing a cisplatin fragment. Eur. J. Med. Chem. 2014, 75, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, M.; Heinemann, F.W.; Markgraf, U.; Steinborn, D. Synthese und Struktur der Kronenetherkomplexe von Kaliumhexachlorodipalladat(II) und-diplatinat(II). Z. Anorg. Allg. Chem. 1997, 623, 1651–1656. [Google Scholar] [CrossRef]
- Qu, Y.; Farrell, N.; Valsecchi, M.; de Greco, L.; Spinelli, S. Multinuclear 195Pt and 15N magnetic resonance spectroscopic studies of the reaction of K[PtCl3(NH3)] with KI and amines. Magn. Reson. Chem. 1993, 31, 920–924. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Nomenclature Committee on Cell Death 2009 Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-loaded mesoporous silica as a biocompatible strategy in cancer treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haikel, Y.; Hassan, M. Crosstalk between Apoptosis and Autophagy: Molecular Mechanisms and Therapeutic Strategies in Cancer. J. Cell Death 2013, 2013, 37–55. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowsk, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1608. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: A molecular switch node in the crosstalk between autophagy and apoptosis. Int. J. Biol. Sci. 2014, 10, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, J.; Xia, M.; Li, H.; Xu, Y.; Ma, C.; Ma, L.; Kang, J.; Yu, H.; Zhang, Z.; et al. Caspase-mediated cleavage of Beclin1 inhibits autophagy and promotes apoptosis induced by S1 in human ovarian cancer SKOV3 cells. Apoptosis Int. J. Program. Cell Death 2016, 21, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Bette, M.; Kalbitz, J.; Fuchs, P.; Fulda, S.; Mier, W.; Paschke, R. In vitro anticancer studies of alpha- and beta-d-glucopyranose betulin anomers. Chem. Biol. Interact. 2010, 185, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Mueller, T.; Paschke, R.; Kalinowski, B.; Behlendorf, T.; Reipsch, F.; Fruehauf, A.; Schmoll, H.-J.; Kloft, C.; Voigt, W. 2-(4-(Tetrahydro-2H-pyran-2-yloxy)-undecyl)-propane-1,3-diamminedichloroplatinum(II): A novel platinum compound that overcomes cisplatin resistance and induces apoptosis by mechanisms different from that of cisplatin. J. Med. Chem. 2008, 51, 5413–5422. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluđerović, G.N.; Bulatović, M.; Krajnović, T.; Paschke, R.; B. Zmejkovski, B.; Maksimović-Ivanić, D.; Mijatović, S. (18-Crown-6)potassium(I) Trichlorido[28-acetyl-3-(tris-(hydroxylmethyl)amino-ethane)betulinic ester-κN]platinum(II): Synthesis and In Vitro Antitumor Activity. Inorganics 2017, 5, 56. https://doi.org/10.3390/inorganics5030056
Kaluđerović GN, Bulatović M, Krajnović T, Paschke R, B. Zmejkovski B, Maksimović-Ivanić D, Mijatović S. (18-Crown-6)potassium(I) Trichlorido[28-acetyl-3-(tris-(hydroxylmethyl)amino-ethane)betulinic ester-κN]platinum(II): Synthesis and In Vitro Antitumor Activity. Inorganics. 2017; 5(3):56. https://doi.org/10.3390/inorganics5030056
Chicago/Turabian StyleKaluđerović, Goran N., Mirna Bulatović, Tamara Krajnović, Reinhard Paschke, Bojana B. Zmejkovski, Danijela Maksimović-Ivanić, and Sanja Mijatović. 2017. "(18-Crown-6)potassium(I) Trichlorido[28-acetyl-3-(tris-(hydroxylmethyl)amino-ethane)betulinic ester-κN]platinum(II): Synthesis and In Vitro Antitumor Activity" Inorganics 5, no. 3: 56. https://doi.org/10.3390/inorganics5030056