Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling
Abstract
:1. Introduction
2. Results and Discussion
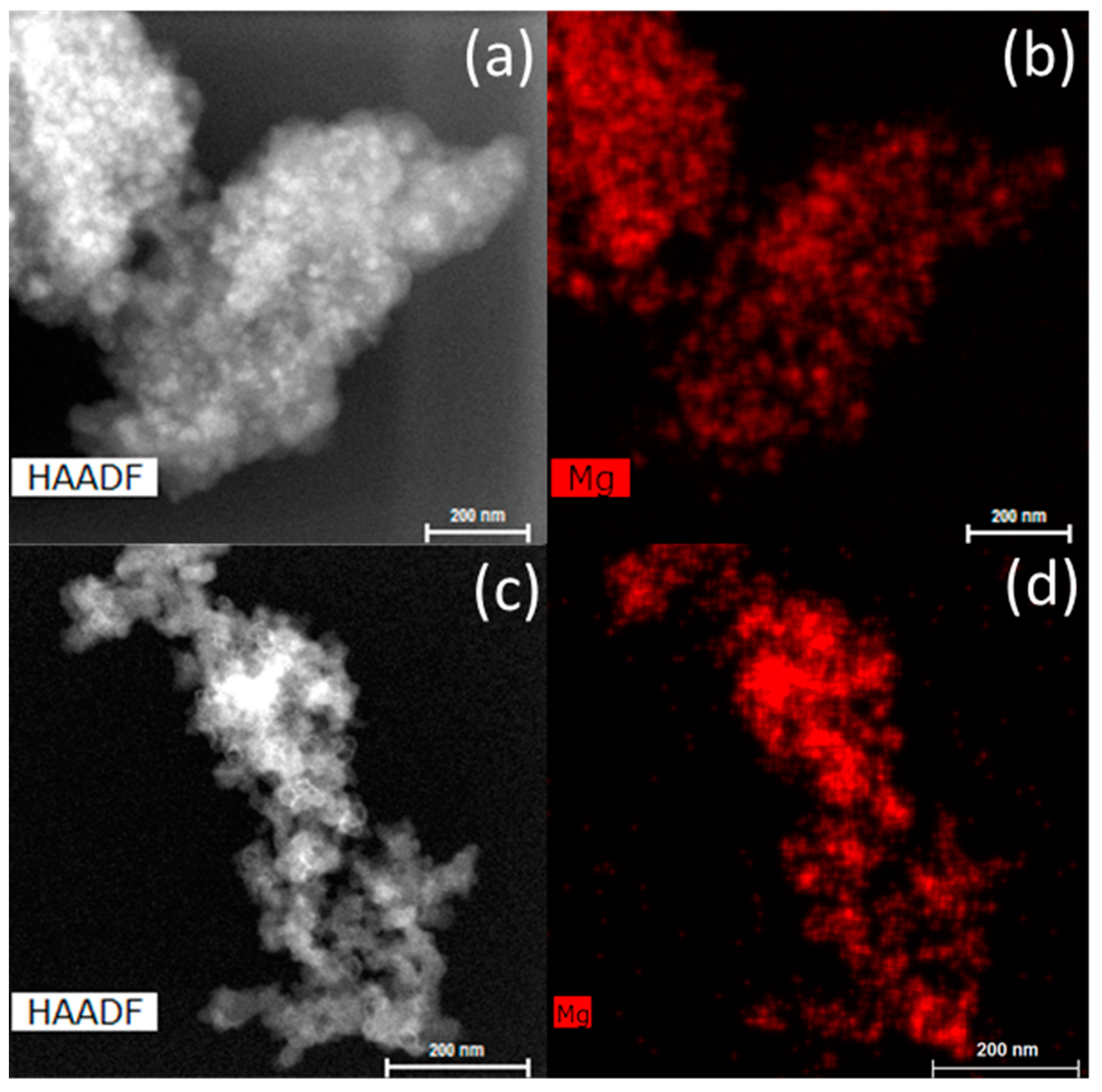
2.1. Porosity of the Nanoporous Scaffolds and Confinement of Magnesium Hydride
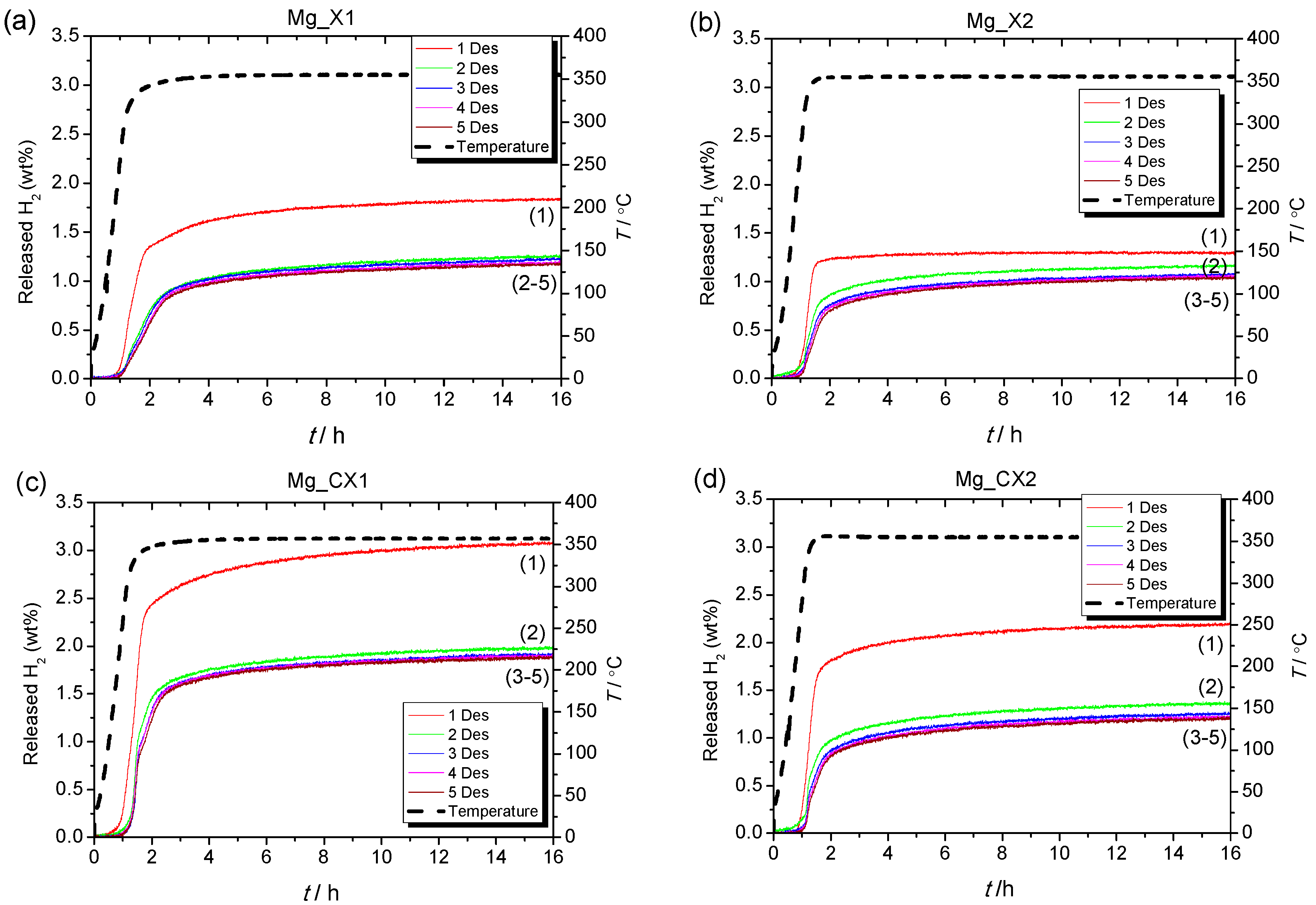
2.2. Hydrogen Storage Capacity upon Cycling
2.2.1. Thermodynamic Considerations
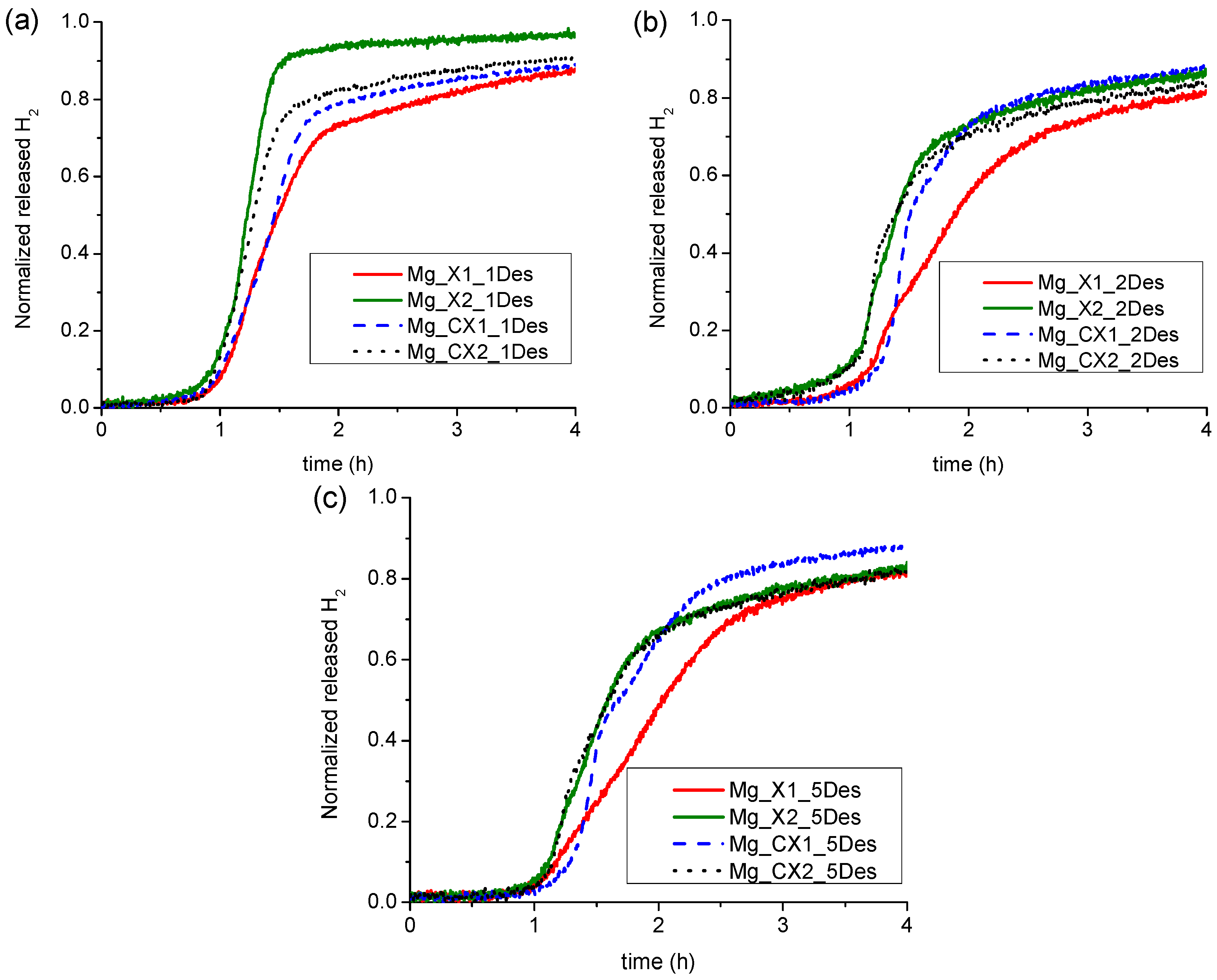
2.2.2. Kinetics of Hydrogen Release of Nanoconfined MgH2
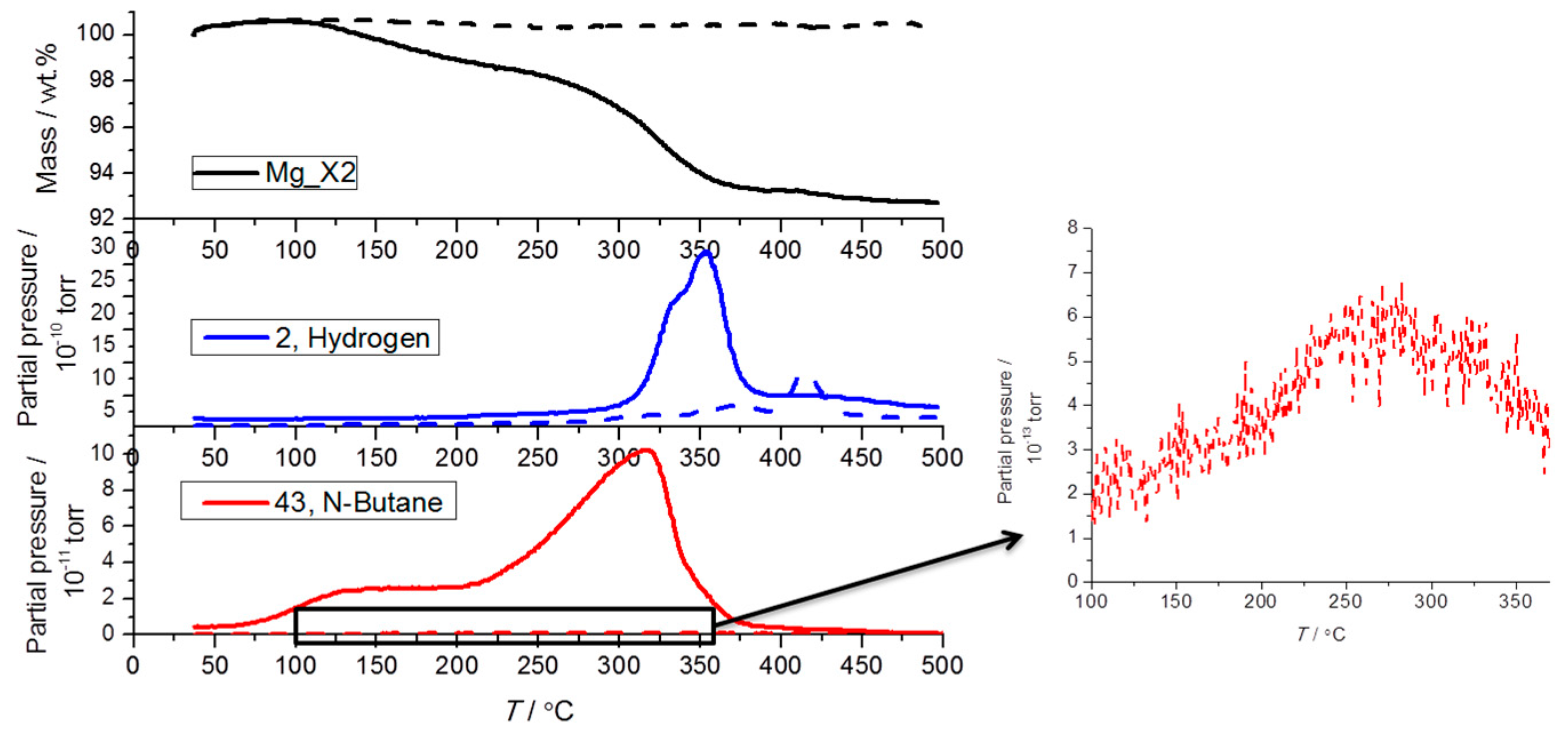
2.3. Analysis of the Released Gases and Samples after Cycling
2.4. Comparison of As-Prepared and Cycled Nanoconfined MgH2
3. Materials and Methods
3.1. Synthesis of Carbon Scaffolds
3.2. Direct Synthesis of Nanoconfined Magnesium Hydride
3.3. Characterisation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Bellosta von Colbe, J.M.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482208689. [Google Scholar]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Manickam, K.; Hirscher, M.; Besenbacher, F.; Jensen, T.R. Confinement of MgH2 Nanoclusters within Nanoporous Aerogel Scaffold Materials. ACS Nano 2009, 3, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gross, A.F.; Van Atta, S.L.; Lopez, M.; Liu, P.; Ahn, C.C.; Vajo, J.J.; Jensen, C.M. The synthesis and hydrogen storage properties of a MgH2 incorporated carbon aerogel scaffold. Nanotechnology 2009, 20, 204027. