Delayed Diagnosis of Osteonecrosis of the Jaw (ONJ) Associated with Bevacizumab Therapy in Colorectal Cancer Patients: Report of Two Cases
Abstract
:1. Introduction
2. Case Report
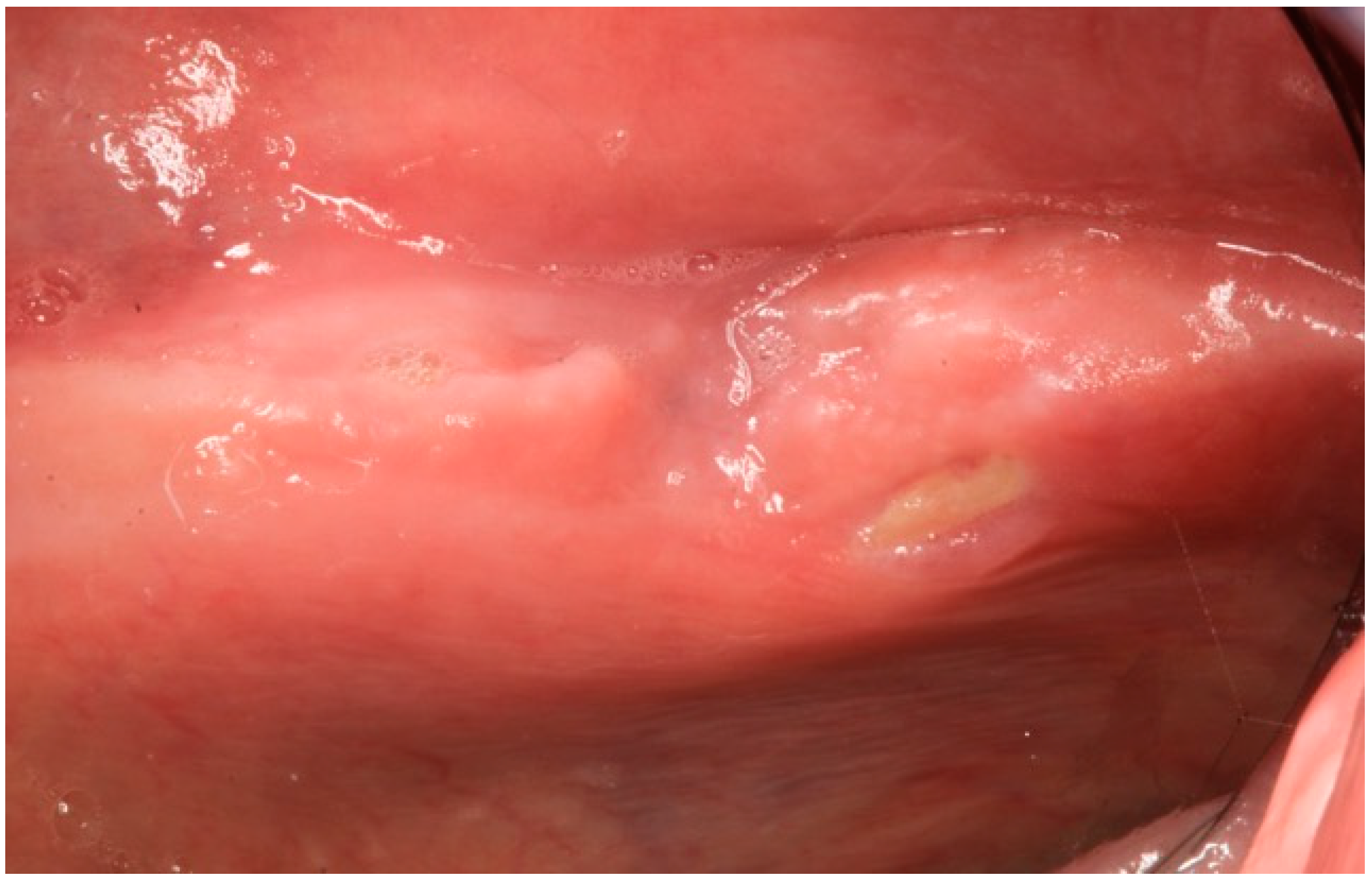
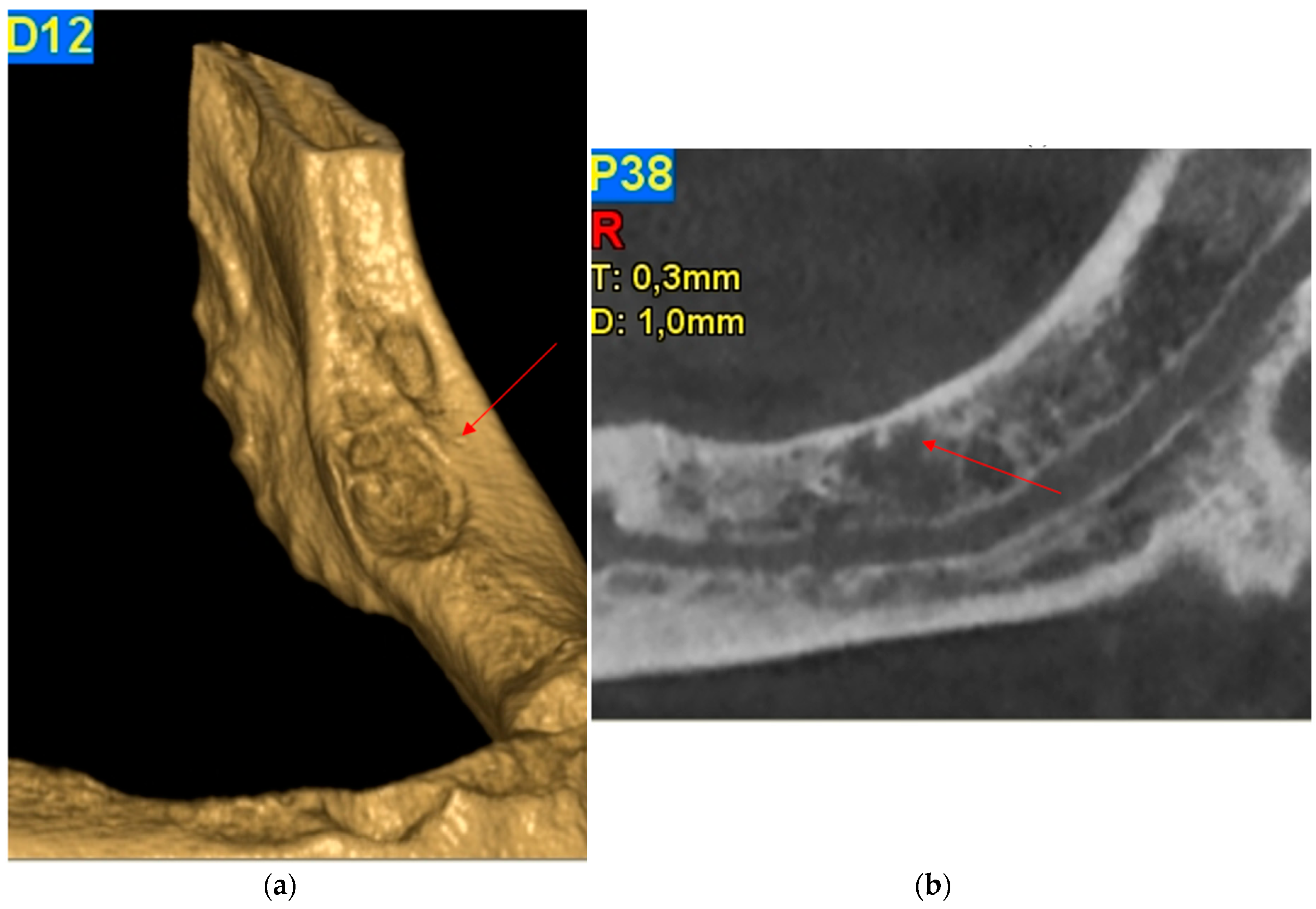
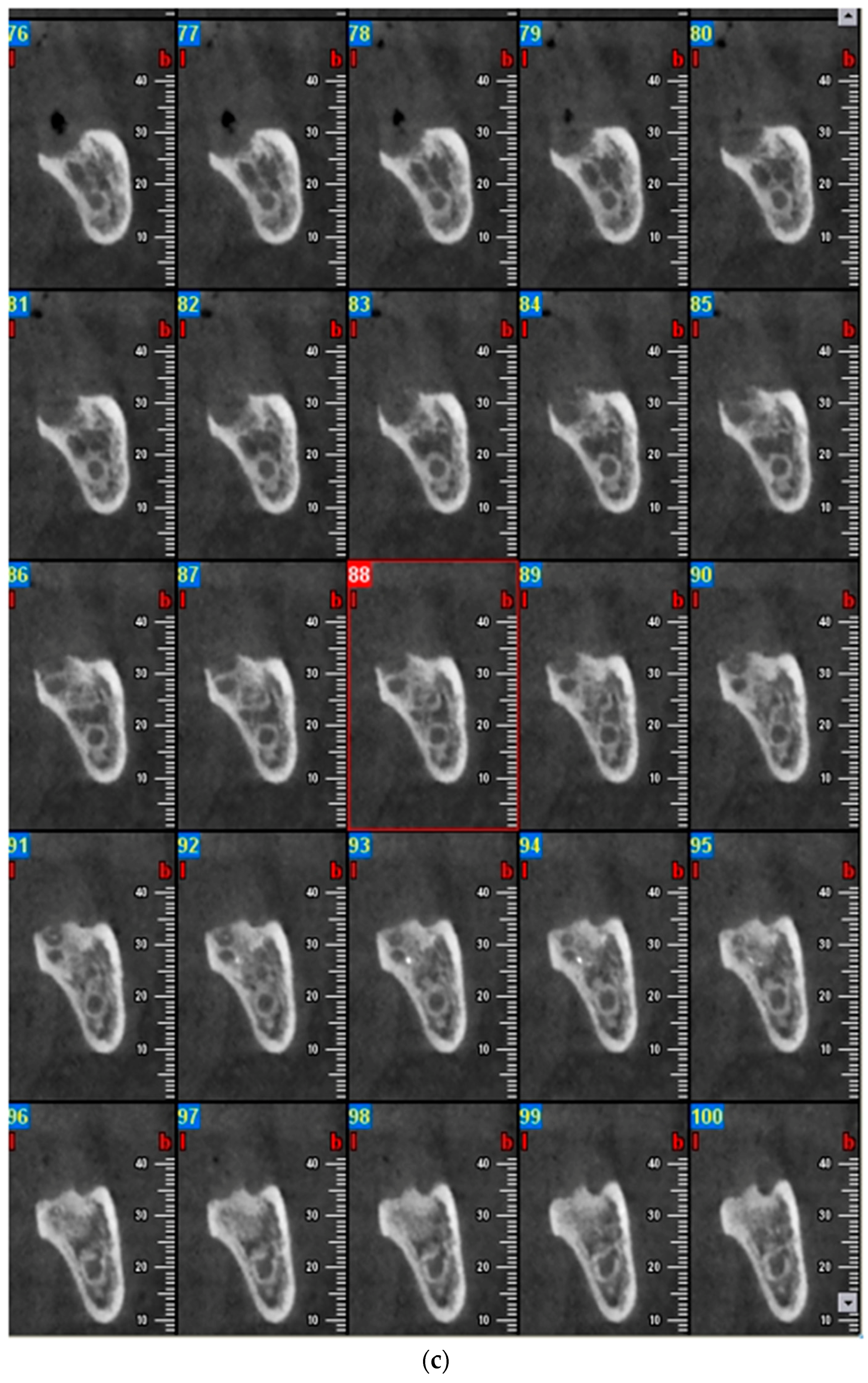
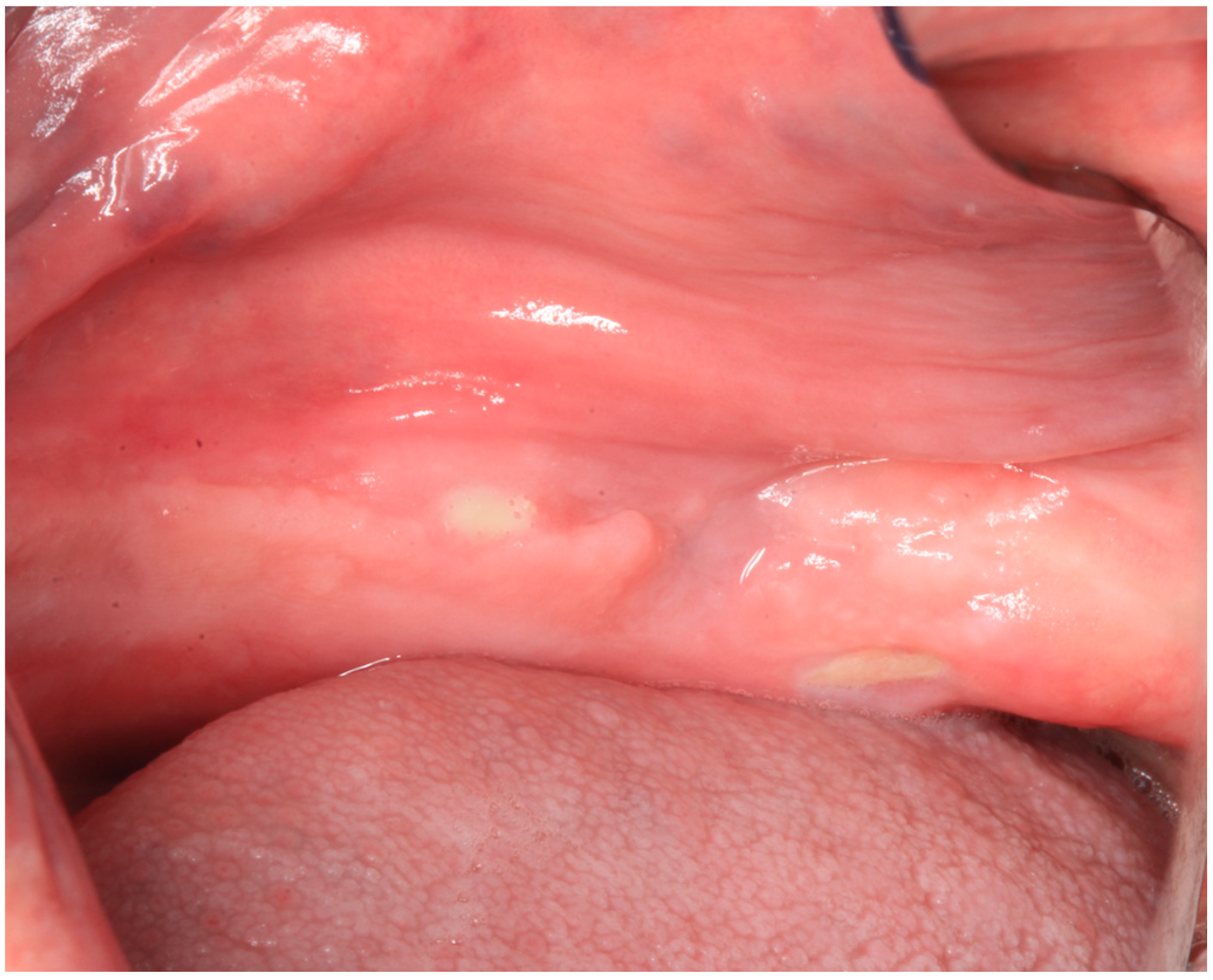
2.1. Case Report 1
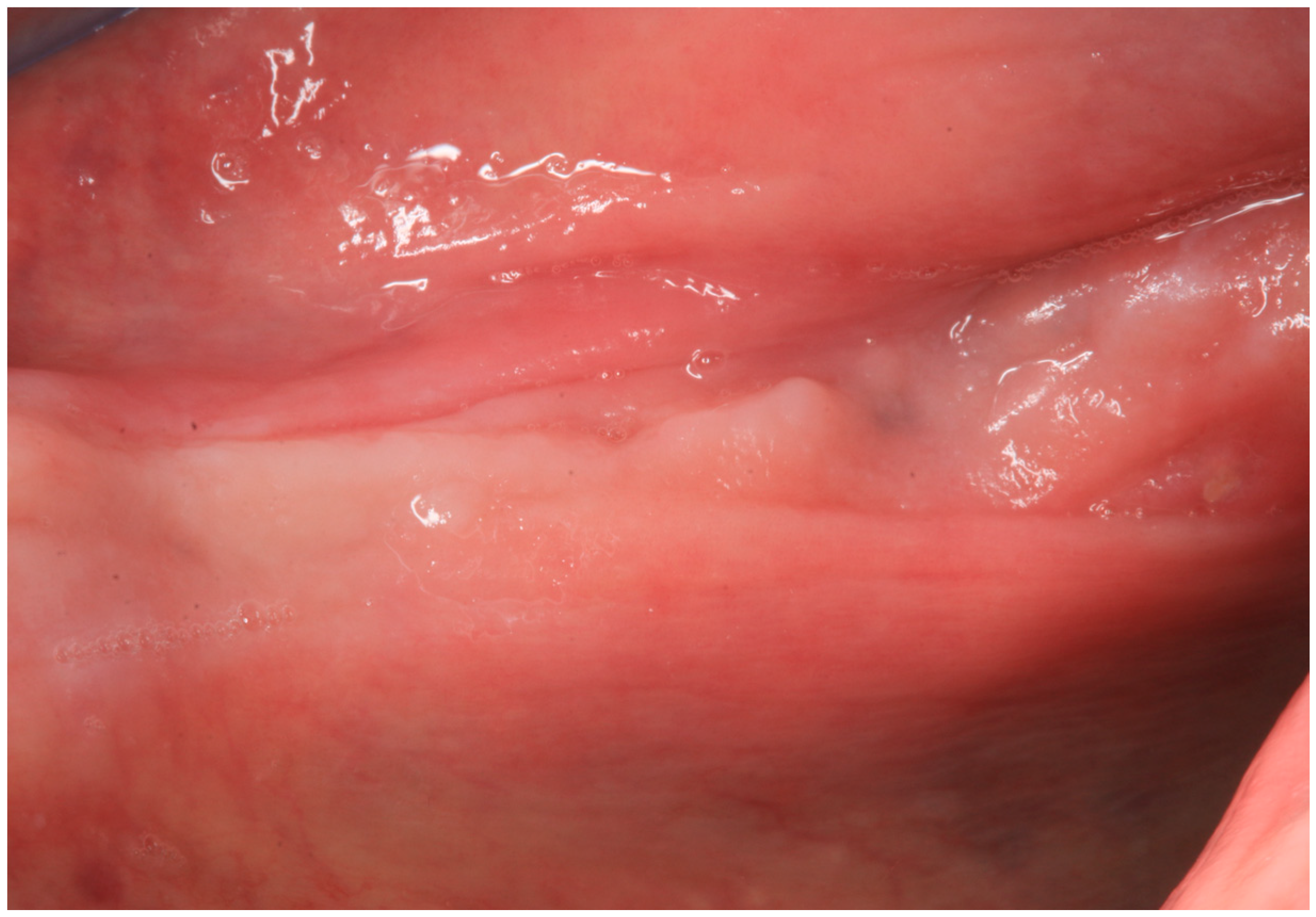
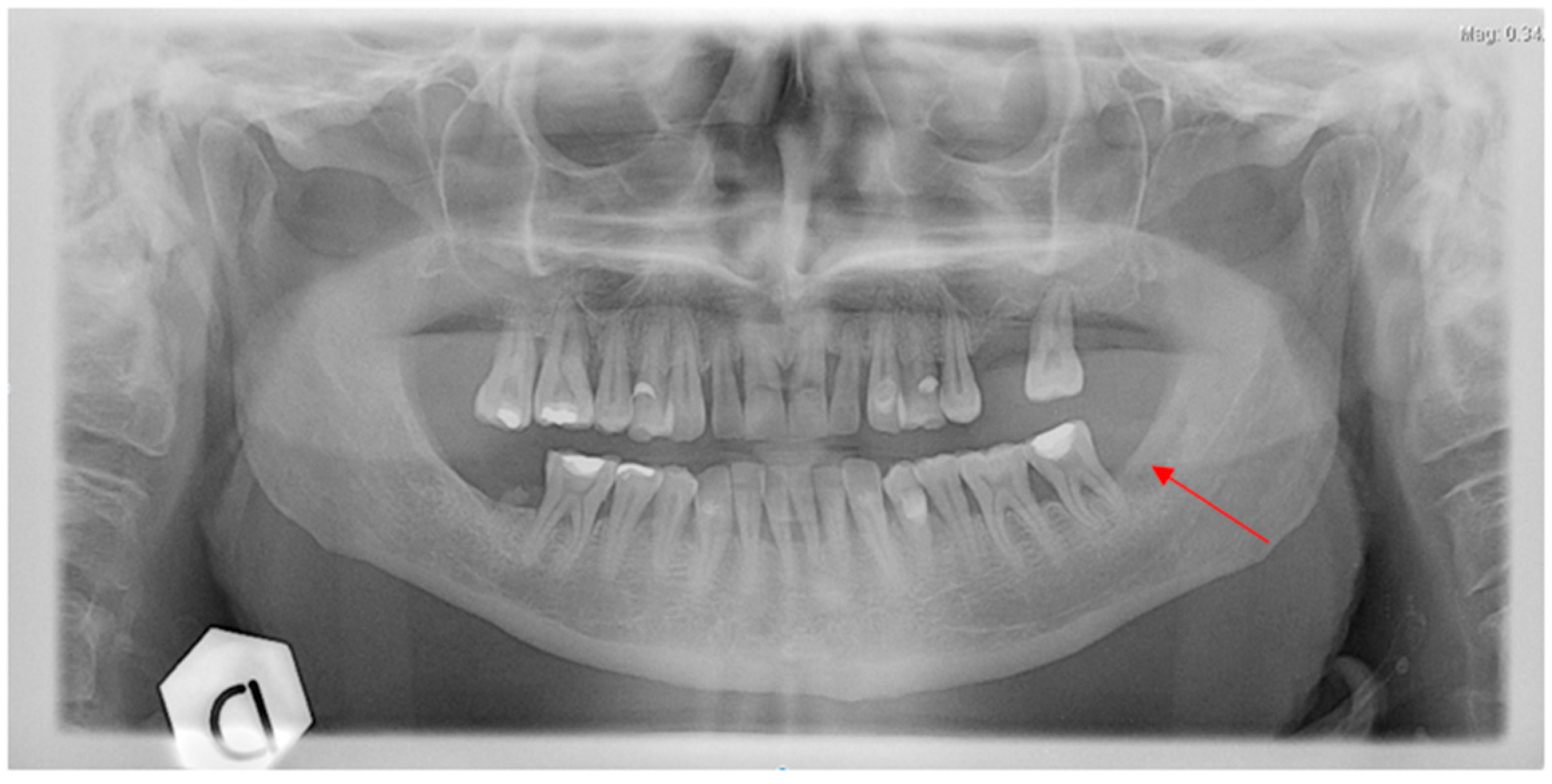
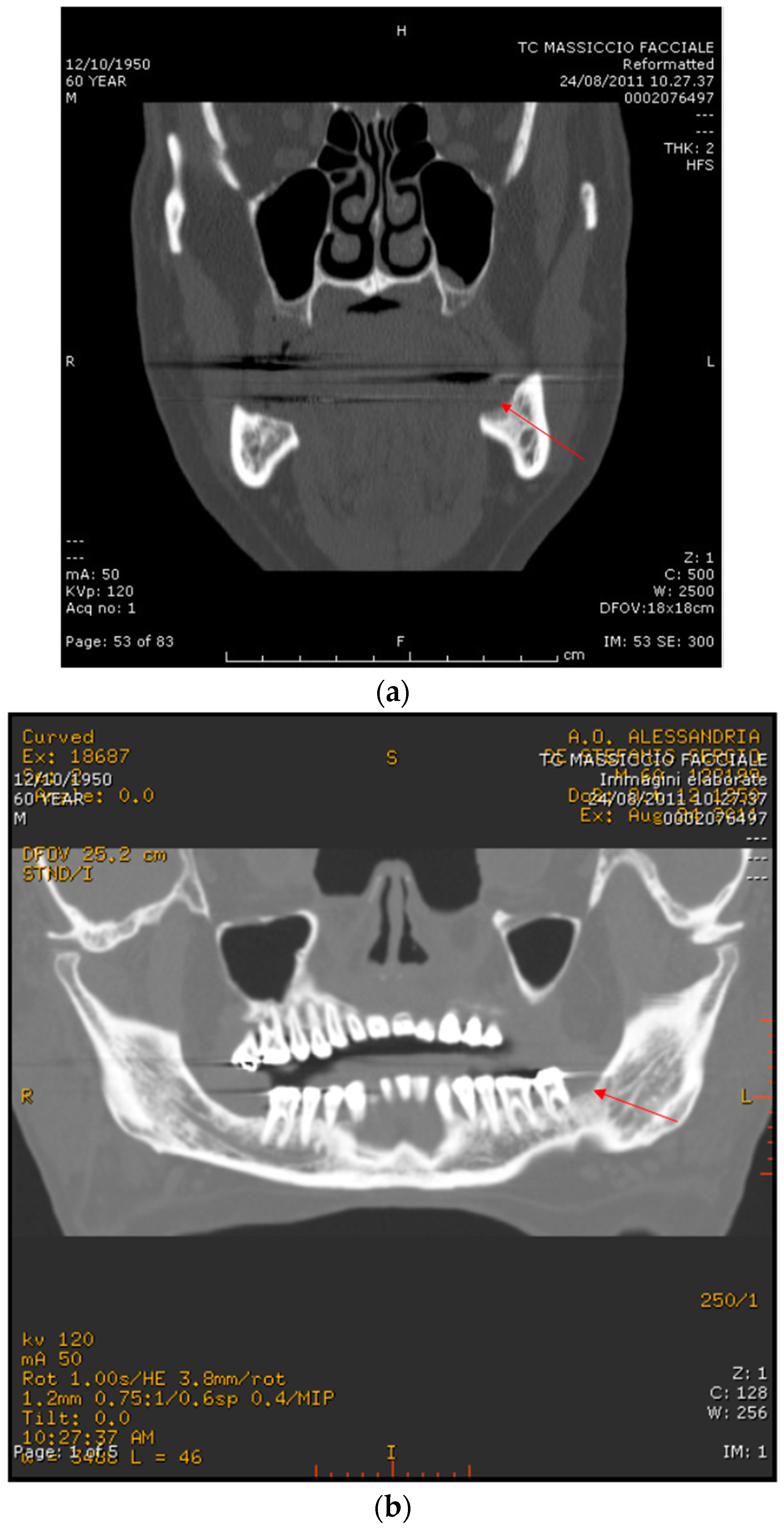
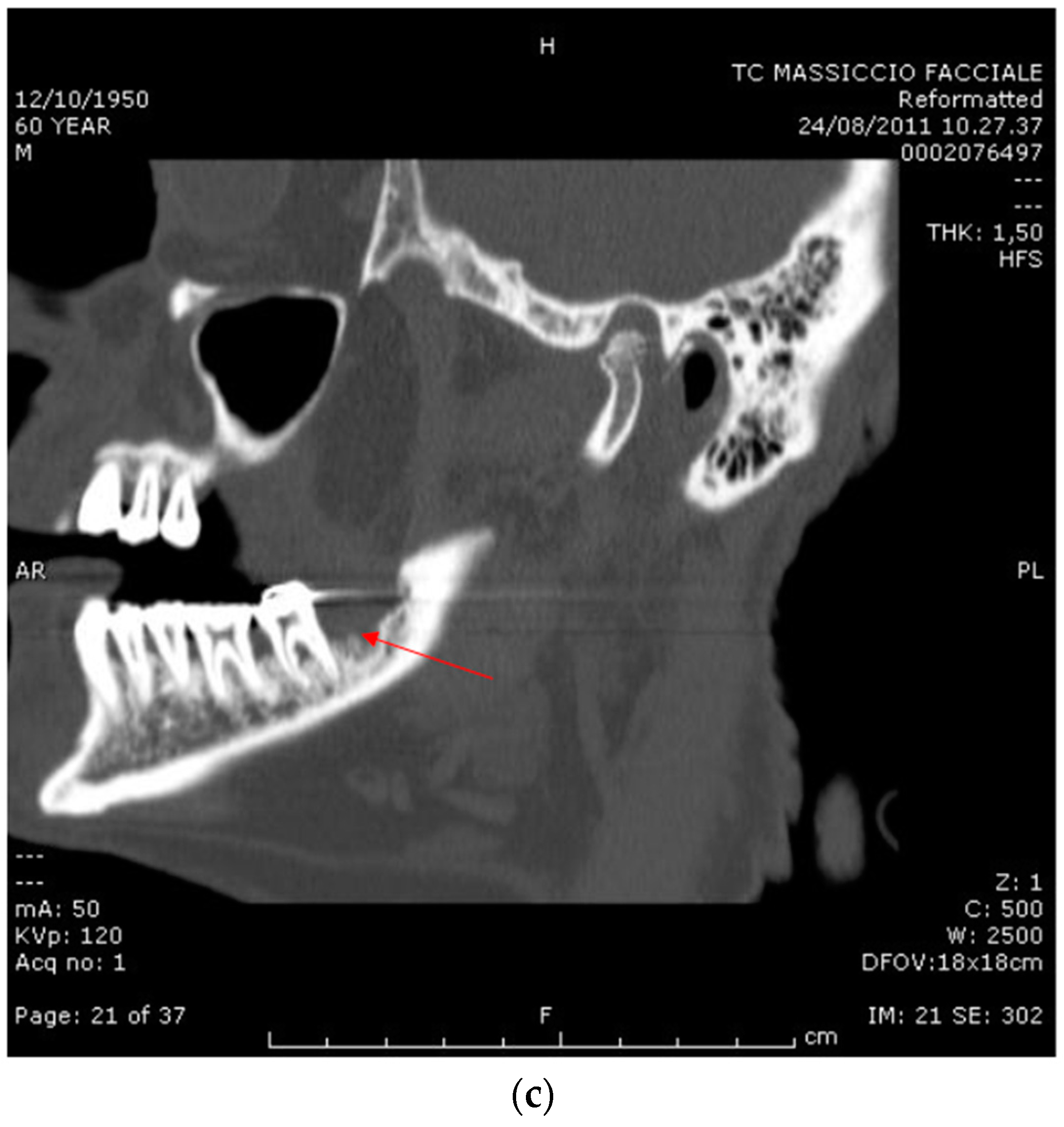
2.2. Case Report 2
3. Discussion
3.1. Origin of ONJ
3.2. ONJ after Bevacizumab Therapy
3.3. Our Two Cases
3.4. Frequency of Bevacizumab-Related ONJ: the Possible Underestimation and the Next Future
3.5. Conclusive Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruggiero, S.L. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): Initial discovery and subsequent development. J. Oral Maxillofac. Surg. 2009, 67, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American association of oral and maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar]
- Colella, G.; Campisi, G.; Fusco, V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-Related Osteonecrosis of the Jaws-2009 update: The need to refine the BRONJ definition. J. Oral Maxillofac. Surg. 2009, 67, 2698–2699. