Effect of Decontamination and Cleaning on the Shear Bond Strength of High Translucency Zirconia
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blatz, M.B.; Alvarez, M.; Sawyer, K.; Brindis, M. How to bond zirconia: The APC Concept. Compend. Cont. Educ. Dent. 2016, 37, 611–617. [Google Scholar]
- Tzanakakis, E.G.; Tzoutzas, I.G.; Koidis, P.T. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, Q.; Zhang, F.; Lu, Y.; Tay, F.R.; Qian, M.; Chen, C. Comparison of resin bonding improvements to zirconia between one-bottle universal adhesives and tribochemical silica coating, which is better? Dent. Mater. 2016, 32, 403–441. [Google Scholar] [CrossRef] [PubMed]
- El-Damanhoury, H.M.; Gaintantzopoulou, M.D. Self-etching ceramic primer versus hydrofluoric acid etching: Etching efficacy and bonding performance. J. Prosthodont. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Román-Rodríguez, J.L.; Perez-Barquero, J.A.; Gonzalez-Angulo, E.; Fons-Font, A.; Bustos-Salvador, J.L. Bonding to silicate ceramics: Conventional technique compared with a simplified technique. J. Clin. Exp. Dent. 2017, 9, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Bielen, V.; Inokoshi, M.; Munck, J.D.; Zhang, F.; Vanmeensel, K.; Minakuchi, S.; Vleugels, J.; Naert, I.; Van Meerbeek, B. Bonding Effectiveness to Differently Sandblasted Dental Zirconia. J. Adhes. Dent. 2015, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E. Influence of Surface Treatments on the Bond Strength of Resin Cements to Monolithic Zirconia. J. Adhes. Dent. 2016, 18, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Al Kheraif, A.A.; Jamaluddin, S.; Elsharawy, M.; Divakar, D.D. Recent Trends in Surface Treatment Methods for Bonding Composite Cement to Zirconia: A Review. J. Adhes. Dent. 2017, 19, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Aladağ, A.; Elter, B.; Çömlekoğlu, E.; Kanat, B.; Sonugelen, M.; Kesercioğlu, A.; Özcan, M. Effect of different cleaning regimens on the adhesion of resin to saliva-contaminated ceramics. J. Prosthodont. 