Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma
Abstract
:1. Introduction
2. Objectives
3. Methods
- What is the prevalence of osteonecrosis (ONJ) relating to primary disease?
- What are the prevalences before and after the adaptions of the AAOMS classification in 2009?
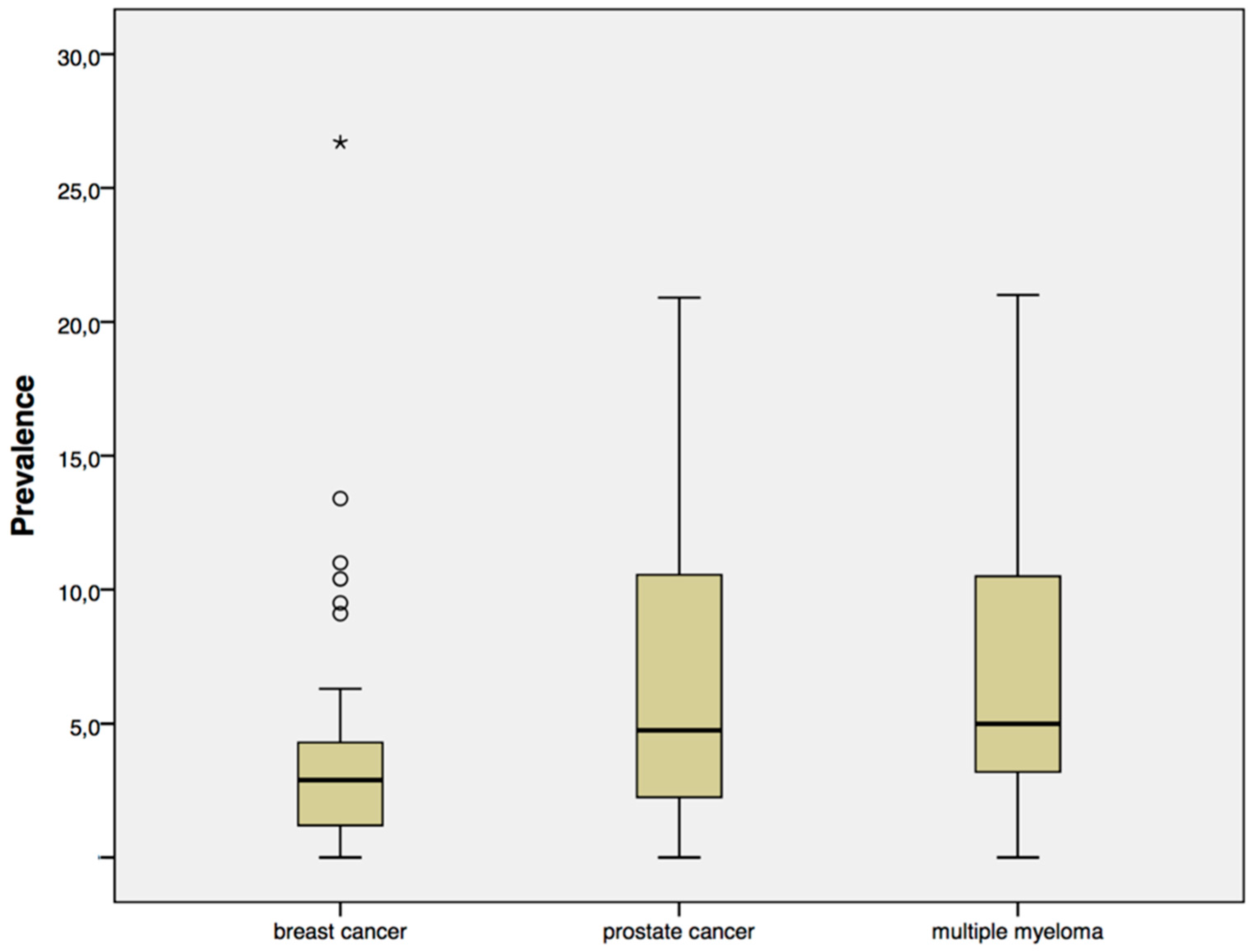
4. Results
- Study population not described in sufficient detail;
- Primary disease for each ONJ case not assignable;
- Oral administration route;
- Double reports;
- Combined analysis of other trials;
- Estimation of prevalence; and
- Special risk situation due to performed intervention.