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.F.; Ahn, C.C.; Van Atta, S.L.; Liu, P.; Vajo, J.J. Fabrication and hydrogen sorption behaviour of nanoparticulate MgH2 incorporated in a porous carbon host. Nanotechnology 2009, 20, 204005. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, C.; Cheng, L.; Abdul Wahab, M.; Cui, J.; Zou, J.; Zhu, M.; Yao, X. Destabilization of Mg–H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2. Phys. Chem. Chem. Phys. 2013, 15, 5814. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Nanoconfined NaAlH4: Determination of Distinct Prolific Effects from Pore Size, Crystallite Size, and Surface Interactions. J. Phys. Chem. C 2012, 116, 21046–21051. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and Nanoconfinement: New Strategies Towards Meeting Hydrogen Storage Goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Gosalawit-Utke, R.; Thiangviriya, S.; Javadian, P.; Laipple, D.; Pistidda, C.; Bergemann, N.; Horstmann, C.; Jensen, T.R.; Klassen, T.; Dornheim, M. Effective nanoconfinement of 2LiBH4–MgH2 via simply MgH2 premilling for reversible hydrogen storages. Int. J. Hydrogen Energy 2014, 39, 15614–15626. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold Mediates Hydrogen Release and the Reactivity of Ammonia Borane. Angew. Chem. 2005, 117, 3644–3648. [Google Scholar] [CrossRef]
- Ngene, P.; van Zwienen, M.; de Jongh, P.E. Reversibility of the hydrogen desorption from LiBH4: A synergetic effect of nanoconfinement and Ni addition. Chem. Commun. 2010, 46, 8201. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Filsø, U.; Karimi, F.; Puszkiel, J.; Pranzas, P.K.; Pistidda, C.; Hoell, A.; Welter, E.; Schreyer, A.; Klassen, T.; et al. Cyclic stability and structure of nanoconfined Ti-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 4159–4167. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Hu, J.; Roth, A.; Wang, D.; Kübel, C.; Lohstroh, W.; Fichtner, M. Altered thermodynamic and kinetic properties of MgH2 infiltrated in microporous scaffold. Chem. Commun. 2010, 46, 8353. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, P.E.; Wagemans, R.W.P.; Eggenhuisen, T.M.; Dauvillier, B.S.; Radstake, P.B.; Meeldijk, J.D.; Geus, J.W.; de Jong, K.P. The Preparation of Carbon-Supported Magnesium Nanoparticles using Melt Infiltration. Chem. Mater. 2007, 19, 6052–6057. [Google Scholar] [CrossRef]
