Development of Next Generation Stevia Sweetener: Rebaudioside M
Abstract
:1. Introduction
Discovery of Rebaudioside M
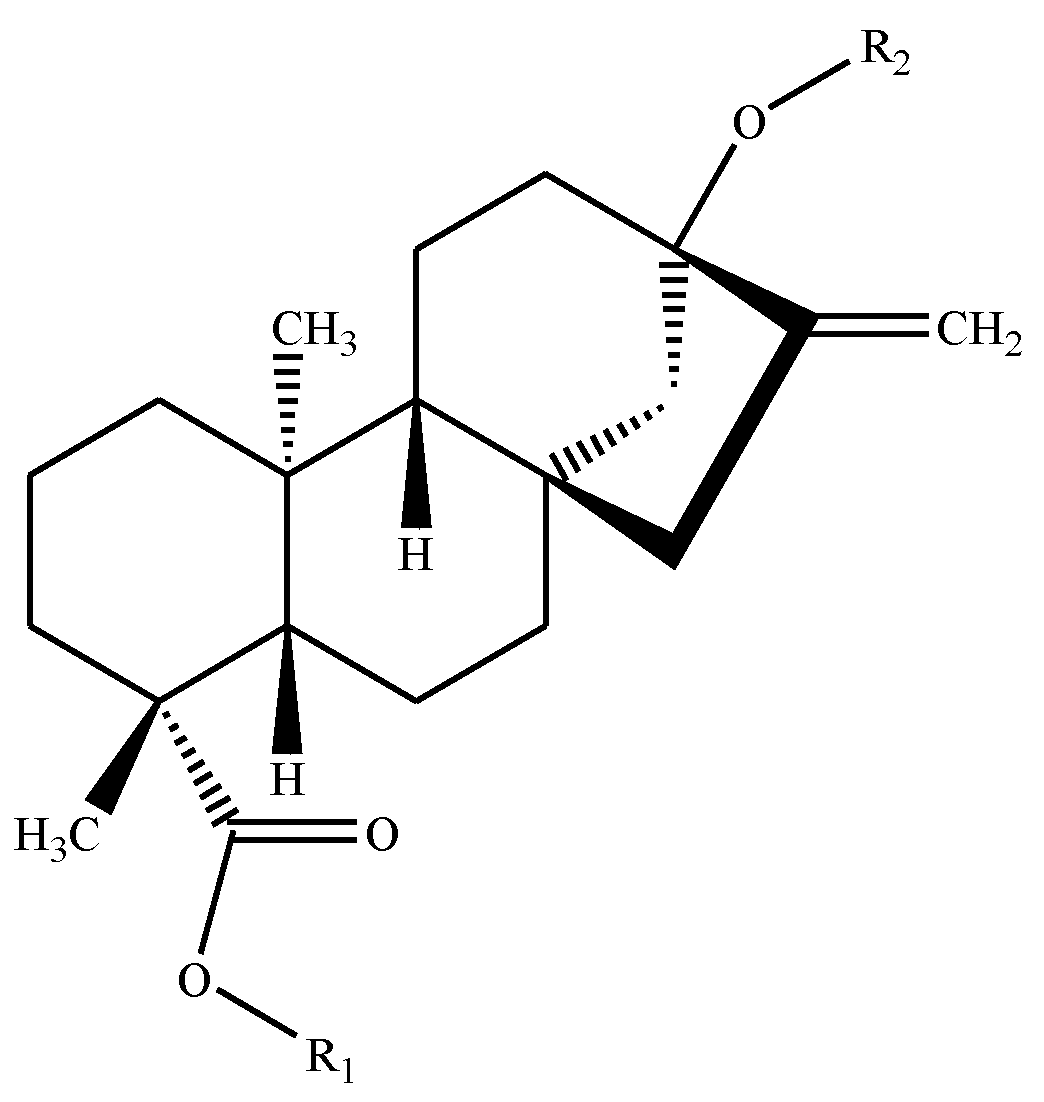
| Sweetener | Reference Number in Text | R-Groups in Backbone Figure Above | Formula | Molecular Weight (g/mol) | Potency * | |
|---|---|---|---|---|---|---|
| R1 | R2 | |||||
| Rebaudioside A | 1 | β-glc- | (β-glc)2-β-glc- | C44H70O23 | 967.01 | 200 |
| Rebaudioside B | 2 | H | (β-glc)2-β-glc- | C38H60O18 | 804.88 | 150 |
| Rebaudioside C | 3 | β-glc- | (β-glc, α-rha-)-β-glc- | C44H70O22 | 951.01 | 30 |