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Fusco, V.; Agrillo, A.; Campisi, G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Oral Dis. 2012, 18, 621–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, S.; Marx, R.E.; Tröltzsch, M.; Ristow, O.; Ziebart, T.; Al-Nawas, B.; Groetz, K.A.; Ehrenfeld, M.; Mercadante, V.; Porter, S.; et al. Comments on “diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus”. J. Bone Miner. Res. 2015, 30, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Woodlock, T.; Kriegelstein, S. Physiology and Pharmacology of Nonbisphosphonate Drugs Implicated in Osteonecrosis of the Jaw. J. Can. Dent. Assoc. 2012, 78, c85. [Google Scholar] [PubMed]
- Fusco, V.; Santini, D.; Armento, G.; Tonini, G.; Campisi, G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert Opin. Drug Saf. 2016, 15, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Estilo, C.L.; Fornier, M.; Farooki, A.; Carlson, D.; Bohle, G., 3rd; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar] [CrossRef] [PubMed]
- Greuter, S.; Schmid, F.; Ruhstaller, T.; Thuerlimann, B. Bevacizumab-associated osteonecrosis of the jaw. Ann. Oncol. 2008, 19, 2091–2092. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Paolantonio, M.; Spoto, G.; Mastrangelo, F.; Tetè, S.; Dolci, M. Bevacizumab-related osteonecrosis of the jaw. Int. J. Immunopathol. Pharmacol. 2009, 22, 1121–1123. [Google Scholar] [PubMed]