2015, 24, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angkasith, P.; Burgess, J.O.; Bottino, M.C.; Lawson, N.C. Cleaning Methods for Zirconia Following Salivary Contamination. J. Prosthodont. 2016, 25, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Oliveira, S.C.; Pereira, G.K.; Beekman, P.; Rippe, M.P.; Kleverlaan, C.J. Silicone Disclosing Material used after Ceramic Surface Treatment Reduces Bond Strength. J. Adhes. Dent. 2016, 18, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, S.A.; Pater, D.; Borges, A.L.S.; Alshehri, E.Z.; Bottino, M.A.; Özcan, M.; Valandro, L.F.; Bottino, M.C. Effect of cleansing methods on saliva-contaminated Zirconia—An evaluation of resin bond durability. Oper. Dent. 2015, 40, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Son, J.S.; Jeong, S.H.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Efficacy of various cleaning solutions on saliva-contaminated zirconia for improved resin bonding. J. Adv. Prosthodont. 2015, 7, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lange-Jansen, H.; Scharnberg, M.; Wolfart, S.; Ludwig, K.; Adelung, R.; Kern, M. Influence of saliva contamination on zirconia ceramic bonding. Dent. Mater. 2008, 24, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, L.; Wang, Y.; Jiao, K.; Liu, Y.; Zhou, W.; Shen, L.; Fang, M.; Li, M.; Zhang, X.; et al. Bonding of Resin Cement to Zirconia with High Pressure Primer Coating. PLoS ONE 2014, 9, e101174. [Google Scholar] [CrossRef] [PubMed]
