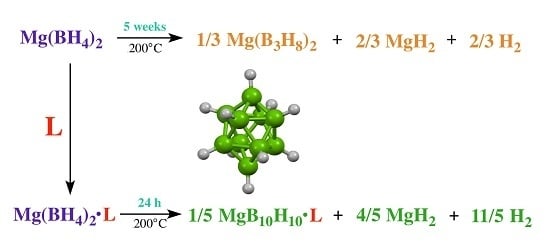
Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release
Abstract
:1. Introduction
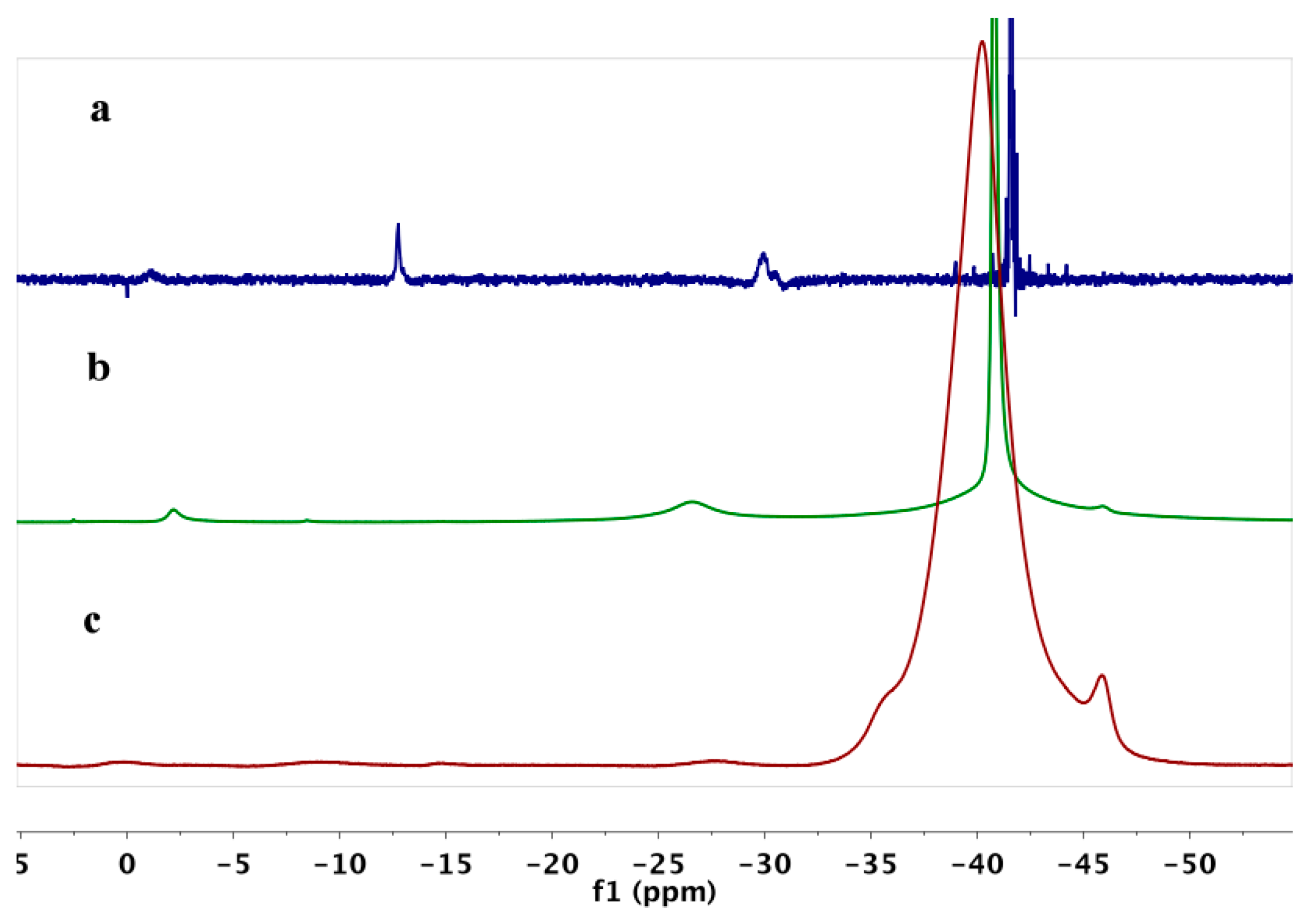
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Mg(BH4)2
4.2. Synthesis of Solvent Adducts of Mg(BH4)2
4.3. Characterization of Mg(BH4)2 Adducts and Decomposition Products by Solution NMR
4.4. Solid State NMR
4.5. In Situ NMR
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and dense magnesium borohydride frameworks: Synthesis, stability, and reversible absorption of guest species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef]
- Hanada, N.; Chłopek, K.; Frommen, C.; Lohstroh, W.; Fichtner, M. Thermal decomposition of Mg(BH4)2 under He flow and H2 pressure. J. Mater. Chem. 2008, 18, 2611–2614. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.C., Jr.; Reiter, J.W.; Rijssenbeek, J.; Soloveichik, G.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. NMR confirmation for formation of [B12H12]2− complexes during hydrogen desorption from metal borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Sato, T.; Nakamori, Y.; Ohba, N.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Synthesis and hydrogen storage properties of a single-phase magnesium borohydride Mg(BH4)2. Mater. Trans. 2008, 49, 2224–2228. [Google Scholar] [CrossRef]
- Matsunaga, T.; Buchter, F.; Mauron, P.; Bielman, M.; Nakamori, Y.; Orimo, S.; Ohba, N.; Miwa, K.; Towata, S.; Züttel, A. Hydrogen storage properties of Mg[BH4]2. J. Alloys Compd. 2008, 459, 583–588. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.-W.; Ohba, N.; Towata, S.; Züttel, A.; Orimo, S. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-principles prediction of a ground state crystal structure of magnesium borohydride. Phys. Rev. Lett. 2008, 100, 135501. [Google Scholar] [CrossRef]
- Severa, G.; Ronnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogen storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Van Setten, M.J.; de Wijs, G.A.; Fichtner, M.; Brocks, G.A. A Density Functional Study of alpha-Mg(BH4)2. Chem. Mater. 2008, 20, 4952–4956. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Nakamori, Y.; Ohba, H.; Miwa, K.; Towata, S.; Orimo, S. Differential scanning calorimetry measurements of magnesium borohydride Mg(BH4)2. Mater. Trans. 2008, 49, 2751–2752. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Deledda, S.; Hauback, B.C. Kinetics studies of the reversible partial decomposition reaction in Mg(BH4)2. Int. J. Hydrog. Energy 2016, 41, 9885–9892. [Google Scholar] [CrossRef]
- Zhang, Y.; Majzoub, E.; Ozoliņš, V.; Wolverton, C. Theoretical prediction of metastable intermediates in the decomposition of Mg(BH4)2. J. Phys. Chem. C 2012, 116, 10522–10528. [Google Scholar] [CrossRef]
- Chłopek, K.; Frommen, C.; Léon, A.; Zabara, O.; Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef]