| Rebaudioside D | 4 | β-glc-β-glc- | (β-glc)2-β-glc- | C50H80O28 | 1129.15 | 221 |
| Rebaudioside E | 5 | β-glc-β-glc- | β-glc-β-glc- | C44H70O23 | 967.01 | 174 |
| Rebaudioside F | 6 | β-glc- | (β-glc, β-xyl)-β-glc- | C43H68O22 | 936.99 | 200 |
| Rebaudioside M | 7 | (β-glc)2-β-glc- | (β-glc)2-β-glc- | C56H90O33 | 1291.3 | 250 |
| Stevioside | 8 | β-glc- | β-glc-β-glc- | C38H60O18 | 804.88 | 210 |
| Steviolbioside | 9 | H | β-glc-β-glc- | C32H50O13 | 642.73 | 90 |
| Rubusoside | 10 | β-glc- | β-glc- | C32H50O13 | 642.73 | 114 |
| Dulcoside A | 11 | β-glc- | α-rha-β-glc- | C38H60O17 | 788.87 | 30 |
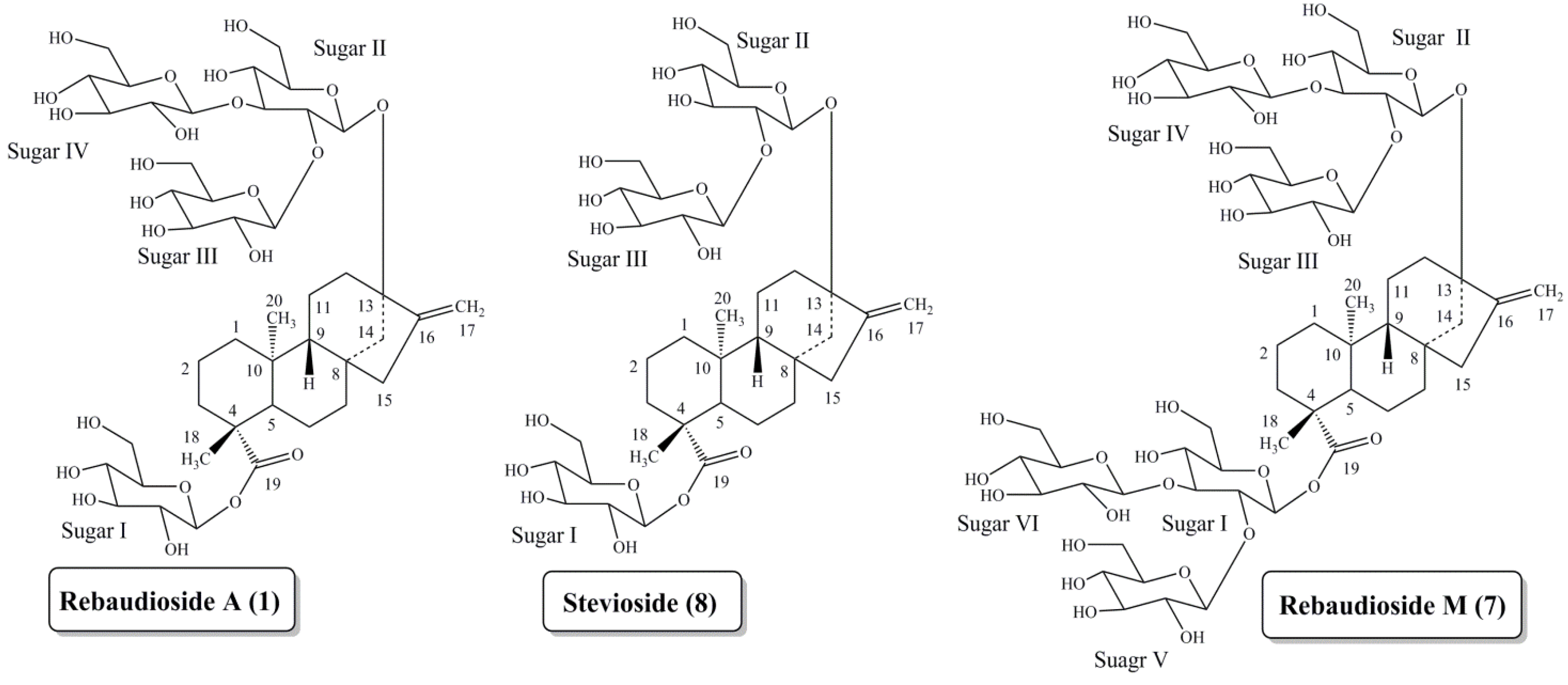
2. Experimental Section
2.1. Purification of Rebaudioside M
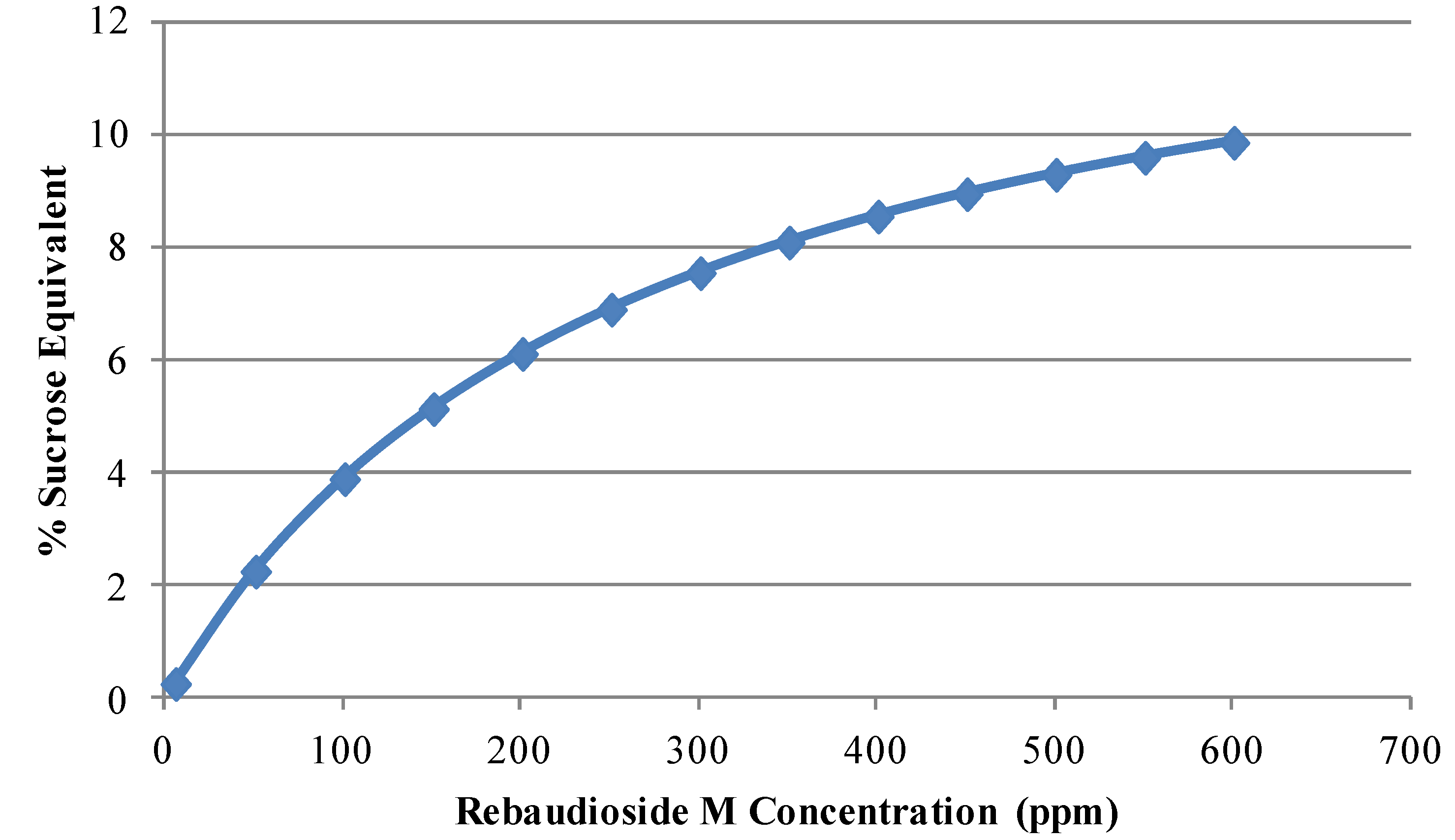
2.2. Properties of Rebaudioside M
2.3. Stability of Rebaudioside M
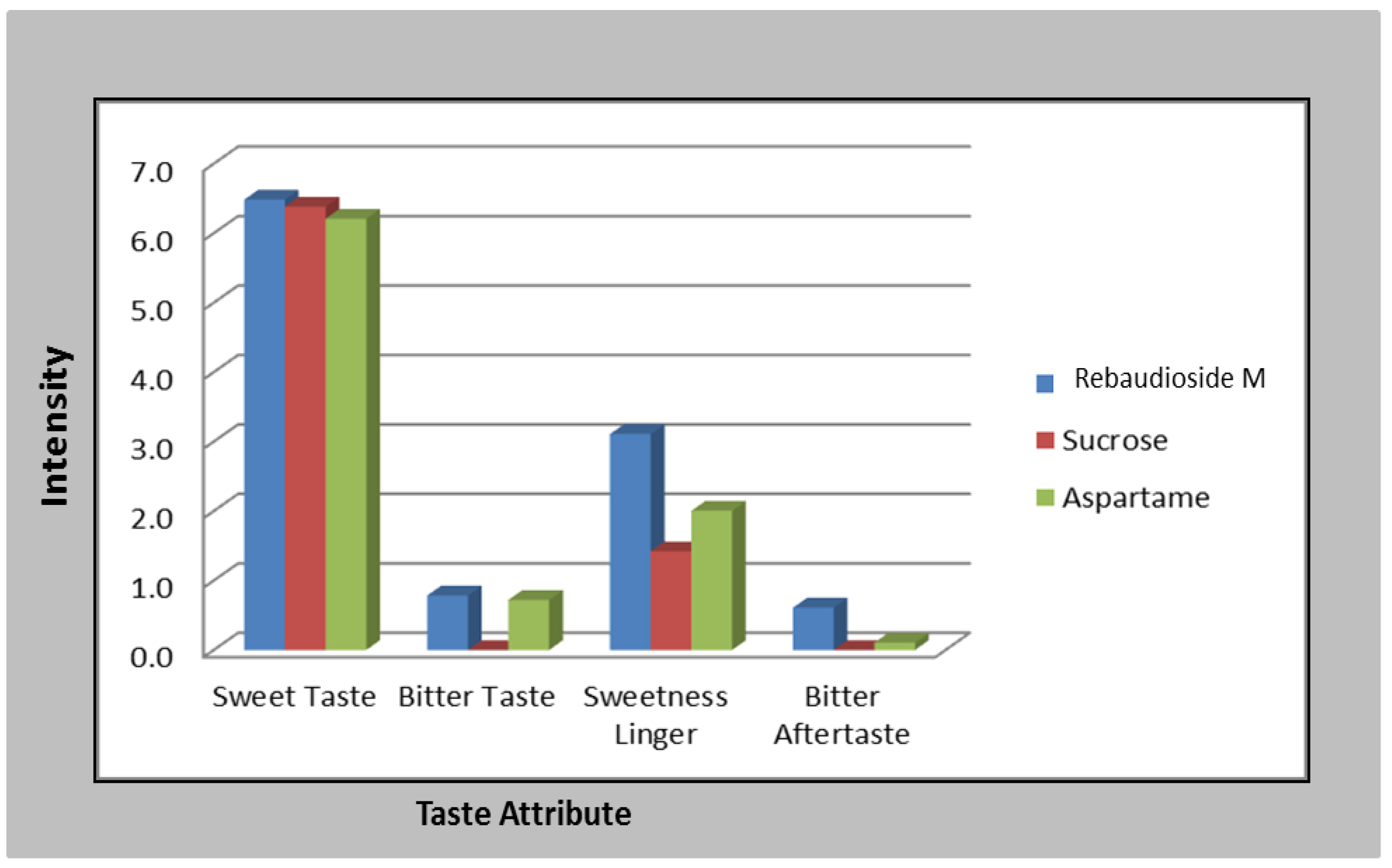
2.4. Sensory Panel for Rebaudioside M and Other Sweeteners
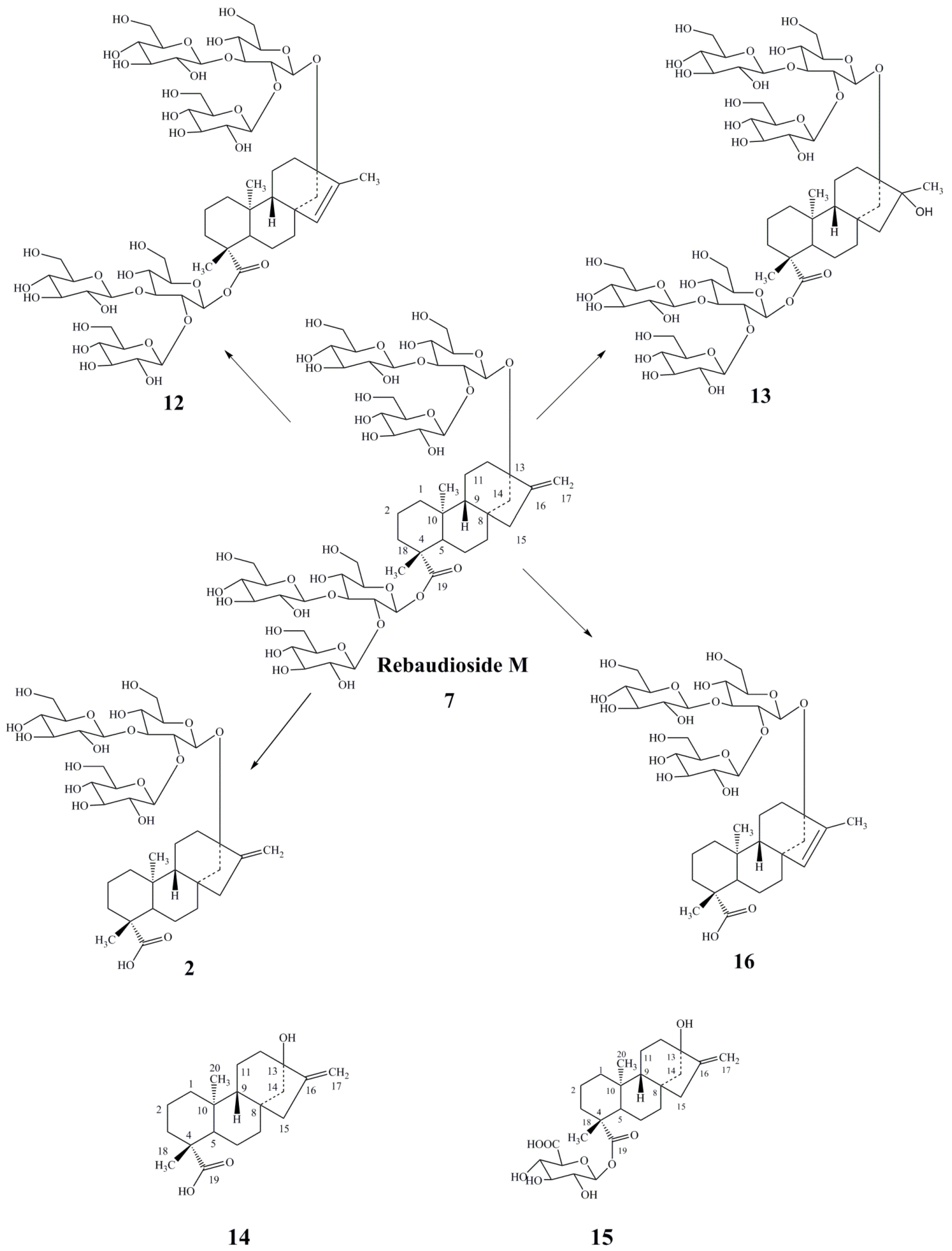
3. Results and Discussion
3.1. Rebaudioside M Sensory Attributes
| Solutions | (%) | (%) |
|---|---|---|
| Water | 99.95 | 99.95 |
| Rebaudioside A 97 (dry basis) | 0.0510 g | |
| Rebaudioside M (dry basis) | 0.0423 g |
