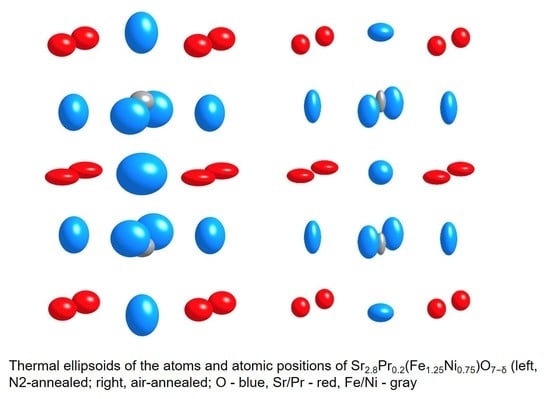
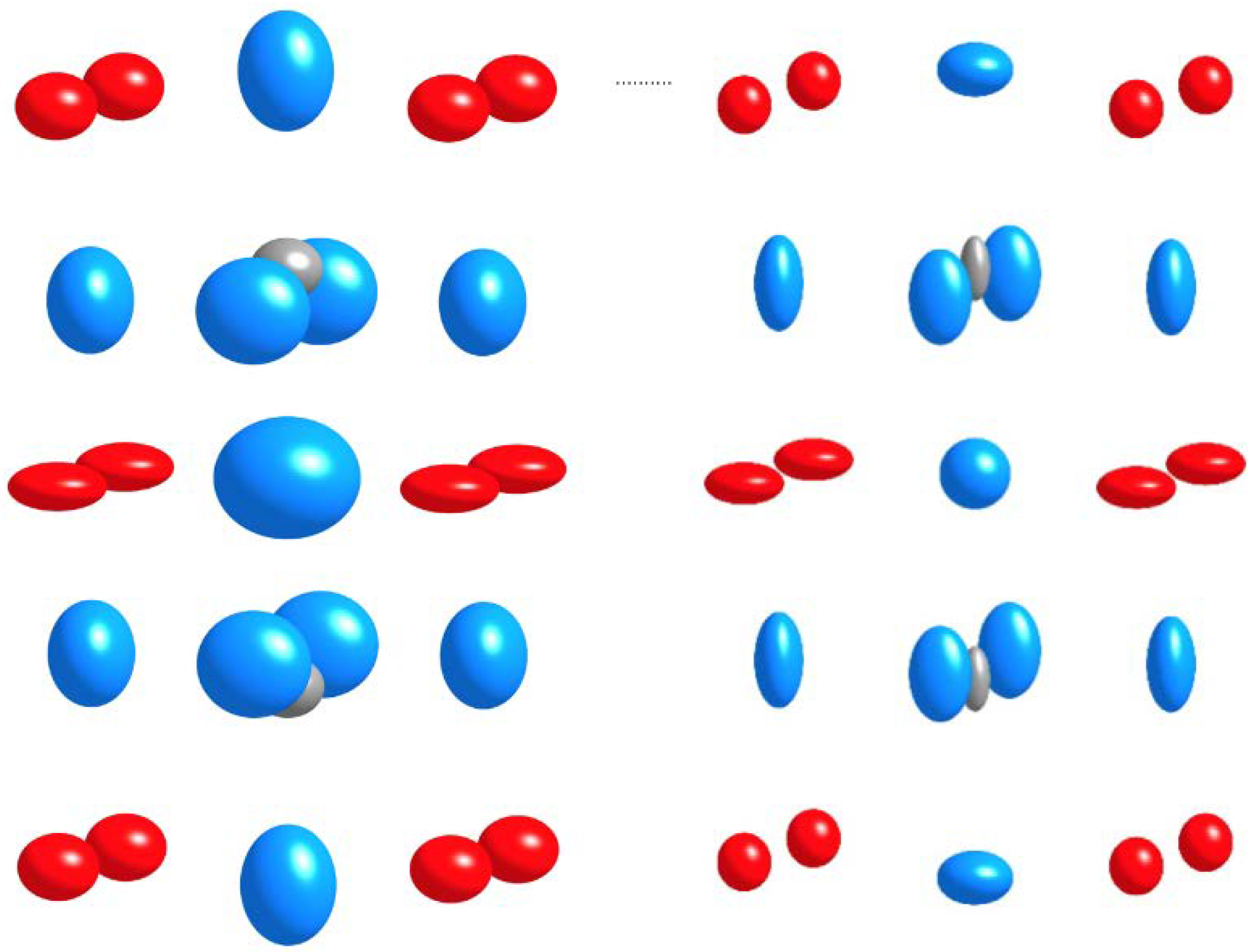
Crystal Structure and Coordination of B-Cations in the Ruddlesden–Popper Phases Sr3−xPrx(Fe1.25Ni0.75)O7−δ (0 ≤ x ≤ 0.4)
Abstract
:1. Introduction
2. Results
2.1. Phase Analysis and Unit Cell Parameters
2.2. Neutron Powder Diffraction Studies
2.3. Refinements Using XRD Data
2.4. Thermal Studies
2.5. Iron-57 Mössbauer Spectroscopy
2.6. High-Temperature Electrical Conductivity
3. Discussion and Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Crystallogr. 1958, 11, 54–55. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Naumovich, E.N.; Marques, F.M.B. Oxygen ion transport in La2NiO4-based ceramics. J. Mater. Chem. 1999, 9, 2623–2629. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Antipov, E.V. Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russ. Chem. Rev. 2013, 82, 686–700. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T.; Currie, D.B. Structure and oxygen stoichiometry in Sr3Fe2O7−y, 0 ≤ y ≤ 1.0. J. Solid State Chem. 1992, 97, 179–185. [Google Scholar] [CrossRef]
- Peets, D.C.; Kim, J.-H.; Dosanj, P.; Reehuis, M.; Aliouane, N.; Ulrich, C.; Keimer, B. Magnetic phase diagram of Sr3Fe2O7−δ. Phys. Rev. B 2013, 87, 214410. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T.; Currie, D.B.; Thomas, M.F.; Al-Rawwa, A.D. Structure and magnetic properties of Sr2FeO4 and Sr3Fe2O7 studied by powder neutron diffraction and Mössbauer spectroscopy. J. Mater. Chem. 1993, 3, 1231–1237. [Google Scholar] [CrossRef]
- Bréard, Y.; Michel, C.; Hervieu, M.; Studer, F.; Maignan, A.; Raveau, B.B. Large Oxygen Deficiency in a n = 2 Member of the RP Series: Sr3FeCoO7−x (x ≤ 1.55). Chem. Mater. 2002, 14, 3128–3135. [Google Scholar] [CrossRef]
- Prado, F.; Armstrong, T.; Caneiro, A.; Manthiram, A. Structural Stability and Oxygen Permeation Properties of Sr3−xLaxFe2−yCoyO7−δ (0 ≤ x ≤ 0.3 and 0 ≤ y ≤ 1.0). J. Electrochem. Soc. 2001, 148, J7–J14. [Google Scholar] [CrossRef]
- Mogni, L.; Prado, F.; Caneiro, A.; Manthiram, A. High temperature properties of the n = 2 Ruddlesden–Popper phases (La, Sr)3(Fe, Ni)2O7−δ. Solid State Ion. 2006, 177, 1807–1810. [Google Scholar] [CrossRef]