- Guarneri, V.; Miles, D.; Robert, N.; Diéras, V.; Glaspy, J.; Smith, I.; Thomssen, C.; Biganzoli, L.; Taran, T.; Conte, P. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010, 122, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Agostino, N.M.; Gingrich, R.; Drabick, J.J. Bevacizumab demonstrates prolonged disease stabilization in patients with heavily pretreated metastatic renal cell carcinoma: A case series and review of the literature. Adv. Urol. 2010, 2010, 687043. [Google Scholar] [CrossRef] [PubMed]
- Bettini, G.; Blandamura, S.; Saia, G.; Bedogni, A. Bevacizumab-related osteonecrosis of the mandible is a self-limiting disease process. BMJ Case Rep. 2012. [Google Scholar] [CrossRef] [PubMed]
- Brunamonti Binello, P.; Bandelloni, R.; Labanca, M.; Buffoli, B.; Rezzani, R.; Rodella, L.F. Osteonecrosis of the jaws and bevacizumab therapy: A case report. Int. J. Immunopathol. Pharmacol. 2012, 25, 789–791. [Google Scholar] [PubMed]
- Pakosch, D.; Papadimas, D.; Munding, J.; Kawa, D.; Kriwalsky, M.S. Osteonecrosis of the mandible due to anti-angiogenic agent, bevacizumab. Oral Maxillofac. Surg. 2013, 17, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.R.; Belizário Rosa, G.A.; Castro Júnior, G.D.; Dias, R.B.; Prado Ribeiro, A.C.; Brandão, T.B. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e32–e36. [Google Scholar] [CrossRef] [PubMed]