5. Discussion
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Tarassoff, P.; Csermak, K. Avascular necrosis of the jaws: Risk factors in metastatic cancer patients. J. Oral Maxillofac. Surg. 2003, 61, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, S2–S12. [Google Scholar]
- Ziebart, T.; Pabst, A.; Klein, M.O.; Kammerer, P.; Gauss, L.; Brullmann, D.; Al-Nawas, B.; Walter, C. Bisphosphonates: Restrictions for vasculogenesis and angiogenesis: Inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin. Oral Investig. 2011, 15, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hoefert, S.; Schmitz, I.; Tannapfel, A.; Eufinger, H. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: A possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin. Oral Investig. 2010, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Rustemeyer, J.; Bremerich, A. Bisphosphonate-associated osteonecrosis of the jaw: What do we currently know? A survey of knowledge given in the recent literature. Clin. Oral Investig. 2010, 14, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67, S61–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar]
- Fedele, S.; Porter, S.R.; D’Aiuto, F.; Aljohani, S.; Vescovi, P.; Manfredi, M.; Arduino, P.G.; Broccoletti, R.; Musciotto, A.; Di Fede, O.; et al. Nonexposed variant of bisphosphonate-associated osteonecrosis of the jaw: A case series. Am. J. Med. 2010, 123, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Almăşan, H.A.; Băciut, M.; Rotaru, H.; Bran, S.; Almăşan, O.C.; Băciut, G. Osteonecrosis of the jaws associated with the use of bisphosphonates. Discussion over 52 cases. Rom. J. Morphol. Embryol. 2011, 52, 1233–1241. [Google Scholar] [PubMed]
- Bantis, A.; Zissimopoulos, A.; Sountoulides, P.; Kalaitzis, C.; Giannakopoulos, S.; Deftereos, S.; Tsakaldimis, G.; Thomaidis, V.; Touloupidis, S. Bisphosphonate-induced osteonecrosis of the jaw in patients with bone metastatic, hormone-sensitive prostate cancer. Risk factors and prevention strategies. Tumori 2011, 97, 479–483. [Google Scholar] [PubMed]
- Bonacina, R.; Mariani, U.; Villa, F.; Villa, A. Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: A review of 282 patients. J. Can. Dent. Assoc. 2011, 77, b147. [Google Scholar] [PubMed]
- Schubert, M.; Klatte, I.; Linek, W.; Müller, B.; Döring, K.; Eckelt, U.; Hemprich, A.; Berger, U.; Hendricks, J. The Saxon Bisphosphonate Register—Therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncol. 2011, 48, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.O.; Toth, B.B.; Altundag, K.; Johnson, M.M.; Warneke, C.L.; Hu, M.; Nooka, A.; Sayegh, G.; Guarneri, V.; Desrouleaux, K.; et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J. Bone Miner. Res. 2008, 23, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; Frickhofen, N.; Gamm, H.; Beck, J.; Reinsch, L.; Blum, C.; Grötz, K.A.; Wagner, W. Prevalence of bisphosphonate associate osteonecrosis of the jaws in multiple myeloma patients. Head Face Med. 2010, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C.; et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.; Katz, M.; Crowley, J. Osteonecrosis of the jaw and bisphosphonates. N. Engl. J. Med. 2005, 353, 99–102. [Google Scholar] [PubMed]
- Guarneri, V.; Donati, S.; Nicolini, M.; Giovannelli, S.; D’Amico, R.; Conte, P.F. Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 Years. Oncologist 2005, 10, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Weikel, D.; Salama, A.; Goloubeva, O.; Schneider, A.; Rapoport, A.; Fenton, R.; Gahres, N.; Sausville, E.; Ord, R.; et al. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J. Clin. Oncol. 2006, 24, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Villas, J.M.; Tapia Torres, M.; Govantes Rodríguez, J.; Carreter de Granda, E.; Sicilia Guillén, F. Osteonecrosis of the jaw in patients with multiple myeloma during and after treatment with zoledronic acid. Med. Clin. 2006, 127, 576–579. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Anagnostopoulos, A.; Melakopoulos, I.; Gika, D.; Moulopoulos, L.A.; Bamia, C.; Terpos, E.; Tsionos, K.; Bamias, A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica 2006, 91, 968–971. [Google Scholar] [PubMed]
