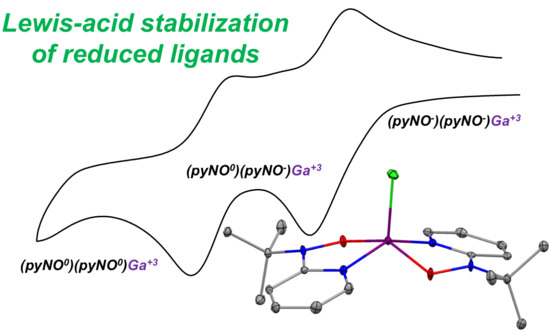
Synthesis and Characterization of (pyNO−)2GaCl: A Redox-Active Gallium Complex
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Spectroscopic Characterization
2.2. Structural Characterization
2.3. Electronic Strucutre of the (pyNO−)2GaCl Complex
2.3.1. Density Functional Theory (DFT) Studies
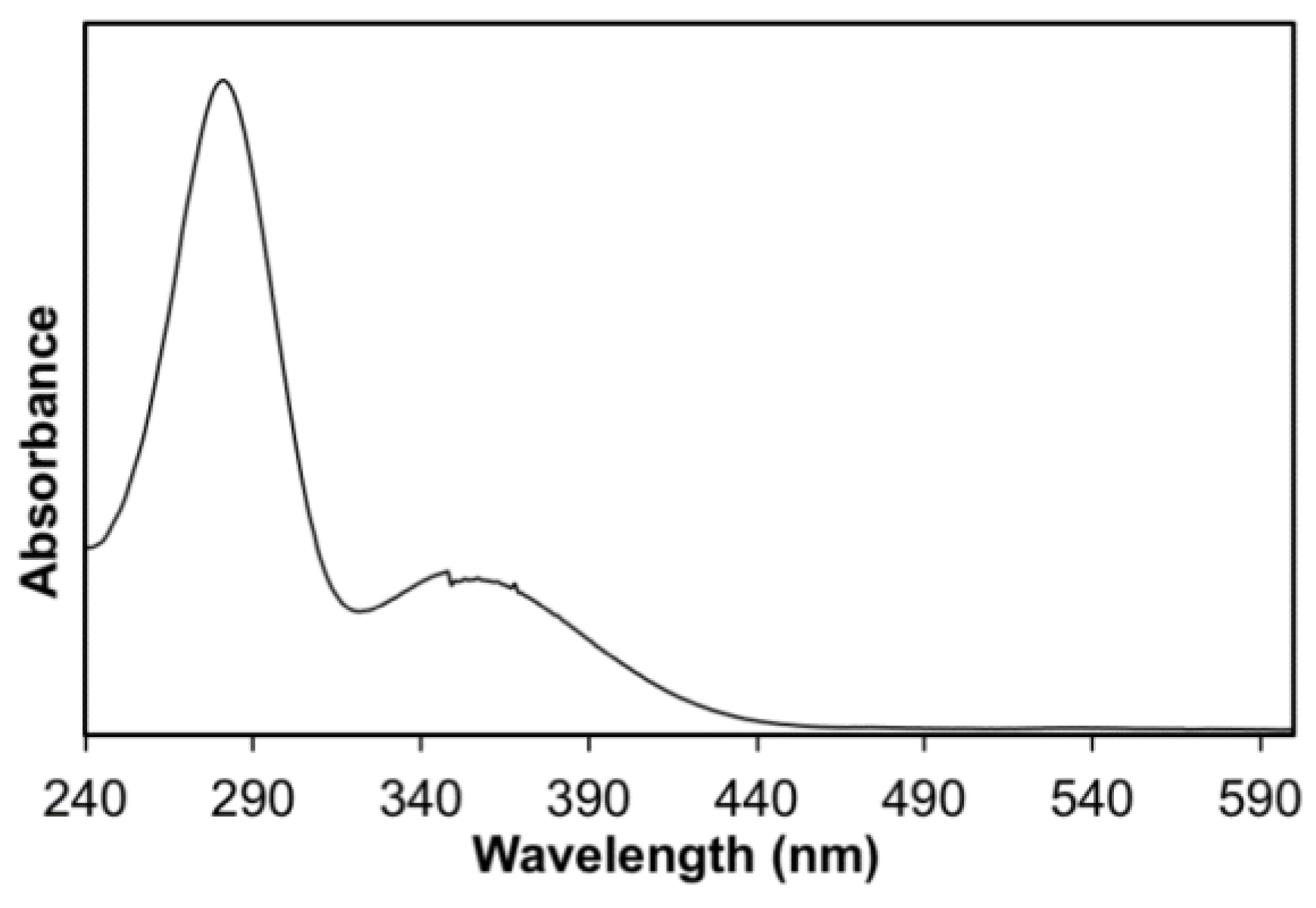
2.3.2. Absorption Spectra
2.3.3. Electrochemistry
3. Discussion
4. Materials and Methods
4.1. Physical Measurements
4.2. Preparation of Compounds
4.3. X-ray Structure Determination
4.4. Computational Details
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Caulton, K.G. Systematics and Future Projections Concerning Redox-Noninnocent Amide/Imine Ligands. Eur. J. Inorg. Chem. 2012, 2012, 435–443. [Google Scholar] [CrossRef]
- Kaim, W. The Shrinking World of Innocent Ligands: Conventional and Non-Conventional Redox-Active Ligands. Eur. J. Inorg. Chem. 2012, 2012, 343–348. [Google Scholar] [CrossRef]
- Luca, O.R.; Crabtree, R.H. Redox-Active Ligands in Catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. [Google Scholar] [CrossRef] [PubMed]
- Lyaskovskyy, V.; de Bruin, B. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. [Google Scholar] [CrossRef]
- Cole, B.E.; Wolbach, J.P.; Dougherty, W.G., Jr.; Piro, N.A.; Kassel, W.S.; Graves, C.R. Synthesis and Characterization of Aluminum-α-diimine Complexes over Multiple Redox States. Inorg. Chem. 2014, 53, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Koellner, C.A.; Piro, N.A.; Kassel, W.S.; Goldsmith, C.R.; Graves, C.R. Synthesis and Characterization of α-Diimine Complexes of Group 13 Metals and Their Catalytic Activity toward the Epoxidation of Alkenes. Inorg. Chem. 2015, 54, 7139–7141. [Google Scholar] [CrossRef] [PubMed]
- Poitras, A.M.; Bogart, J.A.; Cole, B.E.; Carroll, P.J.; Schelter, E.J.; Graves, C.R. Synthesis and Characterization of Aluminum Complexes of Redox-Active Pyridyl Nitroxide Ligands. Inorg. Chem. 2015, 54, 10901–10908. [Google Scholar] [CrossRef] [PubMed]
- Herb, T.M.; Poitras, A.M.; Richardson, K.G.; Cole, B.E.; Bogart, J.A.; Carroll, P.J.; Schelter, E.J.; Graves, C.R. Synthesis and Characterization of Aluminum Nitroxide Complexes. Polyhedron 2016, 114, 194–199. [Google Scholar] [CrossRef]