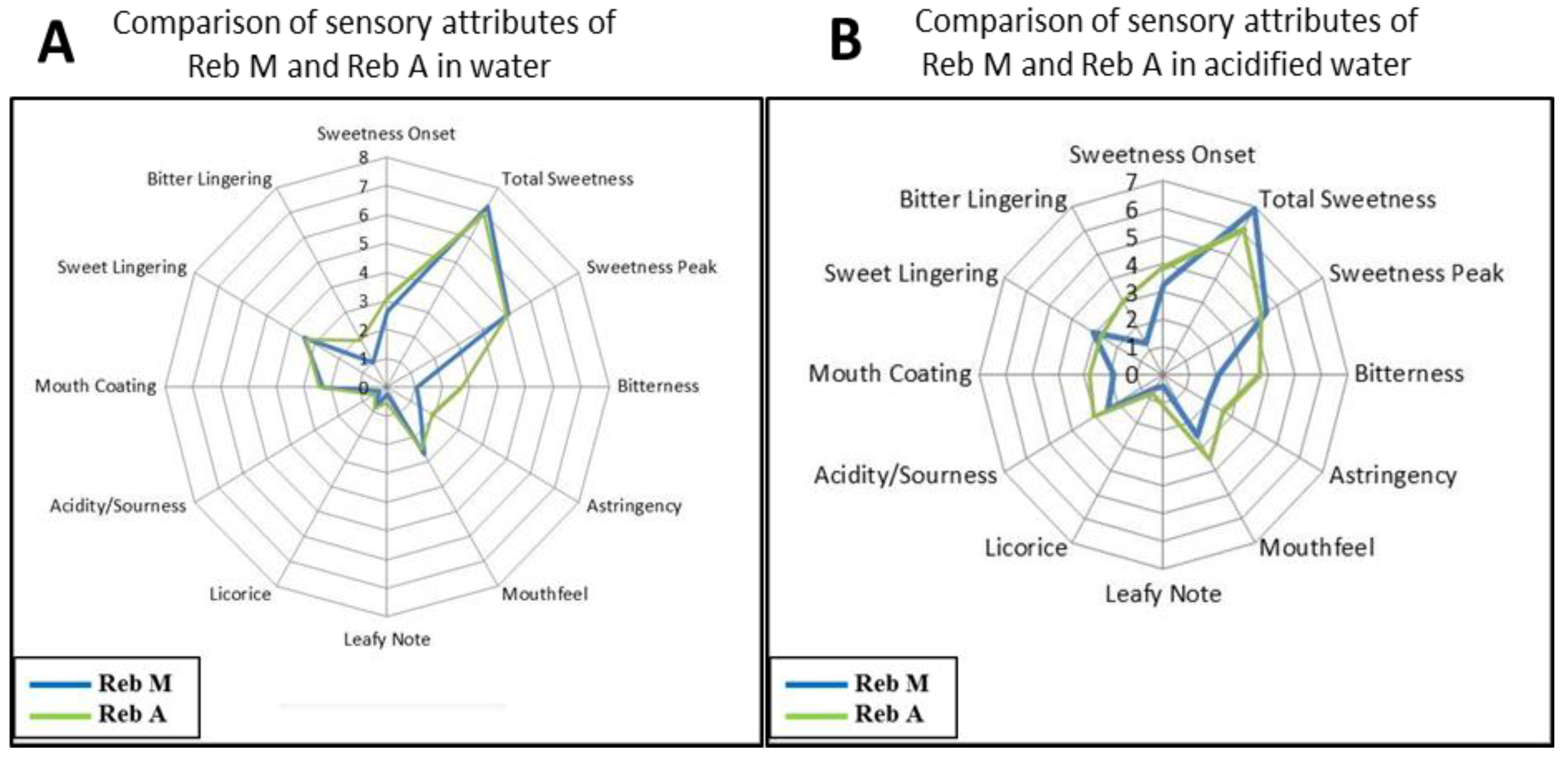
3.2. Blending

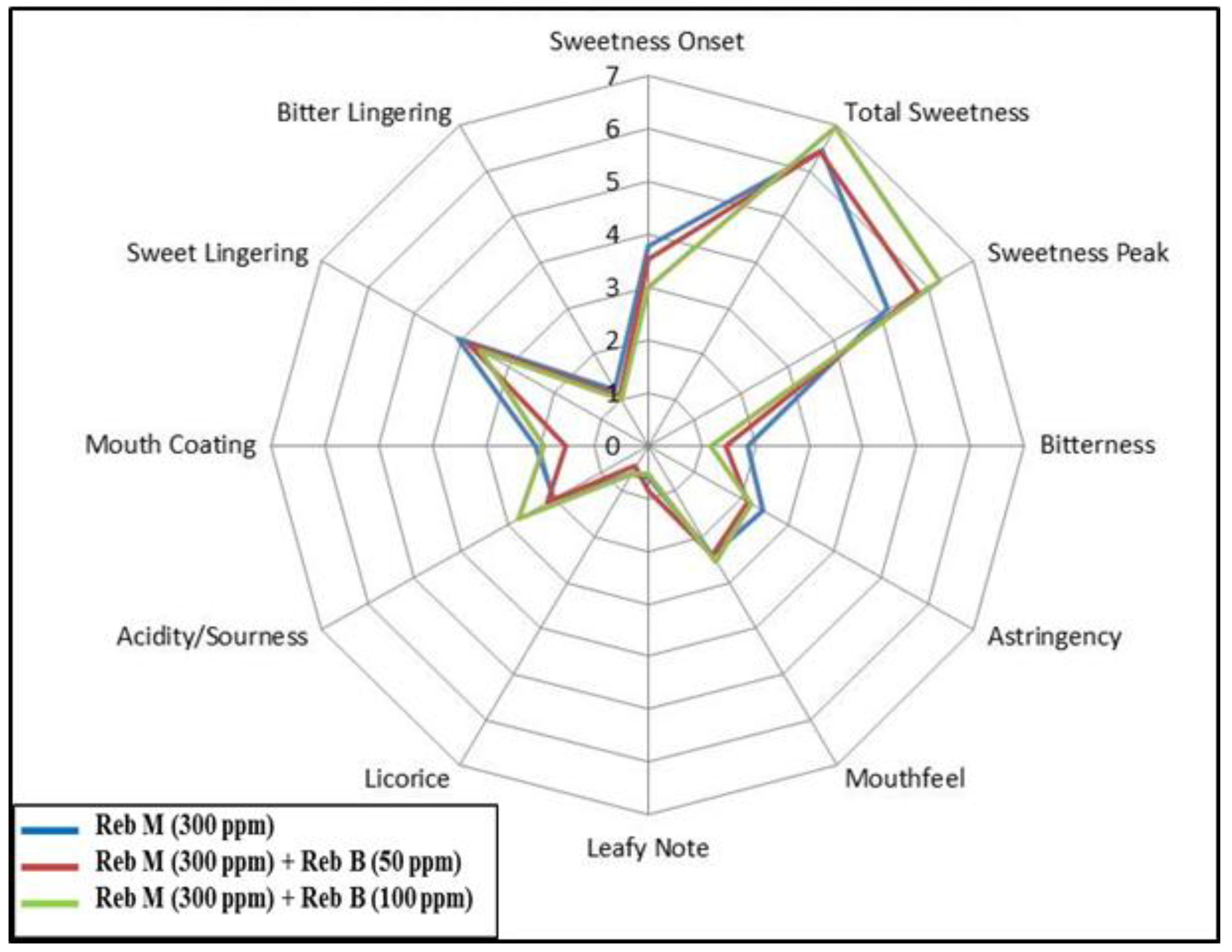
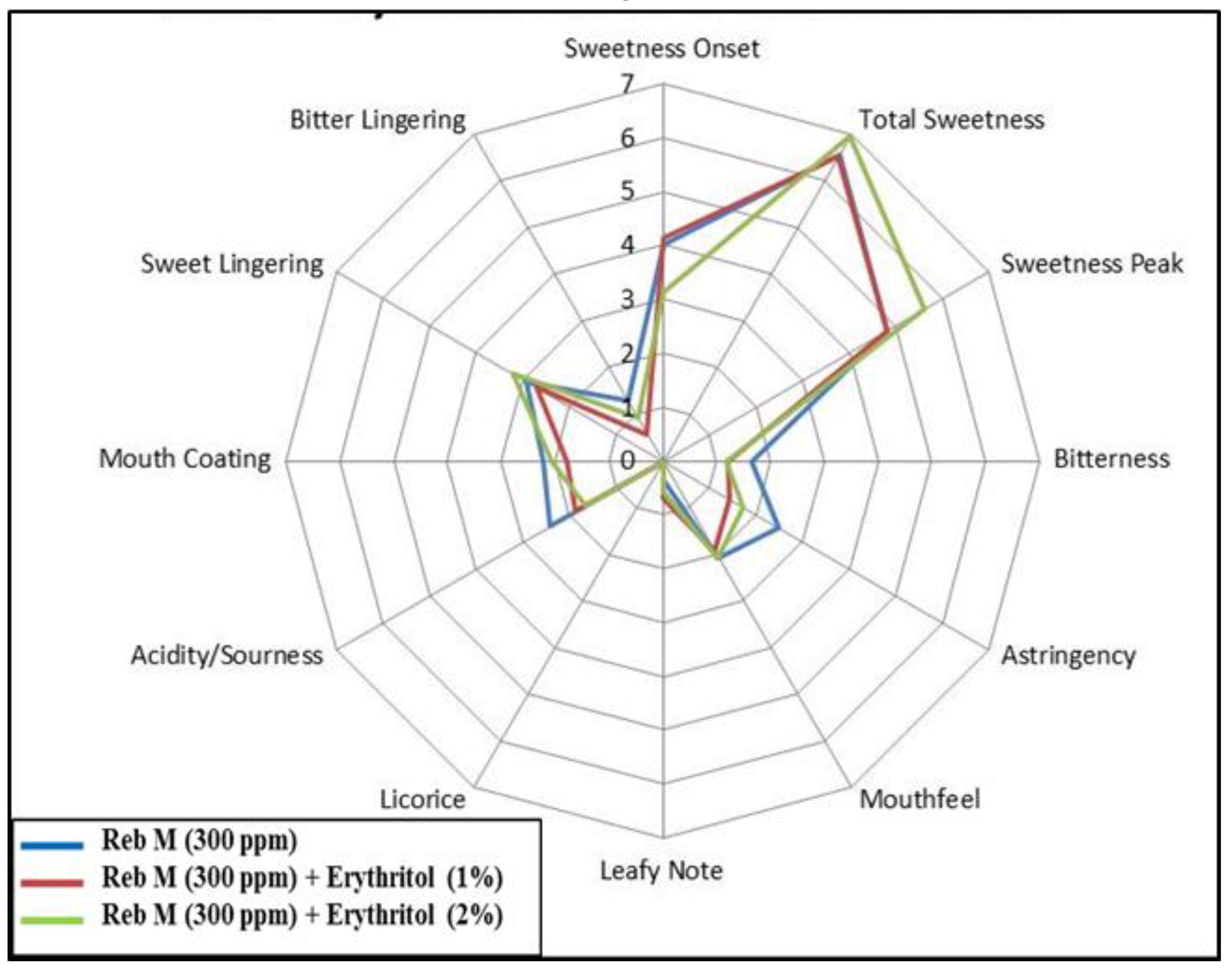
3.3. Food Application
| Product | Range a (mg/kg or mg/L) |
|---|---|
| Carbonated soft drinks | 100–600 |
| Still beverages | 50–600 |
| Powdered soft drinks (as is) | 200–2000 |
| Tabletop (as is) | 800–4000 |
| Bakery products | 200–1000 |
| Dairy products | 150–1000 |
| Chewing gum | 300–6000 |
| Confections | 100–1000 |
| Cereals | 200–1000 |
| Edible gels | 200–1000 |
| Nutraceuticals | 200–1000 |
| Pharmaceuticals | 50–1000 |