- Sanna, G.; Preda, L.; Bruschini, R.; Cossu Rocca, M.; Ferretti, S.; Adamoli, L.; Verri, E.; Franceschelli, L.; Goldhirsch, A.; Nole, F. Bisphosphonates and jaw osteonecrosis in patients with advanced breast cancer. Ann. Oncol. 2006, 17, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Tosi, P.; Zamagni, E.; Cangini, D.; Tacchetti, P.; Di Raimondo, F.; Catalano, L.; D’Arco, A.; Ronconi, S.; Cellini, C.; Offidani, M.; et al. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide-dexamethasone. Blood 2006, 108, 3951–3952. [Google Scholar] [CrossRef] [PubMed]
- Zervas, K.; Verrou, E.; Teleioudis, Z.; Vahtsevanos, K.; Banti, A.; Mihou, D.; Krikelis, D.; Terpos, E. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: A single-centre experience in303patients. Br. J. Haematol. 2006, 134, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; Faggiuolo, R.; Vormola, R.; Montemurro, F.; Nanni, D.; Goia, F.; Aglietta, M. Jaw complications in breast and prostate cancer patients treated with zoledronic acid. Acta Oncol. 2006, 45, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Bujanda, D.; Bohn Sarmiento, U.; Cabrera Suarez, M.A.; Aguiar Morales, J. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann. Oncol. 2007, 18, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Corso, A.; Varettoni, M.; Zappasodi, P.; Klersy, C.; Mangiacavalli, S.; Pica, G.; Lazzarino, M. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia 2007, 21, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Garcia Saenz, J.A.; Lopez Tarruella, S.; Garcia Paredes, B.; Rodriguez Lajusticia, L.; Villalobos, L.; Diaz Rubio, E. Osteonecrosis of the jaw as an adverse bisphosphonate event: Three cases of bone metastatic prostate cancer patients treated with zoledronic acid. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E351–E356. [Google Scholar] [PubMed]
- Jadu, F.; Lee, L.; Pharoah, M.; Reece, D.; Wang, L. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann. Oncol. 2007, 18, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; Montemurro, F.; Faggiuolo, R.; Vormola, R.; Nanni, D.; Goia, F.; Gilardino, M.O.; Aglietta, M. Osteonecrosis of the jaw in prostate cancer patients with bone metastases treated with zoledronate: A retrospective analysis. Acta Oncol. 2007, 46, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, M.T.; Gallucci, C.; Agrillo, A.; Mustazza, M.C.; Foa, R. Role of ozone therapy in the treatment of osteonecrosis of the jaws in multiple myeloma patients. Haematologica 2007, 92, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.P.; Kaban, L.B.; Strewler, G.J.; Raje, N.; Troulis, M.J. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J. Oral Maxillofac. Surg. 2007, 65, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Steger, G.G.; Figueroa, J.; Alvarado, C.; Solal-Celigny, P.; Body, J.J.; de Boer, R.; Berardi, R.; Gascon, P.; Tonkin, K.S.; et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 2007, 25, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, S.; Marcheselli, R.; Sacchi, S.; Baldini, L.; Angrilli, F.; Pennese, E.; Quarta, G.; Stelitano, C.; Caparotti, G.; Luminari, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: A review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leuk. Lymphoma 2007, 48, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Boonyapakorn, T.; Schirmer, I.; Reichart, P.A.; Sturm, I.; Massenkeil, G. Bisphosphonate-induced osteonecrosis of the jaws: Prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008, 44, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Beck, V.; Banys, M.; Lipp, H.P.; Hairass, M.; Reinert, S.; Solomayer, E.F.; Wallwiener, D.; Krimmel, M. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol. Oncol. 2009, 112, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Barbanti, F.; Giorgio-Marrano, G.; Mercatali, L.; Ronconi, S.; Vicini, C.; Amadori, D. Osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: A retrospective study. Oncologist 2008, 13, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; Grotz, K.A.; Thomas, C.; Thuroff, J.W.; Zinser, V.; Gamm, H.; Beck, J.; Wagner, W. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur. Urol. 2008, 54, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Fujiwara, Y.; Minami, H.; Kitagawa, K.; Fujii, H.; Arai, T.; Sohn, W.; Ohkura, M.; Ohtsu, T. Phase 1 trial of denosumab safety, pharmacokinetics, and pharmacodynamics in Japanese women with breast cancer-related bone metastases. Cancer Sci. 2008, 99, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.K.; Bone, H.G.; Chlebowski, R.; Paul, D.; Spadafora, S.; Smith, J.; Fan, M.; Jun, S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008, 26, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Estilo, C.L.; Van Poznak, C.H.; Wiliams, T.; Bohle, G.C.; Lwin, P.T.; Zhou, Q.; Riedel, E.R.; Carlson, D.L.; Schoder, H.; Farooki, A.; et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist 2008, 13, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, V.; Gay, F.; Spina, F.; Miceli, R.; Maniezzo, M.; Teresa Ambrosini, M.; Farina, L.; Piva, S.; Palumbo, A.; Boccadoro, M.; et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk. Lymphoma 2008, 49, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Petrucci, M.T.; Bringhen, S.; Guglielmelli, T.; Caravita, T.; Bongarzoni, V.; Andriani, A.; D’Arena, G.; Balleari, E.; Pietrantuono, G.; et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 2008, 113, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; Du Bois, A.; Buch, L.; Harter, P.; Grötz, K.A. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009, 115, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Ching, J.B.; Ning, Y.M.; Chen, C.C.; Latham, L.; Guadagnini, J.P.; Gulley, J.L.; Arlen, P.M.; Wright, J.J.; Parnes, H.; Figg, W.D.; et al. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Investig. 2009, 27, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cetiner, S.; Sucak, G.T.; Kahraman, S.A.; Aki, S.Z.; Kocakahyaoglu, B.; Gultekin, S.E.; Cetiner, M.; Haznedar, R. Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. J. Bone Miner. Metab. 2009, 27, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.S.; McNulty, R.M.; Kraut, E.H.; Turowski, R.C. Extended use of intravenous bisphosphonate therapy for the prevention of skeletal complications in patients with cancer. Cancer Investig. 2009, 27, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Kastritis, E.; Bamia, C.; Melakopoulos, I.; Gika, D.; Roussou, M.; Migkou, M.; Eleftherakis-Papaiakovou, E.; Christoulas, D.; Terpos, E.; et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann. Oncol. 2009, 20, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Haidar, A.; Jonler, M.; Folkmar, T.B.; Lund, L. Bisphosphonate (zoledronic acid)-induced osteonecrosis of the jaw. Scand. J. Urol. Nephrol. 2009, 43, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.I.; Maniezzo, M.; Campa, T.; Fagnoni, E.; Brunelli, C.; Saibene, G.; Bareggi, C.; Ascani, L.; Cislaghi, E. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann. Oncol. 2009, 20, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katadritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Lipton, A.; Mariette, X.; Body, J.J.; Rahim, Y.; Gralow, J.R.; Gao, G.; Wu, L.; Sohn, W.; Jun, S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009, 27, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.; Nortilli, R.; Molina, A.; Sava, T.; Santo, A.; Caldara, A.; Cetto, G.L. Renal toxicity and osteonecrosis of the jaw in cancer patients treated with bisphosphonates: A long-term retrospective analysis. Med. Oncol. 2009, 27, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Stumpe, M.R.; Chandra, R.K.; Yunus, F.; Samant, S. Incidence and risk factors of bisphosphonate-associated osteonecrosis of the jaws. Head Neck 2009, 31, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, P.; Carlson, K.; Turesson, I.; Fayers, P.; Waage, A.; Vangsted, A.; Mylin, A.; Gluud, C.; Juliusson, G.; Gregersen, H.; et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): A double-blind, randomised controlled trial. Lancet Oncol. 2010, 11, 973–982. [Google Scholar] [CrossRef]