- Mogni, L.; Prado, F.; Caneiro, A. Defect Structure and Electrical Conductivity of the Ruddlesden–Popper Phases Sr3FeMO6+δ (M = Co, Ni). Chem. Mater. 2006, 18, 4163–4170. [Google Scholar] [CrossRef]
- Mogni, L.V.; Prado, F.D.; Cuello, G.J.; Caneiro, A. Study of the Crystal Chemistry of the n = 2 Ruddlesden–Popper Phases Sr3FeMO6+δ (M = Fe, Co, and Ni) Using in Situ High Temperature Neutron Powder Diffraction. Chem. Mater. 2009, 21, 2614–2623. [Google Scholar] [CrossRef]
- Ghosh, S.; Adler, P. Evolution of electronic state, magnetism, and magnetotransport properties in Sr3Fe2−xCoxO7−y (x ≤ 1). J. Mater. Chem. 2002, 12, 511–521. [Google Scholar] [CrossRef]
- Samain, L.; Amshoff, P.; Biendicho, J.J.; Tietz, F.; Mahmoud, A.; Hermann, R.P.; Istomin, S.Y.; Grins, J.; Svensson, G. Crystal structure and high-temperature properties of the Ruddlesden–Popper phases Sr3−xYx(Fe1.25Ni0.75)O7−δ (0 ≤ x ≤ 0.75). J. Solid State Chem. 2015, 227, 45–55. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1981. [Google Scholar]
- Grins, J.; Wardecki, D.; Jansson, K.; Carlson, S.; Biendicho, J.J.; Svensson, G. A structural study of Ruddlesden–Popper phases Sr3−xYx(Fe1.25Ni0.75)O7−δ with x ≤ 0.75 by neutron powder diffraction and EXAFS/XANES spectroscopy. J. Mater. Chem. A 2018, 6, 5313–5323. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Kharton, V.V.; Patrakeev, M.V.; Tsipis, E.V.; Avdeeev, M.; Naumovich, E.N.; Anikina, P.V.; Waerenborgh, J.C. Oxygen nonstoichiometry, chemical expansion, mixed conductivity, and anodic behavior of Mo-substituted Sr3Fe2O7−δ. J. Solid State Ion. 2010, 181, 1052–1063. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry, The Bond Valence Model; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- McCammon, C.A.; Becerro, A.I.; Langenhorst, F.; Angel, R.J.; Marion, S.; Seifert, F. Short-range ordering of oxygen vacancies in CaFexTi1−xO3−x/2 perovskites (0 < x < 0.4). J. Phys. Condens. Matter 2000, 12, 2969–2984. [Google Scholar]
- Waerenborgh, J.C.; Figueiredo, F.M.; Frade, J.R.; Colomer, M.T.; Jurado, J.R. Fe4+ content and ordering of anion vacancies in partially reduced AFexTi1−xO3−y (A = Ca, Sr; x ≤ 0.6) perovskites. An 57Fe Mössbauer spectroscopy study. J. Phys. Condens. Matter 2001, 13, 8171–8187. [Google Scholar] [CrossRef]