4. Conclusions
Conflicts of Interest
References
- Kinghorn, A.D.; Kim, N.-C.; Kim, D.H.L. Terpenoid glycoside sweeteners. In Naturally Occurring Glycosides; Ikan, R., Ed.; John Wiley & Sons: New York, NY, USA, 1999; pp. 399–429. [Google Scholar]
- Kinghorn, A.D.; Wu, C.D.; Soejarto, D.D. Stevioside. In Alternative Sweeteners, 3rd ed.; Revised and Expanded; O’Brien Nabors, L., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 167–183. [Google Scholar]
- Carakostas, M.; Prakash, I.; Kinghorn, A.D.; Wu, C.D.; Soejarto, D.D. Steviol glycosides. In Alternative Sweeteners, 4th ed.; Revised and Expanded; O’Brien Nabors, L., Ed.; Marcel Dekker: New York, NY, USA, 2012; pp. 159–180. [Google Scholar]
- Kinghorn, A.D.; Soejarto, D.D. Current status of stevioside as a sweetening agent for human use. In Economics and Medicinal Plant Research; Wagner, H., Hikino, H., Farnsworth, N.R., Eds.; Academic Press: London, UK, 1985; Volume 1, pp. 1–52. [Google Scholar]
- Lewis, W.H. Early uses of Stevia rebaudiana (Asteraceae) leaves as a sweetener in Paraguay. Econ. Bot. 1992, 46, 336–337. [Google Scholar] [CrossRef]
- Prakash, I.; DuBois, G.E.; Clos, J.F.; Wilkens, K.L.; Fosdick, L.E. Development of Rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 2008, 46, S75–S82. [Google Scholar] [CrossRef]
- Steviol Glycosides. Prepared at the 73rd JECFA (2010) and Published in FAO JECFA Monographs 10 (2010), Superseding Specifications Prepared at the 69th JECFA (2008) and Published in FAO JECFA Monographs 5 (2008). An ADI of 0–4 mg/kg bw (Expressed as Steviol) Was Established at the 69th JECFA (2008).. Available online: http://www.fao.org/ag/agn/jecfa-additives/specs/monograph10/additive-442-m10.pdf (accessed on 1 November 2013).