- Pavkovic, M.; Petrushevska, G.; Jovanovic, R.; Karanfilski, O.; Cevreska, L.; Stankovic, S.; Stojanovic, A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphanates. Prilozi 2010, 31, 39–49. [Google Scholar] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Quispe, D.; Shi, R.; Burton, G. Osteonecrosis of the jaw in patients with metastatic breast cancer: Ethnic and socio-economic aspects. Breast J. 2011, 17, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Fan, Y.; Ma, F.; Li, Q.; Wang, J.; Zhang, P.; Yuan, P.; Xu, B. Prolonged administration of bisphosphonates is well-tolerated and effective for skeletal-related events in Chinese breast cancer patients with bone metastasis. Breast 2012, 21, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Nishimatsu, H.; Kume, H.; Suzuki, M.; Fujimura, T.; Fukuhara, H.; Enomoto, Y.; Ishikawa, A.; Igawa, Y.; Hirano, Y.; et al. Leukopenia as a risk factor for osteonecrosis of the jaw in metastatic prostate cancer treated using zoledronic acid and docetaxel. BJU Int. 2012, 110, E520–E525. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Coleman, R.; Shore, N.; Fizazi, K.; Tombal, B.; Miller, K.; Sieber, P.; Karsh, L.; Damião, R.; et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet 2012, 379, 39–46. [Google Scholar] [CrossRef]
- Thumbigere-Math, V.; Tu, L.; Huckabay, S.; Dudek, A.Z.; Lunos, S.; Basi, D.L.; Hughes, P.J.; Leach, J.W.; Swenson, K.K.; Gopalakrishnan, R. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am. J. Clin. Oncol. 2012, 35, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Rugani, P.; Luschin, G.; Jakse, N.; Kirnbauer, B.; Lang, U.; Acham, S. Prevalence of bisphosphonate-associated osteonecrosis of the jaw after intravenous zoledronate infusions in patients with early breast cancer. Clin. Oral Investig. 2014, 18, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Then, C.; Hörauf, N.; Otto, S.; Pautke, C.; von Tresckow, E.; Röhnisch, T.; Baumann, P.; Schmidmaier, R.; Bumeder, I.; Oduncu, F.S. Incidence and risk factors of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma patients having undergone autologous stem cell transplantation. Onkologie 2012, 35, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.; Bell, R.; Bourgeois, H.; Brufsky, A.; Diel, I.; Eniu, A.; Fallowfield, L.; Fujiwara, Y.; Jassem, J.; Paterson, A.H.; et al. Bone-related complications and quality of life in advanced breast cancer: Results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012, 18, 4841–4849. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Brown, J.E.; Van Poznak, C.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.T.; Smeets, R.; Riecke, B.; Weise, E.; Groebe, A.; Blessmann, M.; Steiner, T.; Wikner, J.; Friedrich, R.E.; Heiland, M.; et al. Incidence of bisphosphonate-related osteonecrosis of the jaw in consideration of primary diseases and concomitant therapies. Anticancer Res. 2013, 33, 3917–3924. [Google Scholar] [PubMed]
- Coleman, R.E.; de Boer, R.; Eidtmann, H.; Llombart, A.; Davidson, N.; Neven, P.; von Minckwitz, G.; Sleeboom, H.P.; Forbes, J.; Barrios, C.; et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann. Oncol. 2013, 24, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Sereika, S.M.; Mathew, A.; Tomifumi, O.; Singh, V.; Rosenzweig, M. Long-term Treatment with Intravenous Bisphosphonates in Metastatic Breast Cancer: A Retrospective Study. Breast J. 2013, 19, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, E.J.; Brown, J.E.; Marshall, H.C.; Collinson, M.; Liversedge, V.; Murden, G.A.; Cameron, D.; Bell, R.; Spensley, S.; Agrawal, R.; et al. Osteonecrosis of the jaw and oral health–related quality of life after adjuvant zoledronic acid: An Adjuvant Zoledronic Acid to Reduce Recurrence Trial subprotocol (BIG01/04). J. Clin. Oncol. 2013, 31, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Lee, P.; Casbard, A.; Abraham, J.; Hood, K.; Coleman, R.; Simmods, P.; Timmins, H.; Wheatley, D.; Griffiths, G.; Murray, N. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metasta-ses from breast cancer: A randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014, 15, 114–122. [Google Scholar] [CrossRef]