- Utke, R.; Thiangviriya, S.; Javadian, P.; Jensen, T.R.; Milanese, C.; Klassen, T.; Dornheim, M. 2LiBH4–MgH2 nanoconfined into carbon aerogel scaffold impregnated with ZrCl4 for reversible hydrogen storage. Mater. Chem. Phys. 2016, 169, 136–141. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Nanoconfined NaAlH4: Prolific effects from increased surface area and pore volume. Nanoscale 2014, 6, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M. Nanoconfinement effects in energy storage materials. Phys. Chem. Chem. Phys. 2011, 13, 21186. [Google Scholar] [CrossRef] [PubMed]
- Roedern, E.; Hansen, B.R.S.; Ley, M.B.; Jensen, T.R. Effect of Eutectic Melting, Reactive Hydride Composites, and Nanoconfinement on Decomposition and Reversibility of LiBH4–KBH4. J. Phys. Chem. C 2015, 119, 25818–25825. [Google Scholar] [CrossRef]
- Bramwell, P.L.; Ngene, P.; de Jongh, P.E. Carbon supported lithium hydride nanoparticles: Impact of preparation conditions on particle size and hydrogen sorption. Int. J. Hydrogen Energy 2017, 42, 5188–5198. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic Changes in Mechanochemically Synthesized Magnesium Hydride Nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Korablov, D.; Latroche, M. Synthesis, structural and hydrogenation properties of Mg-rich MgH2–TiH2 nanocomposites prepared by reactive ball milling under hydrogen gas. Phys. Chem. Chem. Phys. 2012, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Boscherini, F.; Callini, E.; Maurizio, C.; Pasquali, L.; Montecchi, M.; Bonetti, E. Local structure at interfaces between hydride-forming metals: A case study of Mg-Pd nanoparticles by X-ray spectroscopy. Phys. Rev. B 2011, 83, 184111. [Google Scholar] [CrossRef]
- Andreasen, A.; Sørensen, M.B.; Burkarl, R.; Møller, B.; Molenbroek, A.M.; Pedersen, A.S.; Vegge, T.; Jensen, T.R. Dehydrogenation kinetics of air-exposed MgH2/Mg2Cu and MgH2/MgCu2 studied with in situ X-ray powder diffraction. Appl. Phys. A 2006, 82, 515–521. [Google Scholar] [CrossRef]
- Andreasen, A.; Sørensen, M.B.; Burkarl, R.; Møller, B.; Molenbroek, A.M.; Pedersen, A.S.; Andreasen, J.W.; Nielsen, M.M.; Jensen, T.R. Interaction of hydrogen with an Mg–Al alloy. J. Alloys Compd. 2005, 404–406, 323–326. [Google Scholar] [CrossRef]
- Jensen, T.; Andreasen, A.; Vegge, T.; Andreasen, J.; Stahl, K.; Pedersen, A.; Nielsen, M.; Molenbroek, A.; Besenbacher, F. Dehydrogenation kinetics of pure and nickel-doped magnesium hydride investigated by in situ time-resolved powder X-ray diffraction. Int. J. Hydrogen Energy 2006, 31, 2052–2062. [Google Scholar] [CrossRef]