- Kinghorn, A.D.; Soejarto, D.D. Intensely sweet compounds of natural origin. Med. Res. Rev. 1989, 9, 91–115. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Soejarto, D.D. Sweetening agents of plant origin. CRC Crit. Rev. Plant Sci. 1986, 4, 79–120. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Compadre, C.M. Less common high-potency sweeteners. In Alternative Sweeteners, 2nd ed.; Revised and Expanded; O’Brien Nabors, L., Gelardi, R.C., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 197–218. [Google Scholar]
- Kohda, H.; Kasai, R.; Yamasaki, K.; Murakami, K.; Tanaka, O. New sweet diterpene glucosides from Stevia rebaudiana. Phytochemistry 1976, 15, 981–983. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. A new diterpene glycoside from Stevia rebaudiana. Molecules 2011, 15, 2937–2943. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Structures of the novel diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 34, 1057–1060. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpene glycosides from the leaves of Stevia rebaudiana. Phytochem. Lett. 2011, 4, 209–212. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Structures of the novel α-glucosyl linked diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 2034–2038. [Google Scholar] [CrossRef]
- Prakash, I.; Campbell, M.; San Miguel, R.I.; Chaturvedula, V.S.P. Synthesis and sensory evaluation of ent-kaurane diterpene glycosides. Molecules 2012, 17, 8908–8916. [Google Scholar] [CrossRef]
- Prakash, I.; Campbell, M.; San Miguel, R.I.; Chaturvedula, V.S.P. Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, rebaudiosde B, rebaudioside C and rebaudioside D and sensory evaluation of their reduced derivatives. Int. J. Mol. Sci. 2012, 13, 15126–15136. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V.S.P.; Markosyan, A. Isolation, characterization and sensory evaluation of a hexa β-d-glucopyranosyl diterpene from Stevia rebaudiana. Nat. Prod. Commun. 2013, 8, 1523–1526. [Google Scholar]
- Prakash, I.; Markosyan, A.; Chaturvedula, V.S.P.; Campbell, M.; San Miguel, R.; Purkayastha, S.; Johnson, M. Methods for Purifying Steviol Glycosides and Uses of the Same. PCT Patent Application WO 2013/096420, 27 June 2013. [Google Scholar]
- Agency Response Letter GRAS Notice No. GRN 000473. Purified Steviol Glycosides with Rebaudioside X (also Known as Rebaudioside M) as the Principal Component. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm382202 (accessed on 1 June 2013).
- Alvarez, E.R.B. Stevia Plant Named ‘AKH L1’. Patent Application Number 20120090062, 12 April 2012. [Google Scholar]
- Prakash, I.; Markosyan, A.; Chaturvedula, V.S.P.; Ma, G. The stability of rebaudioside M. 2013; Unpublished work. [Google Scholar]
- Purkayastha, S.; Pugh, G.; Lynch, B.; Roberts, A.; Kwok, D.; Tarka, S.M. In virto metabolism of rebaudioside B, D, and M under anaerobic conditions: Comparison with rebaudioside A. Regul. Toxicol. Pharmacol. 2013, 68, 259–268. [Google Scholar]
- Prakash, I.; Chaturvedula, V.S.P.; Markosyan, A. Structural characterization of the degradation products of minor natural sweet ditrpene glycoside rebaudioside M under acidic conditions. Int. J. Mol. Sci. 2014, 15, 1014–1025. [Google Scholar] [CrossRef]
- Lavia, A.; Hill, J. Sweeteners with Masked Saccharin Aftertaste. French Patent 2,087,843, 1972. [Google Scholar]
- DuBois, G.E.; Walters, D.E.; Schiffman, S.S.; Warwick, Z.S.; Booth, B.J.; Pecore, S.D.; Gibes, K.; Carr, B.T.; Brands, L.M. ACS Symposium Series 450. In Sweeteners: Discovery, Molecular Design, and Chemoreception; Walters, D.E., Orthoefer, F.T., DuBois, G.E., Eds.; American Chemical Soceity: Washington, DC, USA, 1990; pp. 261–276. [Google Scholar]
- Paul, T. Physical chemistry of foodstuffs. V. Degree of sweetness of sugars. Chemiker-Zeitung 1921, 45, 38–39. [Google Scholar]
- Schiffman, S.; Booth, B.; Carr, B.; Losee, M.; Sattely-Miller, E.; Graham, B. Investigation of synergism in binary mixtures of sweeteners. Brain Res. Bull. 1995, 38, 105–120. [Google Scholar] [CrossRef]
- Scott, D. Saccharin-Dipeptide Sweetening Compositions. British Patent 1,256,995, 1971. [Google Scholar]
- Verdi, R.J.; Hood, L.L. Advantages of alternative sweetener blends. Food Technol. 1993, 47, 94–102. [Google Scholar]
- Walters, E. High intensity sweetener blends. In Food Product Design; Weeks Publishing Co.: Northbrook, IL, USA, 1993. [Google Scholar]
- Pariza, M.; Ponakala, S.; Gerlat, P.; Andress, S. Predicting the functionality of direct food additives. Food Technol. 1998, 52, 56–60. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prakash, I.; Markosyan, A.; Bunders, C. Development of Next Generation Stevia Sweetener: Rebaudioside M. Foods 2014, 3, 162-175. https://doi.org/10.3390/foods3010162
Prakash I, Markosyan A, Bunders C. Development of Next Generation Stevia Sweetener: Rebaudioside M. Foods. 2014; 3(1):162-175. https://doi.org/10.3390/foods3010162
Chicago/Turabian StylePrakash, Indra, Avetik Markosyan, and Cynthia Bunders. 2014. "Development of Next Generation Stevia Sweetener: Rebaudioside M" Foods 3, no. 1: 162-175. https://doi.org/10.3390/foods3010162