- Coleman, R.; Cameron, D.; Dodwell, D.; Bell, R.; Wilson, C.; Rathbone, E.; Keane, M.; Gil, M.; Burkinshaw, R.; Grieve, R.; et al. Adjuvant zoledronic acid in patients with early breast cancer: Final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014, 15, 997–1006. [Google Scholar] [CrossRef]
- Jackson, G.H.; Morgan, G.J.; Davies, F.E.; Wu, P.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Navarro Coy, N.; Drayson, M.T.; Owen, R.G.; et al. Osteonecrosis of the jaw and renal safety in patients with newly diagnosed multiple myeloma: Medical Research Council Myeloma IX Study results. Br. J. Haematol. 2014, 166, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Knauer, M.; Moik, M.; Jakesz, R.; Seifert, M.; Taucher, S.; Bjelic-Radisic, V.; et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: Final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015, 26, 313–320. [Google Scholar] [PubMed]
- Vidal-Real, C.; Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Gándara-Rey, J.M.; García-García, A. Osteonecrosis of the jaws in 194 patients who have undergone intravenous bisphosphonate therapy in Spain. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e267–e272. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Hering, F.; Imperio, M. Safety of IV Nonnitrogen Bisphosphonates on the Occurrence of Osteonecrosis of the Jaw: Long-Term follow-up on Prostate Cancer Patients. Clin. Genitourin. Cancer 2015, 13, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Fizazi, K.; Body, J.J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer 2016, 24, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Boquete-Castro, A.; Gómez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants Res. 2016, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Mavrokokki, A.; Carter, G.; Stein, B.; Fazzalari, N.L.; Wilson, D.F.; Goss, A.N. The dental implications of bisphosphonates and bone disease. Aust. Dent. J. 2005, 50, S4–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarovici, T.S.; Yahalom, R.; Taicher, S.; Elad, S.; Hardan, I.; Yarom, N. Bisphosphonate-related osteonecrosis of the jaws: A single-center study of 101 patients. J. Oral Maxillofac. Surg. 2009, 67, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Grötz, K.A.; Diel, I.J. Osteonekrose des Kiefers unter Bisphosphonat-Langzeittherapie. Im Focus Onkol. 2005, 3, 52–55. [Google Scholar]
- Abu-Id, M.H.; Warnke, P.H.; Gottschalk, J.; Springer, I.; Wiltfang, J.; Acil, Y.; Russo, P.A.; Kreusch, T. “Bis-phossy jaws”—High and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J. Cranio-Maxillo-Fac. Surg. 2008, 36, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Sturzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws—Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Cranio-Maxillo-Fac. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Suleman, Y.F.; Meer, S.; Lurie, R. Bisphosphonate-induced osteonecrosis of the jaws: Review, clinical implications and case report. Head Neck Pathol. 2007, 1, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, G.; Beninati, F.; Rubino, I.; Vannucchi, A.; Longo, G.; Tonelli, P.; Pini Prato, G. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J. Clin. Periodontol. 2005, 32, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.; Bryant, C.; Popat, S. A study of 225 patients on bisphosphonates presenting to the bisphosphonate clinic at King’s College Hospital. Br. Dent. J. 2013, 214, E18. [Google Scholar] [CrossRef] [PubMed]
- Saia, G.; Blandamura, S.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Bedogni, A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J. Oral Maxillofac. Surg. 2010, 68, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Niibe, K.; Ouchi, T.; Iwasaki, R.; Nakagawa, T.; Horie, N. Osteonecrosis of the jaw in patients with dental prostheses being treated with bisphosphonates or denosumab. J. Prosthodont. Res. 2015, 59, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Nedjat, R.K.; Sagheb, K.; Pabst, A.; Olk, L.; Walter, C. Diabetes Mellitus and Its Association to the Occurrence of Medication-Related Osteonecrosis of the Jaw. Dent. J. 2016, 4, 17. [Google Scholar] [CrossRef]