- Li, H.-W. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- White, J.L.; Newhouse, R.J.; Zhang, J.Z.; Udovic, T.J.; Stavila, V. Understanding and mitigating the effects of stable dodecahydro-closo-dodecaborate intermediates on hydrogen-storage reactions. J. Phys. Chem. C 2016, 120, 25725–25731. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Bardají, E.G.; Hanada, N.; Zabara, O.; Fichtner, M. Effect of several metal chlorides on the thermal decomposition behaviour of α-Mg(BH4)2. Int. J. Hydrog. Energy 2011, 36, 12313–12318. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.; Zhang, J.Z. Reversibility and improved hydrogen release of magnesium borohydride. J. Phys. Chem. C 2010, 114, 5224–5234. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int J. Hydrog. Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Bardají, E.G.; Zhao-Karger, Z.; Boucharat, N.; Nale, A.; van Setten, M.J.; Lohstroh, W.; Röhm, E.; Catti, M.; Fichtner, M. LiBH4−Mg(BH4)2: A physical mixture of metal borohydrides as hydrogen storage material. J. Phys. Chem. C 2011, 115, 6095–6101. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Rapin, J.-P.; Černý, R.; Filinchuk, Y.; Kim, K.C.; Sholl, D.S.; Parker, S.T. New fundamental experimental studies on α-Mg(BH4)2 and other borohydrides. J. Alloys Compd. 2011, 509, S688–S690. [Google Scholar] [CrossRef]
- Nale, A.; Catti, M.; Bardají, E.G.; Fichtner, M. On the decomposition of the 0.6LiBH4–0.4Mg(BH4)2 eutectic mixture for hydrogen storage. Int. J. Hydrog. Energy 2011, 36, 13676–13682. [Google Scholar] [CrossRef]
- Yang, J.; Fu, H.; Song, P.; Zheng, J.; Li, X. Reversible dehydrogenation of Mg(BH4)2–LiH composite under moderate conditions. Int. J. Hydrog. Energy 2012, 37, 6776–6783. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Knoth, W.H. Polyhedral Boranes; Marcel Dekker: New York, NY, USA, 1968. [Google Scholar]
- Chong, M.; Matsuo, M.; Orimo, S.; Autrey, T.; Jensen, C.M. Selective reversible hydrogenation of Mg(B3H8)2/MgH2 to Mg(BH4)2: Pathway to reversible borane-based hydrogen storage? Inorg. Chem. 2015, 54, 4120–4125. [Google Scholar] [CrossRef]
- Chen, J.; Chua, Y.S.; Wu, H.; Xiong, Z.; He, T.; Zhou, W.; Ju, X.; Yang, M.; Wu, G.; Chen, P. Synthesis, structures and dehydrogenation of magnesium borohydride–ethylenediamine composites. Int. J. Hydrog. Energy 2015, 40, 412–419. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Zhang, Y.; Li, Y.; Gao, M.; Pan, H. Hydrogen storage properties and mechanisms of Mg(BH4)2⋅2NH3–xMgH2 combination systems. J. Alloys Compd. 2014, 585, 674–680. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, B.; Sun, N.; Sun, Z.; Zeng, Y.; Meng, L. Improvement in dehydrogenation performance of Mg(BH4)2·2NH3 doped with transition metal: First-principles investigation. Int. J. Hydrog. Energy 2015, 40, 8721–8731. [Google Scholar] [CrossRef]