- Dornheim, M.; Eigen, N.; Barkhordarian, G.; Klassen, T.; Bormann, R. Tailoring Hydrogen Storage Materials Towards Application. Adv. Eng. Mater. 2006, 8, 377–385. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic Mechanism of Transition-Metal Compounds on Mg Hydrogen Sorption Reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Jensen, T.R. MgH2–Nb2O5 investigated by in situ synchrotron X-ray diffraction. Int. J. Hydrogen Energy 2012, 37, 13409–13416. [Google Scholar] [CrossRef]
- Alegre, C.; Sebastián, D.; Baquedano, E.; Gálvez, M.E.; Moliner, R.; Lázaro, M. Tailoring Synthesis Conditions of Carbon Xerogels towards Their Utilization as Pt-Catalyst Supports for Oxygen Reduction Reaction (ORR). Catalysts 2012, 2, 466–489. [Google Scholar] [CrossRef]
- Chumphongphan, S.; Filsø, U.; Paskevicius, M.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Nanoconfinement degradation in NaAlH4/CMK-1. Int. J. Hydrogen Energy 2014, 39, 11103–11109. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Weidenthaler, C.; Schüth, F. Hard-Templating Pathway to Create Mesoporous Magnesium Oxide. Chem. Mater. 2004, 16, 5676–5681. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Carbonization and activation of sol-gel derived carbon xerogels. Carbon 2000, 38, 849–861. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Deboer, J. Studies on pore systems in catalysts VII. Description of the pore dimensions of carbon blacks by the t method. J. Catal. 1965, 4, 649–653. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
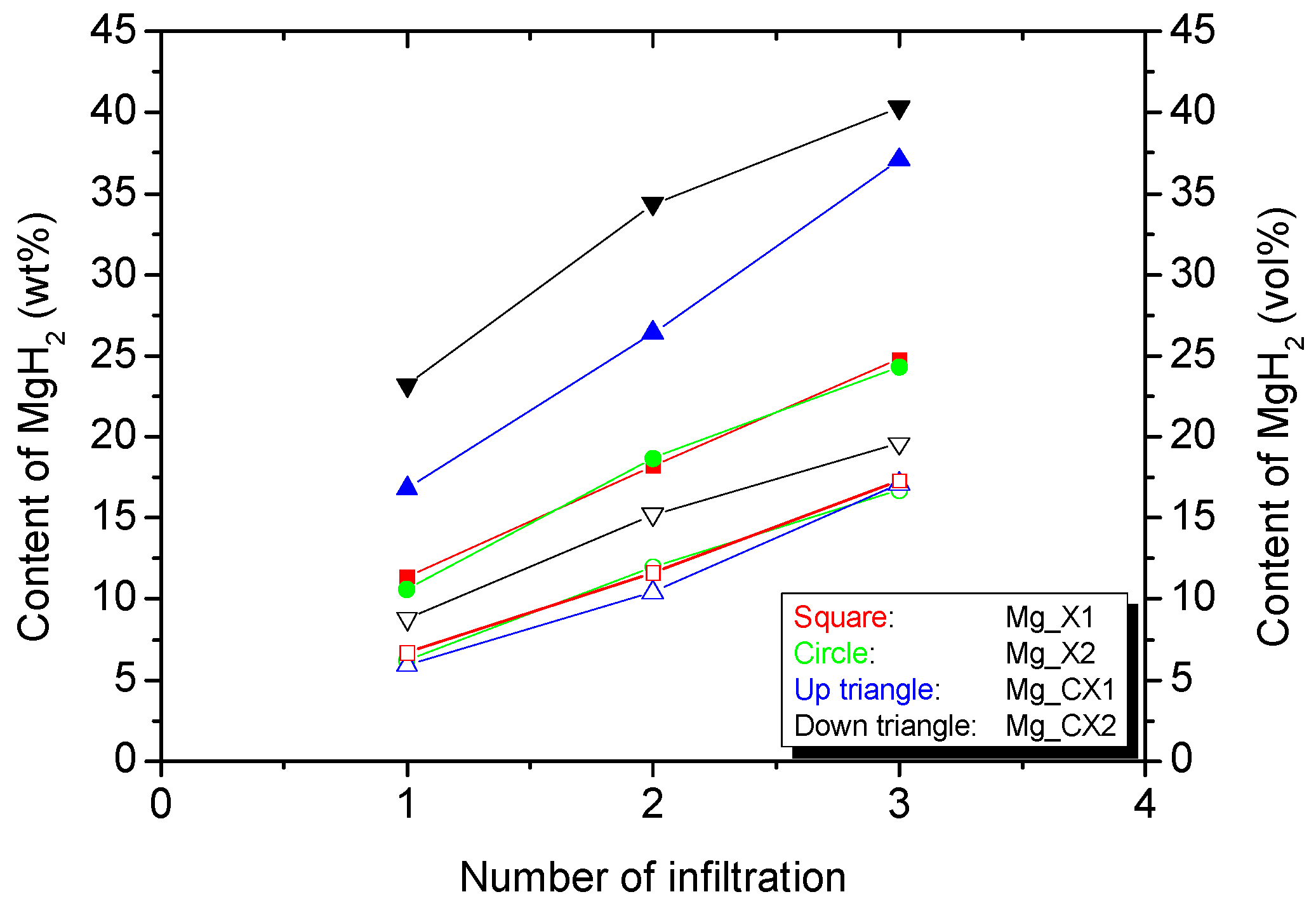

| Carbon Aerogels | SBET (m2/g) | Davg (nm) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vtot (cm3/g) | MgH2 (wt %) a | MgH2 (vol %) b |
|---|---|---|---|---|---|---|---|
| X1 | 829 ± 16 | 16.6 ± 0.5 | 0.23 ± 0.01 | 1.13 ± 0.03 | 1.32 ± 0.04 | 24.8 | 17.3 |
| X2 | 801 ± 16 | 27.1 ± 2.7 | 0.25 ± 0.01 | 1.11 ± 0.08 | 1.32 ± 0.10 | 24.3 | 16.7 |
| CX1 | 1940 ± 131 | 14.7 ± 0.6 | 0.54 ± 0.07 | 1.85 ± 0.06 | 2.37 ± 0.12 | 37.1 | 17.1 |
| CX2 | 1803 ± 30 | 25.0 ± 0.8 | 0.56 ± 0.02 | 1.89 ± 0.04 | 2.38 ± 0.05 | 40.3 | 19.6 |
| Sample | Dmax (nm) | ρm (H2)/(wt %) | Des1 (H2 wt %) | Des2 (H2 wt %) | Des3 (H2 wt %) | Des4 (H2 wt %) | Des5 (H2 wt %) |
|---|---|---|---|---|---|---|---|
| Mg_X1 | 17 | 1.88 | 1.8 (100%) | 1.3 (72%) | 1.2 (67%) | 1.2 (67%) | 1.2 (67%) |
