Effect of Pre-Harvest Sprouting on Physicochemical Properties of Starch in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Sprouting and Sprout Score
| Genotype | Sprout Score | α-Amylase Activity (CU/g) | ||
|---|---|---|---|---|
| Sprouted | Non-Sprouted | ΔD | ||
| RSW | H | - | - | - |
| Hanna | 2.8 | 1.32 | 0.11 | 1.20 |
| Ingot | 7.0 | 2.37 | 0.10 | 2.27 |
| Alsen | 4.8 | 1.82 | 0.09 | 1.57 |
| Briggs | 5.7 | 2.16 | 0.11 | 2.06 |
| Freyr | 4.4 | 1.79 | 0.09 | 1.70 |
| Glenn | 4.0 | 1.68 | 0.08 | 1.61 |
| Granite | 5.3 | 2.09 | 0.13 | 1.96 |
| Kelby | 3.4 | 1.56 | 0.13 | 1.43 |
| Norpro | 6.0 | 2.18 | 0.09 | 2.09 |
| Reeder | 4.4 | 1.76 | 0.08 | 1.68 |
| Steele-ND | 5.0 | 1.93 | 0.08 | 1.85 |
| Knudson | 5.4 | 2.12 | 0.10 | 2.02 |
| Mean | 4.8 | 1.90 | 0.10 | 1.79 |
| HWSW | ||||
| 99S0155-14W | 2.5 | 1.36 | 0.12 | 1.24 |
| Otis | 7.8 | 2.47 | 0.11 | 2.37 |
| AC Snowbird | 2.8 | 1.39 | 0.08 | 1.31 |
| AC Vista | 5.8 | 2.13 | 0.09 | 2.04 |
| Argent | 4.8 | 1.98 | 0.16 | 1.83 |
| CS3100L | 6.8 | 2.33 | 0.18 | 2.16 |
| CS3100Q | 6.8 | 2.44 | 0.14 | 2.3 |
| Explorer | 6.9 | 2.37 | 0.16 | 2.21 |
| Lolo | 5.7 | 2.17 | 0.12 | 2.05 |
| MT9420 | 6.9 | 2.33 | 0.14 | 2.19 |
| NDSW0602 | 6.3 | 2.37 | 0.12 | 2.24 |
| Pristine | 5.0 | 1.99 | 0.18 | 1.81 |
| Mean | 5.7 | 2.11 | 0.13 | 1.98 |
| LSD | 1.4 | 0.39 | 0.05 | 0.40 |
2.3. α-Amylase Activity
2.4. Pasting Properties
2.5. Scanning Electron Microscopy (SEM) Analysis of Starch Granules
2.6. High Performance Size Exclusion Chromatography (HP-SEC) Analysis of Starch
2.7. Statistical Analysis
3. Results and Discussion
3.1. α-Amylase Activity in Non-Sprouted and Sprouted HRS and HWS Wheat Samples
3.2. Pasting Properties of Non-sprouted and Sprouted HRS and HWS Wheat
| Wheat Class | Genotype | Peak Viscosity | Hot Paste Viscosity | Final Viscosity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sprouted | Non-Sprouted | ΔD | Sprouted | Non-Sprouted | ΔD | Sprouted | Non-Sprouted | ΔD | ||
| Carrington | ||||||||||
| HRSW | Hanna | 11.2 | 222.7 | 212.8 | 2.3 | 134.8 | 133.2 | 3.5 | 258.5 | 255.4 |
| Ingot | 3.5 | 233.4 | 230.4 | 2.3 | 139.9 | 137.6 | 2.9 | 256.7 | 253.8 | |
| Mean | 4.4 | 224.4 | 221.4 | 1.4 | 126.5 | 125.6 | 1.7 | 244.5 | 243.1 | |
| HWSW | AC Snowbird | 11.4 | 252.4 | 239.3 | 1.9 | 125.2 | 124.2 | 3.0 | 237.8 | 235.5 |
| Lolo | 2.2 | 210.4 | 205.4 | 0.6 | 93.0 | 90.8 | 0.6 | 202.9 | 200.3 | |
| Mean | 3.1 | 209.3 | 207.1 | 2.0 | 99.2 | 97.8 | 2.4 | 215.7 | 213.9 | |
| LSD | 1.8 | 19.3 | 19.5 | 1.3 | 18.1 | 18.6 | 1.3 | 28.2 | 28.6 | |
| Casselton | ||||||||||