- Wilson, H.H.; Koellner, C.A.; Hannan, Z.M.; Endy, C.B.; Bezpalko, M.W.; Piro, N.A.; Kassel, W.S.; Sonntag, M.D.; Graves, C.R. Synthesis and Characterization of Neutral Ligand α-Diimine Complexes of Aluminum with Tunable Redox Energetics. Inorg. Chem. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Lee, H.B.; Boreen, M.A.; Jun, M.; Schelter, E.J. Fine-Tuning the Oxidative Ability of Persistent Radicals: Electrochemical and Computational Studies of Substituted 2-Pyridylhydroxylamines. J. Org. Chem. 2013, 78, 6344–6349. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Ishida, T.; Yoshii, S.; Nojiri, H. Single-molecule magnet [Tb(hfac)3(2pyNO)] (2pyNO = t-butyl 2-pyridyl nitroxide) with a relatively high barrier of magnetization reversal. Dalton Trans. 2013, 42, 13968–13973. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Lewis, A.J.; Medling, S.A.; Piro, N.A.; Carroll, P.J.; Booth, C.H.; Schelter, E.J. Homoleptic Cerium(III) and Cerium(IV) Nitroxide Complexes: Significant Stabilization of the 4+ Oxidation State. Inorg. Chem. 2013, 52, 11600–11607. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Lewis, A.J.; Boreen, M.A.; Lee, H.B.; Medling, S.A.; Carroll, P.J.; Booth, C.H.; Schelter, E.J. A Ligand Field Series for the 4f-Block from Experimental and DFT Computed Ce(IV/III) Electrochemical Potentials. Inorg. Chem. 2015, 54, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- McSkimming, A.; Su, J.; Cheisson, T.; Gau, M.R.; Carroll, P.J.; Batista, E.R.; Yang, P.; Schelter, E.J. Coordination Chemistry of a Strongly-Donating Hydroxylamine with Early Actinides: An Investigation of Redox Properties and Electronic Structure. Inorg. Chem. 2018, 57, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Murakami, R.; Kanetomo, T.; Nojiri, H. Magnetic Study on Radical-Gadolinium(III) Complexes. Relationship Between the Exchange Coupling and Coordination Structure. Polyhedron 2013, 66, 183–187. [Google Scholar] [CrossRef]
- Okazawa, A.; Nogami, T.; Ishida, T. tert-Butyl 2-Pyridyl Nitroxide Available as a Paramagnetic Chelate Ligand for Strongly Exchange-Coupled Metal–Radical Compounds. Chem. Mater. 2007, 19, 2733–2735. [Google Scholar] [CrossRef]
- Okazawa, A.; Nogami, T.; Ishida, T. Strong Intramolecular Ferromagnetic Couplings in Nickel(II) and Copper(II) Complexes Chelated with tert-Butyl 5-Methoxy-2-Pyridyl Nitroxide. Polyhedron 2009, 28, 1917–1921. [Google Scholar] [CrossRef]