- Figueiredo, F.M.; Waerenborgh, J.; Kharton, V.V.; Näfe, H.; Frade, J.R. On the relationships between structure, oxygen stoichiometry and ionic conductivity of CaTi1−xFexO3−δ (x = 0.05, 0.20, 0.40, 0.60). Solid State Ion. 2003, 156, 371–381. [Google Scholar] [CrossRef]
- Delattre, J.L.; Stacy, A.M.; Young, V.G., Jr.; Long, G.J.; Hermann, R.; Grandjean, F. Study of the Structural, Electronic, and Magnetic Properties of the Barium-Rich Iron(IV) Oxides, Ba2FeO4 and Ba3FeO5. Inorg. Chem. 2002, 41, 2834–2838. [Google Scholar] [CrossRef] [PubMed]
- Battle, P.D.; Gibb, T.C.; Nixon, S. A study of charge disproportionation in the nonstoichiometric perovskite Sr2LaFe3O8+y by Mössbauer spectroscopy. J. Solid State Chem. 1988, 77, 124–131. [Google Scholar] [CrossRef]
- Adler, P. Electronic state, magnetism, and electrical transport behavior of Sr3−xAxFe2O7 (x ≤ 0.4, A = Ba, La). J. Solid State Chem. 1997, 130, 129–139. [Google Scholar] [CrossRef]
- Adler, P. Charge disproportionation in iron (IV) oxides: Electronic properties and magnetism in Sr3Fe2−xTixO7−y annealed at high oxygen pressures. J. Mater. Chem. 1999, 9, 471–477. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Naumovich, E.N.; Patrakeev, M.V.; Anikina, P.V.; Waerenborgh, J.C.; Kharton, V.V. Defect Interactions in Sr3La(Fe, Al)3O10−δ by Computer Simulations and Mössbauer Spectroscopy. Chem. Mater. 2009, 21, 5072–5078. [Google Scholar] [CrossRef]
- Biendicho, J.J.; Shafeie, S.; Frenck, L.; Gavrilova, D.; Böhme, S.; Bettanini, A.M.; Svedlindh, P.; Hull, S.; Zhao, Z.; Istomin, S.Y.; et al. Synthesis and characterization of perovskite-type Sr(x)Y1−xFeO(3−δ) (0.63 ≤ x < 1.0) and Sr0.75Y0.25Fe1−yMyO3−δ (M = Cr, Mn, Ni), (y = 0.2, 0.33, 0.5). J. Solid State Chem. 2013, 200, 30–38. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Carvajal, J. FULLPROF: A program for Rietveld refinement and pattern matching analysis. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Bordeaux, France, 19–28 July 1990. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS). In Los Alamos National Laboratory Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]







| N2-Annealed a | Air-Annealed b | N2-Annealed c | Air-Annealed d | |
|---|---|---|---|---|