- Tzermpos, F.; Ismail, A.; Pavli, M.; Tosios, K.I. Osteonecrosis of the mandible in a patient with lung adenocarcinoma undergoing anti-angiogenic therapy with bevacizumab. Oral Surg. 2016, 9, 40–46. [Google Scholar] [CrossRef]
- Dişel, U.; Beşen, A.A.; Özyılkan, Ö.; Er, E.; Canpolat, T. A case report of bevacizumab-related osteonecrosis of the jaw: Old problem, new culprit. Oral Oncol. 2012, 48, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ono, F.; Yamamura, A.; Onochi, S. A case of osteonecrosis of the jaw during treatment by bevacizumab for sigmoid colon cancer. Nihon Shokakibyo Gakkai Zasshi 2013, 110, 655–659. [Google Scholar] [PubMed]
- Santini, D.; Galluzzo, S.; Vincenzi, B.; Schiavon, G.; Fratto, E.; Pantano, F.; Tonini, G. New developments of aminobisphosphonates: The double face of Janus. Ann. Oncol. 2007, 18 (Suppl. S6), vi164–vi167. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67 (Suppl. S5), 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenzi, B.; Napolitano, A.; Zoccoli, A.; Iuliani, M.; Pantano, F.; Papapietro, N.; Denaro, V.; Santini, D.; Tonini, G. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. J. Hematol. Oncol. 2012, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Menegatti, E.; Scoletta, M.; Battaglio, C.; Mozzati, M.; Chiecchio, A.; Berardi, D.; Vandone, A.M.; Donadio, M.; Gandolfo, S.; et al. Vascular endothelial growth factor genetic polymorphisms and haplotypes in female patients with bisphosphonate-related osteonecrosis of the jaws. J. Oral Pathol. Med. 2011, 40, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Pabst, A.; Ziebart, T.; Klein, M.; Al-Nawas, B. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2011, 17, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Kühl, S.; Walter, C.; Acham, S.; Pfeffer, R.; Lambrecht, J.T. Bisphosphonate-related osteonecrosis of the jaws—A review. Oral Oncol. 2012, 48, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Fedele, S.; Fusco, V.; Pizzo, G.; Di Fede, O.; Bedogni, A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future Oncol. 2014, 10, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Ziebart, T.; Ackermann, M.; Konerding, M.A.; Walter, C. Bisphosphonates’ antiangiogenic potency in the development of bisphosphonate-associated osteonecrosis of the jaws: Influence on microvessel sprouting in an in vivo 3D Matrigel assay. Clin. Oral Investig. 2014, 18, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Walter, C.; Hansen, T.; Jäger, E.; Wagner, W. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac. Surg. 2011, 15, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, A.; Pinta, F.; Spadi, R.; Mecca, C.; Fanchini, L.; Zanini, M.; Ciuffreda, L.; Racca, P. Jaw osteonecrosis associated with aflibercept, irinotecan and fluorouracil: Attention to oral district. Tumori 2015. [Google Scholar] [CrossRef] [PubMed]
- Antonuzzo, L.; Lunghi, A.; Giommoni, E.; Brugia, M.; Di Costanzo, F. Regorafenib Also Can Cause Osteonecrosis of the Jaw. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Campisi, G.; Numico, G.; Migliorati, C.A.; Santini, D.; Bedogni, A. RE: Regorafenib Also Can Cause Osteonecrosis of the Jaw. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Renk, M.; Crino’, L. Advances in anti-VEGF and anti-EGFR therapy for advanced non-small cell lung cancer. Lung Cancer 2009, 63, 1–9. [Google Scholar]
- Gordon, C.R.; Rojavin, Y.; Patel, M.; Zins, J.E.; Grana, G.; Kann, B.; Simons, R.; Atabek, U. A review on bevacizumab and surgical wound healing. An important warning to all surgeons. Ann. Plast. Surg. 2009, 62, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Scappaticci, F.A.; Fehrenbacher, L.; Cartwright, T.; Hainsworth, J.D.; Heim, W.; Berlin, J.; Kabbinavar, F.; Novotny, W.; Sarkar, S.; Hurwitz, H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 2005, 91, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kaneda, T.; Arakawa, T.; Morita, S.; Sato, T.; Yomada, T.; Hanada, K.; Kumegawa, M.; Hakeda, Y. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000, 473, 161–164. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, Y.; Dellian, M.; Safabakhsh, N.; Ferrara, N.; Jain, R.K. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl. Acad. Sci. USA 1996, 93, 14765–14770. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Razis, E.; Galiti, D.; Galitis, E.; Labropoulos, S.; Tsimpidakis, A.; Sgouros, J.; Karampeazis, A.; Migliorati, C. Periodontaldiseaseprecedingosteonecrosis of the jaw (ONJ) in cancer patients receiving antiresorptives alone or combined with targeted therapies: Report of 5 cases and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Garant, A.; Des Groseilliers, S.; Martin, L.; Ferland, É.; Vuong, T. Late anastomotic dehiscence during bevacizumab therapy for patients with colorectal cancer. Clin. Oncol. (R. Coll. Radiol.) 2011, 23, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Bevacizumab, sunitinib: Osteonecrosis of the jaw. Prescrire Int. 2011, 20, 155.
- Hompes, D.; Ruers, T. Review: Incidence and clinical significance of bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur. J. Surg. Oncol. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons. Position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67 (Suppl. S5), 2–12. [Google Scholar] [PubMed]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Bedogni, G.; Scoletta, M. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br. J. Oral Maxillofac. Surg. 2015, 53, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Spanidis, M.; Engel, C.; Roos, F.C.; Frees, S.; Neisius, A.; Hampel, C.; Rubenwolf, P.; Thüroff, J.W.; Walter, C.; et al. Bone scintigraphy predicts bisphosphonate-induced osteonecrosis of the jaw (BRONJ) in patients with metastatic castration-resistant prostate cancer (mCRPC). Clin. Oral Investig. 