- Okazawa, A.; Hashizume, D.; Ishida, T. Ferro- and Antiferromagnetic Coupling Switch Accompanied by Twist Deformation around the Copper(II) and Nitroxide Coordination Bond. J. Am. Chem. Soc. 2010, 132, 11516–11524. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.W.; Holmes, A.L.; Berben, L.A. Redox Routes to Substitution of Aluminum(III): Synthesis and Characterization of (IP−)2AlX (IP = α-iminopyridine, X = Cl, Me, SMe, S2CNMe2, C ≡ CPh, N3, SPh, NHPh). Inorg. Chem. 2012, 51, 8997–9004. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.W.; Berben, L.A. Countercations Direct One- or Two-Electron Oxidation of an Al(III) Complex and Al(III)-oxo Intermediates Activate C–H Bonds. J. Am. Chem. Soc. 2011, 133, 11865–11867. [Google Scholar] [CrossRef] [PubMed]
- Cates, C.D.; Myers, T.W.; Berben, L.A. (IP)2GaIII Complexes Facilitate Net Two-Electron Redox Transformations (IP = α-Iminopyridine). Inorg. Chem. 2012, 51, 11891–11897. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-Sulfur Donor Ligands: The Crystal and Molecular Structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Ershova, I.V.; Bogomyakov, A.S.; Starikov, A.G.; Fukin, G.K.; Cherkasov, V.K. Indirect Magnetic Exchange between o-Iminosemiquinonate Ligands Controlled by Apical Substituent in Pentacoordinated Gallium(III). Inorg. Chem. 2015, 54, 6090–6099. [Google Scholar] [CrossRef] [PubMed]
- Specklin, D.; Fliedel, C.; Hild, F.; Mameri, S.; Karmazin, L.; Bailly, C.; Dagorne, S. Mononuclear Salen-Gallium Complexes for iso-Selective Ring-Opening Polymerization (ROP) of rac-Lactide. Dalton Trans. 2017, 46, 12824–12834. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Marchand, P.; Parkin, I.P.; Carmalt, C.J. Group 13 β-ketoiminate Compounds: Gallium Hydride Derivatives as Molecular Precursors to Thin Films of Ga2O3. Inorg. Chem. 2012, 51, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Timoshkin, A.Y.; Bodensteiner, M.; Sevastianova, T.N.; Lisovenko, A.S.; Davydova, E.I.; Scheer, M.; Grassl, C.; Butlak, A.V. Do Solid-State Structures Reflect Lewis Acidity Trends of Heavier Group 13 Trihalides? Experimental and Theoretical Case Study. Inorg. Chem. 2012, 51, 11602–11611. [Google Scholar] [CrossRef] [PubMed]
- Nogai, S.; Schriewer, A.; Schmidbaur, H. Reactions of trichlorogermane HGeCl3 and Dichlorogallane HGaCl2 with Pyridine Donors. Dalton Trans. 2003, 16, 3165–3171. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Lustig, C.; Woski, M. Different Modes of Aggregation in Organoaluminum and -gallium Hydroxylamides. Dalton Trans. 2004, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Rose, R.P. Synthesis and Characterisation of Tetramethylpiperidinyloxide (TEMPO) complexes of Group 13 Metal Hydrides. New J. Chem. 2007, 31, 1484–1487. [Google Scholar] [CrossRef]