- Soloveichik, G.; Her, J.H.; Stephens, P.W.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Zhao, J.-C. Ammine magnesium borohydride complex as a New material for hydrogen storage: Structure and properties of Mg(BH4)2·2NH3. Inorg. Chem. 2008, 47, 4290–4298. [Google Scholar] [CrossRef]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrog. Energy 2016, 41, 14387–14404. [Google Scholar] [CrossRef]
- Zanella, P.; Crociani, L.; Masciocchi, N.; Giunchi, G. Facile high-yield synthesis of pure, crystalline Mg(BH4)2. Inorg. Chem. 2007, 46, 9039–9041. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Dobrowolski, M.A.; Dobrzycki, Ł.; Cyrański, M.K.; Grochala, W. Organic derivatives of Mg(BH4)2 as precursors towards MgB2 and novel inorganic mixed-cation borohydrides. Dalton Trans 2016, 45, 14370–14377. [Google Scholar] [CrossRef]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A. The role of MgB12H12 in the hydrogen desorption process of Mg(BH4)2. Chem. Commun. 2015, 51, 700–702. [Google Scholar] [CrossRef]
- Hoyt, D.W.; Turcu, R.V.F.; Sears, J.A.; Rosso, K.; Burton, S.D.; Felmy, A.R.; Hu, J.-Z. High-pressure magic angle spinning nuclear magnetic resonance. J. Magn. Res. 2011, 212, 378–385. [Google Scholar] [CrossRef]
- Turcu, R.V.F.; Hoyt, D.W.; Rosso, K.M.; Sears, J.A.; Loring, J.S.; Felmy, A.R.; Hu, J.-Z. Rotor design for high pressure magic angle spinning nuclear magnetic resonance. J. Magn. Res. 2013, 226, 64–69. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Y.H.; Li, Q.; Yu, X.B. The comparison in dehydrogenation properties and mechanism between MgCl2(NH3)/LiBH4 and MgCl2(NH3)/NaBH4 systems. J. Phys. Chem. C 2010, 114, 9534–9540. [Google Scholar] [CrossRef]
- Marks, T.J.; Kolb, J.R. Covalent transition metal, lanthanide, and actinide tetrahydroborate complexes. Chem. Rev. 1977, 77, 263–293. [Google Scholar]

| Solvate | Mg:Ligand Ratio |
|---|---|
| Mg(BH4)2·DMS § | 1:0.34 |
| Mg(BH4)2·TEA | 1:1.8 |
| Mg(BH4)2·Et2O | 1:0.36 |
| Mg(BH4)2·Digly | 1:1.18 |
| Mg(BH4)2·DME | 1:2.2 |
| Mg(BH4)2·THF | 1:2.8 |
| Sample | B10H102− | B3H8− | B12H122− | BH4− |
|---|---|---|---|---|
| Mg(BH4)2 | 3 | 93 | ||
| Mg(BH4)2·TEA | 2 | 6 | 89 | |
| Mg(BH4)2·Et2O | 4 | 4 | 88 | |
| Mg(BH4)2·Digl | 5 | 2 | 3 | 82 |
| Mg(BH4)2·DME | 46 | 14 | 4 | 30 |
| Mg(BH4)2·THF | 31 | 12 | 3 | 39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, M.; Autrey, T.; Jensen, C.M. Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release. Inorganics 2017, 5, 89. https://doi.org/10.3390/inorganics5040089
Chong M, Autrey T, Jensen CM. Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release. Inorganics. 2017; 5(4):89. https://doi.org/10.3390/inorganics5040089
Chicago/Turabian StyleChong, Marina, Tom Autrey, and Craig M. Jensen. 2017. "Lewis Base Complexes of Magnesium Borohydride: Enhanced Kinetics and Product Selectivity upon Hydrogen Release" Inorganics 5, no. 4: 89. https://doi.org/10.3390/inorganics5040089