- Beninati, F.; Pruneti, R.; Ficarra, G. Bisphosphonate-related osteonecrosis of the jaws (Bronj). Med. Oral Patol. Oral Cir. Bucal 2013, 18, e752–e758. [Google Scholar] [CrossRef] [PubMed]
- Sim Ie, W.; Sanders, K.M.; Borromeo, G.L.; Seymour, J.F.; Ebeling, P.R. Declining Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Cancer. J. Clin. Endocrinol. Metab. 2015, 100, 3887–3893. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.V.; Jimenez, Y.; Gomez, D.; Sirera, R.; Poveda, R.; Scully, C. Collagen telopeptide (serum CTX) and its relationship with the size and number of lesions in osteonecrosis of the jaws in cancer patients on intravenous bisphosphonates. Oral Oncol. 2008, 44, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Cillo, J.E., Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar] [CrossRef] [PubMed]

| Year | Author | Study Design | Disease | Patients (n) | ONJ Cases | Prevalence (%) | Used Agent |
|---|---|---|---|---|---|---|---|
| 2005 | Bamias [17] | pros | breast ca | 70 | 2 | 2.86 | Z, PZ, ZI |
| pros | mult myel | 111 | 11 | 9.91 | Z, PZ, ZI | ||
| pros | prostate ca | 46 | 3 | 6.52 | Z, PZ, ZI | ||
| Durie [18] | web survey | mult myel | 904 | 62 | 6.86 | Z, P | |
| web survey | breast ca | 299 | 13 | 4.35 | |||
| Guarneri [19] | retro | breast ca | 48 | 3 | 6.25 | P | |
| 2006 | Badros [20] | retro | mult myel | 340 | 11 | 3.24 | P, Z, PZ |
| Calvo-Villas [21] | retro | mult myel | 64 | 7 | 10.94 | Z | |
| Dimopoulos [22] | pros | mult myel | 202 | 15 | 7.43 | Z | |
| Sanna [23] | pros | breast ca | 81 | 5 | 6.17 | P, Z | |
| Tosi [24] | retro | mult myel | 259 | 9 | 3.47 | Z | |
| Zervas [25] | pros | mult myel | 254 | 28 | 11.02 | Z, P, ZP | |
| Ortega [26] | ? | breast ca | 126 | 2 | 1.59 | Z | |
| 2007 | Aguiar Bujanda [27] | css | breast ca | 35 | 4 | 11.43 | Z |
| Corso [28] | retro | mult myel | 106 | 8 | 7.55 | Z, PZ | |
| García Sáenz [29] | pros | prostate ca | 104 | 3 | 2.88 | Z | |
| Jadu [30] | retro | mult myel | 655 | 21 | 3.21 | P | |
| Ortega [31] | retro | prostate ca | 52 | 6 | 11.54 | Z | |
| Petrucci [32] | ? | mult myel | 311 | 22 | 7.07 | Z, P, PZ | |
| Wang [33] | retro | mult myel | 292 | 11 | 3.77 | Z, P, Z | |
| retro | breast ca | 81 | 2 | 2.47 | Z, P, Z | ||
| retro | prostate ca | 69 | 2 | 2.9 | Z, P, Z | ||
| Lipton [34] | prosp | breast ca | 211 | 0 | 0 | D | |
| Pozzi [35] | retro | mult myel | 1402 | 28 | 2 | Z, PZ | |
| 2008 | Boonyapakorn [36] | pros | mult myel | 58 | 10 | 17.24 | P, PZ, IZ, Z |
| Fehm [37] | retro | breast ca | 233 | 10 | 4.29 | Z, ICPZ | |
| Ibrahim [38] | retro | breast ca | 220 | 5 | 2.27 | PZ, Z | |
| retro | mult myel | 59 | 2 | 3.39 | PZ, Z | ||
| Walter [39] | css | prostate ca | 43 | 8 | 18.6 | IZ, PZ, Z | |
| Yonemori [40] | prosp | breast ca | 18 | 0 | 0 | D | |
| Ellis [41] | prosp | breast ca | 106 | 0 | 0 | D | |
| Christodoulou [42] | retro | breast ca | 75 | 2 | 2.67 | Z, I | |
| retro | prostate ca | 11 | 1 | 9.1 | Z, I | ||
| Estilo [43] | retro | breast ca | 134 | 18 | 13.43 | P, Z, PZ | |
| retro | prostate ca | 31 | 4 | 12.9 | P, Z, PZ | ||
| retro | mult myel | 145 | 6 | 4.14 | P, Z, PZ | ||
| Hoff [14] | retro | breast ca | 1338 | 16 | 1.2 | P, Z | |
| retro | mult myel | 548 | 13 | 2..37 | P, Z | ||
| Montefusco [44] | retro | mult myel | 178 | 9 | 5.06 | BP | |
| Musto [45] | prosp | mult myel | 81 | 1 | 1.23 | Z | |
| 2009 | Walter [46] | css | breast ca | 75 | 4 | 5.33 | Z, PZI |
| Aragon-Ching [47] | pros | prostate ca | 60 | 11 | 18.33 | Z | |
| Cetiner [48] | pros | mult myel | 32 | 5 | 15.63 | Z | |
| Crawford [49] | retro | breast ca | 113 | 10 | 3.5 | P, PZ, Z | |
| Dimopoulos [50] | pros | mult myel | 128 | 16 | 12.5 | Z | |
| Haidar [51] | retro | prostate ca | 51 | 2 | 3.92 | Z | |
| Ripamonti [52] | retro | breast ca | 590 | 18 | 3.05 | P, PZ, Z | |
| prosp | breast ca | 112 | 2 | 1.79 | P, PZ, Z | ||
| Vahtsevanos [53] | retro | breast ca | 1041 | 32 | 3.07 | Z, P, I, PZ, IZ | |