- Attia, A. Bond strength of three luting agents to zirconia ceramic—Influence of surface treatment and thermocycling. J. Appl. Oral. Sci. 2011, 19, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.A.; Pasha, N.; Hilal, M.; Amarnath, G.S.; Kundapur, V.; Anand, M.; Singh, S. To Evaluate Effect of Airborne Particle Abrasion using Different Abrasives Particles and Compare Two Commercial Available Zirconia on Flexural Strength on Heat Treatment. Int. J. Biomed. Sci. 2017, 13, 93–112. [Google Scholar] [PubMed]
- Scribante, A.; Contreras-Bulnes, R.; Montasser, M.A.; Vallittu, P.K. Orthodontics: Bracket Materials, Adhesives Systems, and Their Bond Strength. Biomed. Res. Int. 2016, 2016, 1329814. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.R. A review of direct orthodontic bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Giannini, M.; Soares, C.J.; de Carvalho, R.M. Ultimate tensile strength of tooth structures. Dent. Mater. 2004, 20, 322–329. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Attin, T. Enzymes in the acquired enamel pellicle. Eur. J. Oral. Sci. 2005, 113, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef] [PubMed]
- Pitta, J.; Branco, T.C.; Portugal, J. Effect of saliva contamination and artificial aging on different primer/cement systems bonded to zirconia. J. Prosthet. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, F.; Cardenas, A.M.; Gutierrez, M.F.; Malaquias, P.; Hass, V.; Reis, A.; Loguercio, A.D.; Perdigão, J. Laboratory Performance of Universal Adhesive Systems for Luting CAD/CAM Restorative Materials. J. Adhes. Dent. 2016, 18, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Proff, P.; Kolbeck, C.; Langrieger, S.; Kunze, J.; Handel, G.; Rosentritt, M. The bond strength of the resin-to-zirconia interface using different bonding concepts. J. Mech. Behav. Biomed. Mater. 2011, 4, 2–8. [Google Scholar] [CrossRef] [PubMed]
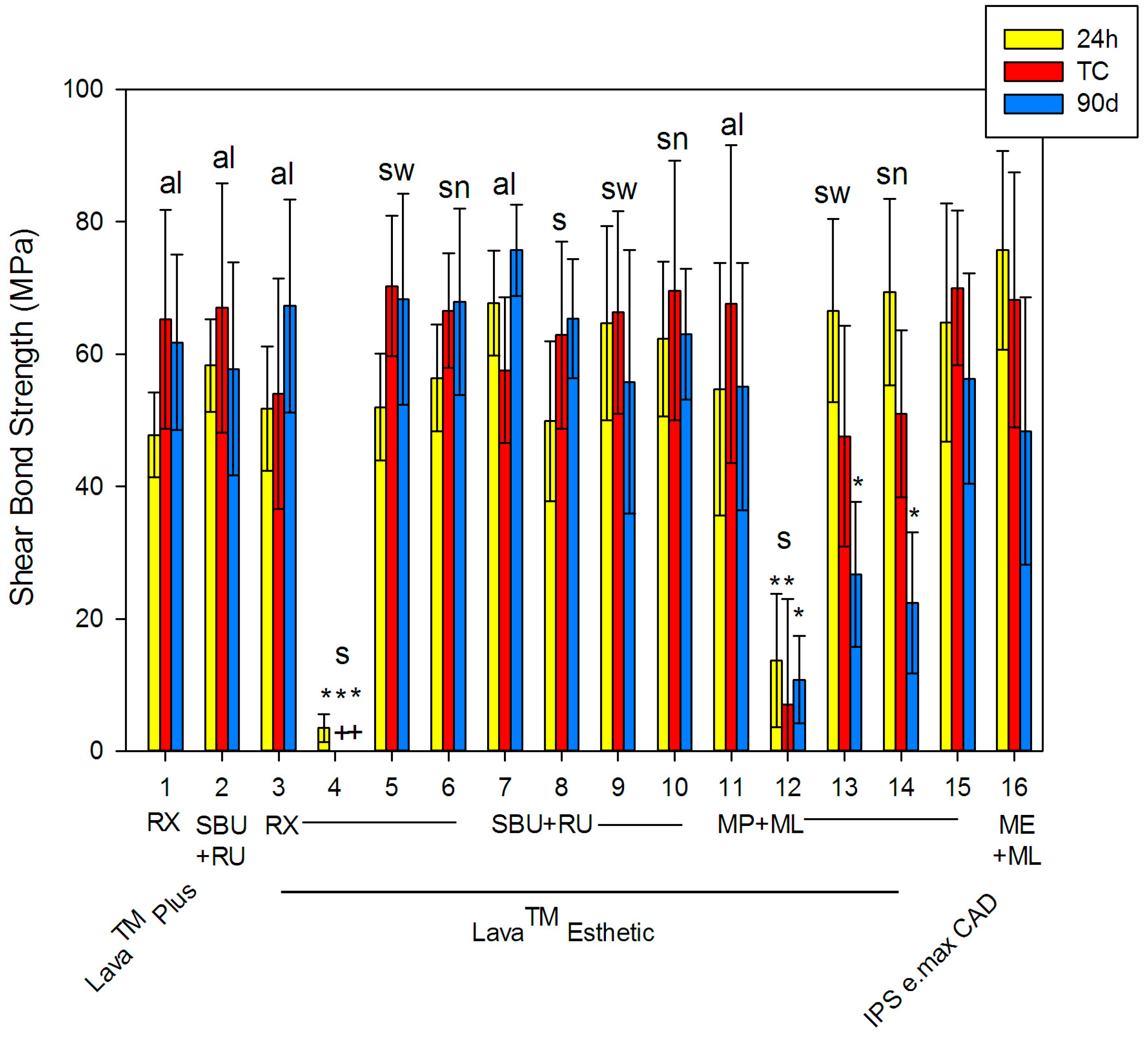
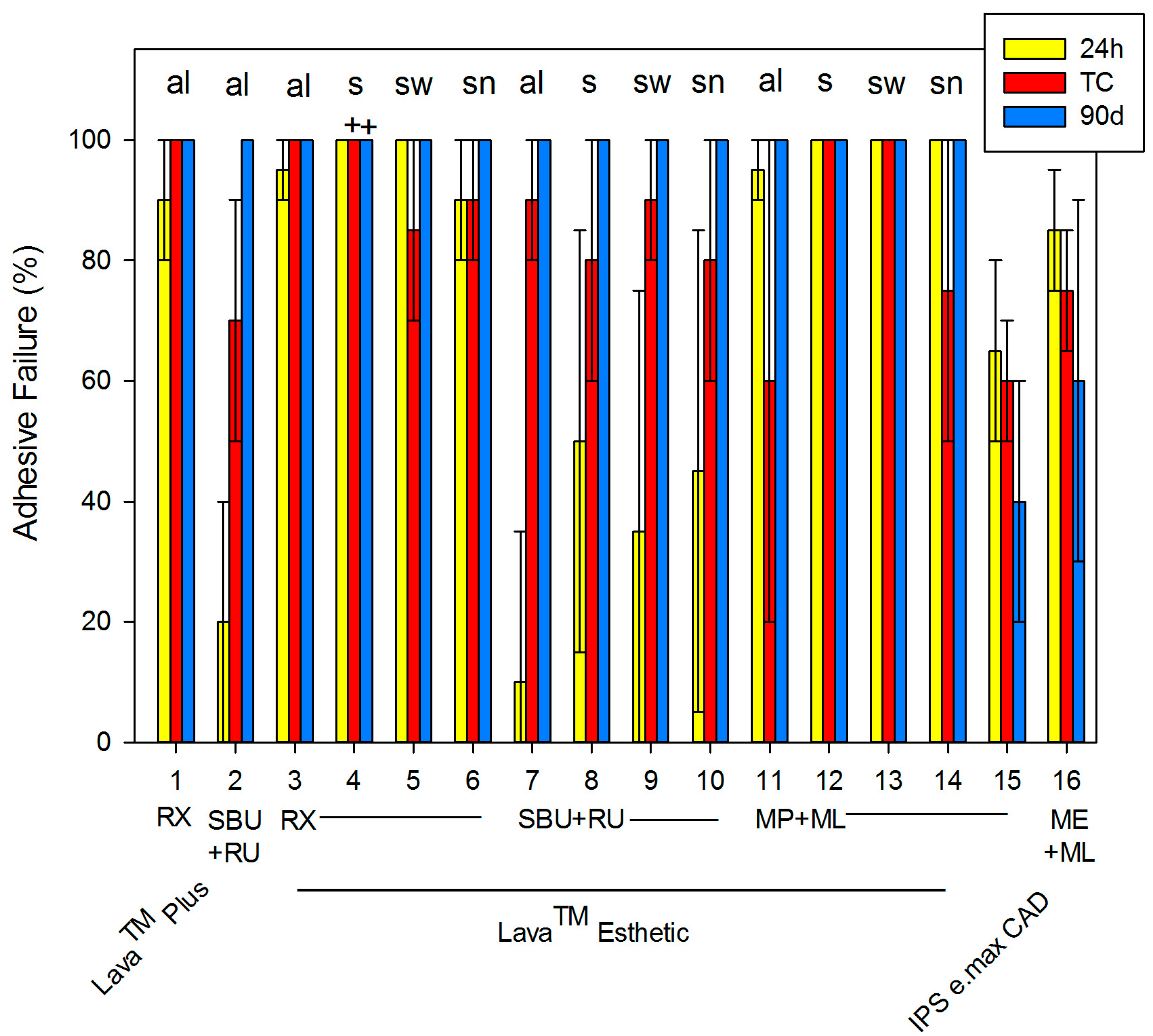