2016, 20, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Bedogni, A.; Addeo, A.; Campisi, G. Definition and estimation of osteonecrosis of jaw (ONJ), and optimal duration of antiresorptive treatment in bone metastatic cancer patients: Supplementary data from the denosumab extension study? Support. Care Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erovigni, F.; Gambino, A.; Cabras, M.; Fasciolo, A.; Bianchi, S.D.; Bellini, E.; Fusco, V. Delayed Diagnosis of Osteonecrosis of the Jaw (ONJ) Associated with Bevacizumab Therapy in Colorectal Cancer Patients: Report of Two Cases. Dent. J. 2016, 4, 39. https://doi.org/10.3390/dj4040039
Erovigni F, Gambino A, Cabras M, Fasciolo A, Bianchi SD, Bellini E, Fusco V. Delayed Diagnosis of Osteonecrosis of the Jaw (ONJ) Associated with Bevacizumab Therapy in Colorectal Cancer Patients: Report of Two Cases. Dentistry Journal. 2016; 4(4):39. https://doi.org/10.3390/dj4040039
Chicago/Turabian StyleErovigni, Francesco, Alessio Gambino, Marco Cabras, Antonella Fasciolo, Silvio Diego Bianchi, Elisa Bellini, and Vittorio Fusco. 2016. "Delayed Diagnosis of Osteonecrosis of the Jaw (ONJ) Associated with Bevacizumab Therapy in Colorectal Cancer Patients: Report of Two Cases" Dentistry Journal 4, no. 4: 39. https://doi.org/10.3390/dj4040039