| x | 0 | 0 | 0.2 | 0.2 |
| Site e,f | z/occupancy | z/occupancy | z/occupancy | z/occupancy |
| A1 2b | 0 | 0 | 0 | 0 |
| Sr/Pr | - | - | 0.840(7) Sr + 0.160(7) Pr | 0.89(1) Sr + 0.11(1) Pr |
| A2 4e | 0.68335(4) | 0.68202(4) | 0.68280(4) | 0.6821(1) |
| Sr/Pr | - | - | 0.980(3) Sr + 0.020(3) Pr | 0.956(7) Sr + 0.044(7) Pr |
| B 4e g | 0.10043(3) | 0.09898(3) | 0.10041(2) | 0.09881(3) |
| O1 2a | 0 | 0 | 0 | 0 |
| occ | 0.335(5) | 0.552(6) | 0.274(4) | 0.640(6) |
| O2 4e | 0.19502(5) | 0.19406(5) | 0.19562(5) | 0.19437(6) |
| occ | 1 | 1 | 1 | 1 |
| O3 8b | 0.08683(4) | 0.09094(4) | 0.08487(4) | 0.09072(1) |
| occ | 0.813(3) | 1 | 0.852(2) | 1 |
| N2-Annealed | N2-Annealed | Air-Annealed | Air-Annealed | |
|---|---|---|---|---|
| x | 0 | 0.2 | 0 | 0.2 |
| A1–O1 × 4 | 2.7147(1) | 2.7283(2) | 2.7170(1) | 2.7182(1) |
| A1–O3 × 8 | 2.6121(1) | 2.5820(5) | 2.6522(1) | 2.6485(6) |
| A2–O2 × 1 | 2.4790(1) | 2.458(1) | 2.4919(1) | 2.481(1) |
| A2–O2 × 4 | 2.7248(1) | 2.7406(2) | 2.7277(1) | 2.7293(2) |
| A2–O3 × 4 | 2.7428(1) | 2.7645(7) | 2.6537(1) | 2.6579(9) |
| B–O1 × 1 | 2.0438(1) | 2.0301(5) | 1.9901(1) | 1.9847(7) |
| B–O2 × 1 | 1.9207(1) | 1.9252(9) | 1.9113(1) | 1.919(1) |
| B–O3 × 4 | 1.9388(1) | 1.9546(2) | 1.9280(1) | 1.9289(2) |
| B–O3–B | 163.86(1) | 161.50(6) | 170.38(1) | 170.33(7) |
| N2-Annealed | Air-Annealed | |||
|---|---|---|---|---|
| x | 0.0 | 0.2 | 0.0 | 0.2 |
| A1 | 1.96 | 2.17 | 2.32 | 2.43 |
| A2 | 1.74 | 1.74 | 2.07 | 2.07 |
| B | 2.89 | 2.96 | 3.62 | 3.54 |
| x | 0.1 | 0.2 | 0.3 |
|---|---|---|---|
| 150–900 °C | 23.4 | 22.5 | 19.5 |
| 150–400 °C | 17.9 | 17.7 | 15.6 |
| 400–900 °C | 26.2 | 24.9 | 21.5 |
| x | 0 | 0.2 | 0.4 | |
|---|---|---|---|---|
| Site I Pentahedral (1) Fe3+ | δ | 0.40(1) | 0.38(5) | 0.37(2) |
| Δ | −0.27(2) | −0.10(2) | −0.23(5) | |
| Γ | 0.52(1) | 0.8(3) | 0.68(1) | |
| Bhf (T) | 46.2(1) | 45.6(5) | 49.1(2) | |
| Area (%) b | 37(3) | 50(3) | 94(1) | |
| Site II 5-fold (2) Fe3+ | δ | 0.39(1) | 0.39(2) | - |
| Δ | −0.26(2) | −0.28(4) | - | |
| Γ | 0.52(1) | 0.36(5) | - | |
| Bhf (T) | 49.1(1) | 49.3(2) | - | |
| Area (%) b | 24(1) | 43(1) | - | |
| Site III Tetrahedral Fe3+ | δ | 0.26(2) | - | - |
| Δ | −0.06(2) | - | - | |
| Γ | 0.52(1) | - | - | |