| Mg_X2 | 26 | 1.85 | 1.3 (100%) | 1.2 (92%) | 1.1 (85%) | 1.1 (85%) | 1.0 (77%) |
| Mg_CX1 | 15 | 2.82 | 3.1 (100%) | 2.0 (67%) | 1.9 (61%) | 1.9 (61%) | 1.9 (61%) |
| Mg_CX2 | 25 | 3.06 | 2.2 (100%) | 1.4 (64%) | 1.3 (59%) | 1.2 (55%) | 1.2 (55%) |
| Sample | Dmax (nm) | As Infiltrated | After Five Cycles | ||
|---|---|---|---|---|---|
| MgH2 Cryst. Size (nm) | MgH2 Cryst. wt % | MgH2 Cryst. Size (nm) | MgH2 Cryst. wt % | ||
| Mg_X1 | 17 | 13 | 0.38 | 210 | 0.21 |
| Mg_X2 | 26 | 10 | 0.40 | 248 | 0.19 |
| Mg_CX1 | 15 | 8 | 0.48 | 300 | 0.35 |
| Mg_CX2 | 25 | 13 | 0.23 | 95 | 0.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huen, P.; Paskevicius, M.; Richter, B.; Ravnsbæk, D.B.; Jensen, T.R. Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling. Inorganics 2017, 5, 57. https://doi.org/10.3390/inorganics5030057
Huen P, Paskevicius M, Richter B, Ravnsbæk DB, Jensen TR. Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling. Inorganics. 2017; 5(3):57. https://doi.org/10.3390/inorganics5030057
Chicago/Turabian StyleHuen, Priscilla, Mark Paskevicius, Bo Richter, Dorthe B. Ravnsbæk, and Torben R. Jensen. 2017. "Hydrogen Storage Stability of Nanoconfined MgH2 upon Cycling" Inorganics 5, no. 3: 57. https://doi.org/10.3390/inorganics5030057