| # | Ceramic | Pre-Treatment | Procedure | Primer | Cement | Mean (SD) | ||
|---|---|---|---|---|---|---|---|---|
| 24 h | TC | 90 days | ||||||
| 1 | Lava™ Plus | sandblasting | al | none | RX | 47.8 (6.4) | 65.3 (16.5) | 61.8 (13.3) |
| 2 | SBU | RU | 58.3 (7.0) | 67.0 (18.8) | 57.8 (16.1) | |||
| 3 | Lava™ Esthetic | al | none | RX | 51.8 (9.4) | 54.0 (17.4) | 67.3 (16.1) | |
| 4 | s | 3.5 * (2.1) | 0 * (0) | 0 * (0) | ||||
| 5 | sw | 52.0 (8.1) | 70.3 (10.6) | 68.3 (15.9) | ||||
| 6 | sn | 56.4 (8.1) | 66.6 (8.7) | 67.9 (14.1) | ||||
| 7 | al | SBU | RU | 67.7 (7.9) | 57.6 (11.0) | 75.7 (6.9) | ||
| 8 | s | 49.9 (12.1) | 62.9 (14.1) | 65.4 (9.0) | ||||
| 9 | sw | 64.7 (14.7) | 66.3 (15.3) | 55.8 (19.9) | ||||
| 10 | sn | 62.3 (11.7) | 69.6 (19.6) | 63.0 (9.9) | ||||
| 11 | al | MP | ML | 54.7 (19.1) | 67.6 (24.0) | 55.1 (18.7) | ||
| 12 | s | 13.7 * (10.1) | 7.0 * (16.0) | 10.8 * (6.6) | ||||
| 13 | sw | 66.6 (13.8) | 47.6 (16.7) | 26.7 * (11.0) | ||||
| 14 | sn | 69.4 (14.1) | 51.0 (12.6) | 22.4 * (10.7) | ||||
| 15 | IPS e.max CAD | 5% HF | none | MP | 64.8 (18.0) | 70.0 (11.7) | 56.3 (15.9) | |
| 16 | none | ME | 75.7 (15.0) | 68.2 (19.3) | 48.4 (20.2) | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krifka, S.; Preis, V.; Rosentritt, M. Effect of Decontamination and Cleaning on the Shear Bond Strength of High Translucency Zirconia. Dent. J. 2017, 5, 32. https://doi.org/10.3390/dj5040032
Krifka S, Preis V, Rosentritt M. Effect of Decontamination and Cleaning on the Shear Bond Strength of High Translucency Zirconia. Dentistry Journal. 2017; 5(4):32. https://doi.org/10.3390/dj5040032
Chicago/Turabian StyleKrifka, Stephanie, Verena Preis, and Martin Rosentritt. 2017. "Effect of Decontamination and Cleaning on the Shear Bond Strength of High Translucency Zirconia" Dentistry Journal 5, no. 4: 32. https://doi.org/10.3390/dj5040032