- Boesing, P.; Willner, A.; Pape, T.; Hepp, A.; Mitzel, N.W. Structural Diversity in Bishydroxylamine Complexes of Ggallium. Dalton Trans. 2008, 19, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Lustig, C.; Mitzel, N.W. The Highly Flexible Bis(hydroxylamine) Ligand [ON(Me)]2CH22− and its Different Behavior in the Chemistry of Aluminum and Gallium. Angew. Chem. Int. Ed. 2001, 40, 4390–4392. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; p. 1355. [Google Scholar]
- Ogawa, A.; Fujimoto, H. Lewis Acidity of Gallium Halides. Inorg. Chem. 2002, 41, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.K.; Scott, B.L.; Morris, D.E.; Kiplinger, J.L. Synthesis, Structure, Spectroscopy and Redox Energetics of a Series of Uranium(IV) Mixed-Ligand Metallocene Complexes. C. R. Chim. 2010, 13, 790–802. [Google Scholar] [CrossRef]
- APEX II, v. 2012.10-0 or v. 2013.4-1; Bruker AXS: Madison, WI, USA, 2012.
- Bruker APEX 3, v2016.1-0; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Bruker SAINT, v8.37a; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2007. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K and Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]





| Solid-State | Theoretical | |
|---|---|---|
| Ga(1)–Cl(1) | 2.2215(7) | 2.2918 |
| Ga(1)–O(1) Ga(1)–O(2) | 1.8890(18) 1.9011(18) | 1.8936 |
| Ga(1)–N(1) Ga(1)–N(3) | 1.991(2) 2.006(2) | 2.0028 |
| N(2)–O(1) N(4)–O(2) | 1.397(3) 1.397(3) | 1.4265 |
| O(1)–Ga(1)–N(1) O(1)–Ga(1)–N(3) O(2)–Ga(1)–N(1) O(2)–Ga(1)–N(3) | 81.00(8) 91.61(8) 88.87(8) 80.28(8) | 81.22 90.12 |
| O(1)–Ga(1)–O(2) | 145.14(8) | 138.53 |
| N(1)–Ga(1)–N(3) | 149.25(9) | 155.45 |
| O(1)–Ga(1)–Cl(1) O(2)–Ga(1)–Cl(1) | 108.00(6) 106.86(6) | 110.74 |
| N(1)–Ga(1)–Cl(1) N(3)–Ga(1)–Cl(1) | 106.23(6) 104.44(6) | 102.28 |
| Ga(1) | 1.124 | Cl(1) | −0.418 |
| N(1)/N(3) | −0.470 | N(2)/N(4) | −0.076 |
| O(1)/O(2) | −0.565 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsh, J.M.; Woodside, A.J.; Manor, B.C.; Carroll, P.J.; Rablen, P.R.; Graves, C.R. Synthesis and Characterization of (pyNO−)2GaCl: A Redox-Active Gallium Complex. Inorganics 2018, 6, 50. https://doi.org/10.3390/inorganics6020050
Kirsh JM, Woodside AJ, Manor BC, Carroll PJ, Rablen PR, Graves CR. Synthesis and Characterization of (pyNO−)2GaCl: A Redox-Active Gallium Complex. Inorganics. 2018; 6(2):50. https://doi.org/10.3390/inorganics6020050
Chicago/Turabian StyleKirsh, Jacob M., Audra J. Woodside, Brian C. Manor, Patrick J. Carroll, Paul R. Rablen, and Christopher R. Graves. 2018. "Synthesis and Characterization of (pyNO−)2GaCl: A Redox-Active Gallium Complex" Inorganics 6, no. 2: 50. https://doi.org/10.3390/inorganics6020050