| Bhf (T) | 42.7(2) | - | - | |
| Area (%) b | 33(1) | - | - | |
| Site IV Paramagnetic | δ | 0.45(1) | 0.44(2) | 0.5(1) |
| Γ | 1.29(1) | 1.0(1) | 0.97(1) | |
| Area (%) b | 6(1) | 7(1) | 6(1) |
| x | 0 | 0.2 | 0.4 | |
|---|---|---|---|---|
| Site V Fe3+ octahedral | δ | 0.65(4) | - | 0.46(8) |
| Δ | 0.80(2) | - | 0.09(1) | |
| Γ | 0.50(4) | - | 0.28(3) | |
| Bhf (T) | 33(1) | - | 40.5(6) | |
| Area (%) b | 21(1) | - | 36(2) | |
| Site VI Fe3+/Fe4+ | δ | 0.17(1) | 0.21(7) | 0.06(6) |
| Δ | 0.50(4) | −0.06(1) | −0.07(1) | |
| Γ | 0.50(4) | 0.40(2) | 0.28(-) | |
| Bhf (T) | 25(1) | 38.5(7) | 32.1(2) | |
| Area (%) b | 28(4) | 57(1) | 46(3) | |
| Site VII Fe5+ octahedral | δ | −0.03(1) | −0.03(1) | −0.02(2) |
| Δ | −0.2(1) | −0.1(1) | −0.4(1) | |
| Γ | 0.28(1) | 0.25(1) | 0.28(1) | |
| Bhf (T) | 31.6(9) | 32.0(9) | 39(1) | |
| Area (%) b | 51(2) | 43(2) | 18 (3) | |
| O-content | 6.4 | 6.8 | 6.5 |
| x | 0 | 0.2 | 0.4 | |
|---|---|---|---|---|
| Ideal a | Fe3+/Ni2+ | 5.63 | 5.73 | 5.83 |
| Ideal a | Fe3+/Ni3+ | 6.00 | 6.10 | 6.20 |
| Ideal a | Fe4+/Ni3+ | 6.63 | 6.73 | 6.83 |
| MB b—N2 | Fe3+/Ni2+ | 5.63 | 5.73 | 5.83 |
| MB b—air | Fe3+/Fe4+/Fe5+/Ni2+ | 6.6 | 6.8 | 6.5 |
| N2-NPD*/XRD** c | - | 5.56* | 5.68* | 5.90** |
| air-NPD*/XRD** c | - | 6.55* | 6.64* | 6.68** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, G.; Samain, L.; Biendicho, J.J.; Mahmoud, A.; Hermann, R.P.; Istomin, S.Y.; Grins, J. Crystal Structure and Coordination of B-Cations in the Ruddlesden–Popper Phases Sr3−xPrx(Fe1.25Ni0.75)O7−δ (0 ≤ x ≤ 0.4). Inorganics 2018, 6, 89. https://doi.org/10.3390/inorganics6030089
Svensson G, Samain L, Biendicho JJ, Mahmoud A, Hermann RP, Istomin SY, Grins J. Crystal Structure and Coordination of B-Cations in the Ruddlesden–Popper Phases Sr3−xPrx(Fe1.25Ni0.75)O7−δ (0 ≤ x ≤ 0.4). Inorganics. 2018; 6(3):89. https://doi.org/10.3390/inorganics6030089
Chicago/Turabian StyleSvensson, Gunnar, Louise Samain, Jordi Jacas Biendicho, Abdelfattah Mahmoud, Raphaël P. Hermann, Sergey Ya. Istomin, and Jekabs Grins. 2018. "Crystal Structure and Coordination of B-Cations in the Ruddlesden–Popper Phases Sr3−xPrx(Fe1.25Ni0.75)O7−δ (0 ≤ x ≤ 0.4)" Inorganics 6, no. 3: 89. https://doi.org/10.3390/inorganics6030089