| retro | mult myel | 539 | 46 | 8.53 | P, PZ, Z | ||
| retro | prostate ca | 41 | 2 | 4.88 | P, Z, ZI | ||
| Fizazi [54] | prosp | prostate | 17 | 0 | 0 | P, Z | |
| prosp | breast ca | 16 | 0 | 0 | P, Z | ||
| prosp | prostate ca | 33 | 0 | 0 | D | ||
| prosp | breast ca | 30 | 0 | 0 | D | ||
| Bonomi [55] | retro | breast ca | 238 | 7 | 2.94 | P, PZ, Z | |
| retro | protate ca | 46 | 1 | 2.17 | P, PZ, Z | ||
| Stumpe [56] | retro | mult myel | 128 | 3 | 2.34 | P, Z, PZ | |
| retro | breast ca | 241 | 1 | 0.41 | P, Z, PZ | ||
| retro | prostate ca | 128 | 1 | 0.78 | P, Z, PZ | ||
| 2010 | Walter [16] | retro | mult myel | 81 | 4 | 4.94 | U, PZ |
| css | mult myel | 78 | 16 | 20.51 | Z, PZ, IZ, PZI | ||
| Bantis [11] | retro | prostate ca | 60 | 9 | 15 | Z | |
| Gimsing [57] | retro | breast ca | 250 | 8 | 3.2 | P normal dose (90 mg) | |
| retro | breast ca | 252 | 2 | 0.79 | P low dose (30 mg) | ||
| Pakovic [58] | retro | mult myel | 190 | 2 | 1.05 | P, PI, I | |
| Stopeck [59] | prosp | breast ca | 1020 | 20 | 1.96 | D | |
| prosp | breast ca | 1013 | 14 | 1.38 | Z | ||
| 2011 | Fizazi [60] | prosp | prostate ca | 950 | 22 | 2.32 | D |
| prosp | prostate ca | 951 | 12 | 1.26 | Z | ||
| Quispe [61] | retro | breast ca | 110 | 10 | 9.09 | Z | |
| 2012 | Ding [62] | retro | breast ca | 181 | 1 | 0.55 | P, I, Z |
| Miyazaki [63] | retro | prostate ca | 111 | 9 | 8.11 | Z | |
| Smith [64] | prosp | prostate ca | 716 | 33 | 4.61 | D | |
| Thumbigere-Math [65] | retro | breast ca | 190 | 8 | 4.21 | P, PZ, Z | |
| retro | mult myel | 83 | 6 | 7.23 | P, PZ, Z | ||
| retro | prostate ca | 84 | 2 | 2.38 | P, PZ, Z | ||
| Rugani [66] | retro | breast ca | 48 | 5 | 10.42 | Z | |
| Then [67] | retro | mult myel | 120 | 23 | 19.17 | P, Z, I | |
| Martin [68] | prosp | breast ca | 1026 | 0 | 0 | D | |
| Henry [69] + Saad [70] | prosp | mult myel | 180 | 6 | 3.33 | D,Z | |
| 2013 | Assaf [71] | retro | breast ca | 95 | 9 | 9.47 | P, I, Z, ZI, PI |
| retro | mult myel | 42 | 5 | 11.9 | P, I, Z, ZI, PI | ||
| Coleman [72] | prosp | breast ca | 1065 | 5 | 0.47 | Z | |
| Brufsky [73] | retro | breast ca | 159 | 6 | 3.77 | P, Z, PZ | |
| retro | breast ca | 62 | 1 | 1.61 | P, Z, PZ | ||
| Rathbone [74] | prosp | breast ca | 1681 | 26 | 1.55 | Z | |
| 2014 | Barrett-Lee [75] | prosp | breast ca | 697 | 9 | 1.29 | Z |
| Coleman [76] | prosp | breast ca | 1685 | 26 | 1.54 | Z | |
| Jackson [77] | prosp | mult myel | 981 | 36 | 3.67 | Z | |
| Gnant [78] | prosp | breast ca | 900 | 0 | 0 | Z | |
| 2015 | Vidal-Real [79] | retro | prostate ca | 43 | 9 | 20.93 | Z |
| retro | breast ca | 15 | 4 | 26.67 | Z | ||
| retro | mult myel | 18 | 0 | 0 | Z | ||
| Rodrigues [80] | prosp | prostate ca | 324 | 2 | 0.62 | Z | |
| 2016 | Stopeck [81] | prosp | breast ca | 318 | 20 | 6.29 | D |
| prosp | breast ca | 334 | 18 | 5.39 | ZD | ||
| prosp | prostate ca | 147 | 12 | 8.16 | D | ||
| prosp | prostate ca | 118 | 7 | 5.93 | ZD |
| Breast Cancer | Prostate Cancer | Multiple Myeloma | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases | prev | n | Cases | prev | n | Cases | prev | n | Cases | prev | |
| −2009 | 5531 | 156 | 2.82% | 732 | 44 | 6.01% | 6796 | 344 | 5.06% | 13,059 | 544 | 4.17% |
| 2010+ | 11,101 | 192 | 1.73% | 3504 | 117 | 3.34% | 1773 | 98 | 5.53% | 16,378 | 407 | 2.49% |
| Total | 16,632 | 348 | 2.09% | 4236 | 161 | 3.80% | 8569 | 442 | 5.16% | 29,437 | 951 | 3.23% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugani, P.; Walter, C.; Kirnbauer, B.; Acham, S.; Begus-Nahrman, Y.; Jakse, N. Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma. Dent. J. 2016, 4, 32. https://doi.org/10.3390/dj4040032
Rugani P, Walter C, Kirnbauer B, Acham S, Begus-Nahrman Y, Jakse N. Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma. Dentistry Journal. 2016; 4(4):32. https://doi.org/10.3390/dj4040032
Chicago/Turabian StyleRugani, Petra, Christian Walter, Barbara Kirnbauer, Stephan Acham, Yvonne Begus-Nahrman, and Norbert Jakse. 2016. "Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma" Dentistry Journal 4, no. 4: 32. https://doi.org/10.3390/dj4040032