| HRSW | Hanna | 20.5 | 197.9 | 177.4 | 3.1 | 128.6 | 125.4 | 4.8 | 237.5 | 232.4 |
| Ingot | 10.6 | 210.1 | 198.6 | 2.5 | 133.6 | 130.6 | 3.1 | 233.2 | 229.6 | |
| Mean | 12.4 | 204.5 | 192.3 | 2.3 | 122.0 | 119.5 | 3.1 | 223.1 | 219.8 | |
| HWSW | AC Snowbird | 14.8 | 242.0 | 228.6 | 1.9 | 129.8 | 128.3 | 3.1 | 236.1 | 233.8 |
| Lolo | 10.6 | 213.9 | 200.0 | 2.8 | 112.0 | 108.4 | 3.5 | 216.2 | 212.0 | |
| Mean | 10.6 | 188.9 | 177.5 | 2.6 | 94.0 | 91.3 | 3.3 | 193.7 | 190.1 | |
| LSD | 5.3 | 25.5 | 25.4 | 2.1 | 20.4 | 19.9 | 2.8 | 37.9 | 37.4 | |
| Prosper | ||||||||||
| HRSW | Hanna | 14.7 | 200.2 | 183.2 | 1.8 | 129.1 | 125.6 | 3.0 | 240.8 | 236.2 |
| Ingot | 6.8 | 236.2 | 227.6 | 2.0 | 153.7 | 150.4 | 2.6 | 274.4 | 270.6 | |
| Mean | 8.4 | 201.7 | 192.9 | 2.0 | 119.9 | 117.8 | 2.4 | 228.2 | 225.9 | |
| HWSW | AC Snowbird | 17.9 | 289.0 | 268.2 | 1.9 | 163.7 | 160.9 | 3.3 | 294.6 | 290.5 |
| Lolo | 8.6 | 203.5 | 193.8 | 3.5 | 103.5 | 100.8 | 3.7 | 209.6 | 206.7 | |
| Mean | 8.1 | 189.8 | 180.8 | 2.3 | 95.1 | 92.4 | 2.8 | 203.3 | 200.3 | |
| LSD | 2.3 | 24.1 | 24.3 | 1.2 | 19.5 | 19.3 | 1.3 | 30.9 | 30.8 | |
| Pasting Characteristics | Sprout | Sound | PHS | ∆D | |
|---|---|---|---|---|---|
| Score | α-Amylase | α-Amylase | α-Amylase | ||
| Carrington | |||||
| Non-Sprouted | Peak Viscosity | NS | −0.45 * | NS | NS |
| Hot Paste Viscosity | −0.47 * | −0.50 * | −0.51 * | −0.45 * | |
| Final Viscosity | NS | NS | −0.43 * | NS | |
| Sprouted | Peak Viscosity | −0.64 *** | NS | −0.61 ** | −0.60 ** |
| ∆D | Peak Viscosity | NS | −0.45 * | NS | NS |
| Hot Paste Viscosity | −0.48 * | −0.50 * | −0.51 * | −0.45 * | |
| Final Viscosity | NS | NS | −0.43 * | NS | |
| Casselton | |||||
| Non-Sprouted | Peak Viscosity | −0.41 * | −0.67 *** | NS | NS |
| Hot Paste Viscosity | −0.44 * | −0.68 *** | NS | NS | |
| Final Viscosity | NS | −0.54 ** | NS | NS | |
| Sprouted | Peak Viscosity | −0.56 ** | NS | −0.54 ** | −0.54 ** |
| ∆D | Peak Viscosity | NS | −0.68 *** | NS | NS |
| Hot Paste Viscosity | −0.43 * | −0.69 *** | NS | NS | |
| Final Viscosity | NS | −0.55 ** | NS | NS | |
| Prosper | |||||
| Non-Sprouted | Peak Viscosity | NS | −0.64 *** | NS | NS |
| Hot Paste Viscosity | NS | −0.68 *** | NS | NS | |
| Final Viscosity | NS | −0.57 ** | NS | NS | |
| Sprouted | Peak Viscosity | −0.76 *** | NS | −0.69 *** | −0.68 *** |
| ∆D | Peak Viscosity | NS | −0.66 *** | NS | NS |
| Hot Paste Viscosity | NS | −0.69 *** | NS | NS | |
| Final Viscosity | NS | −0.57 ** | NS | NS | |
3.3. Starch Granule Morphology of Non-sprouted and Sprouted HRS and HWS Wheat
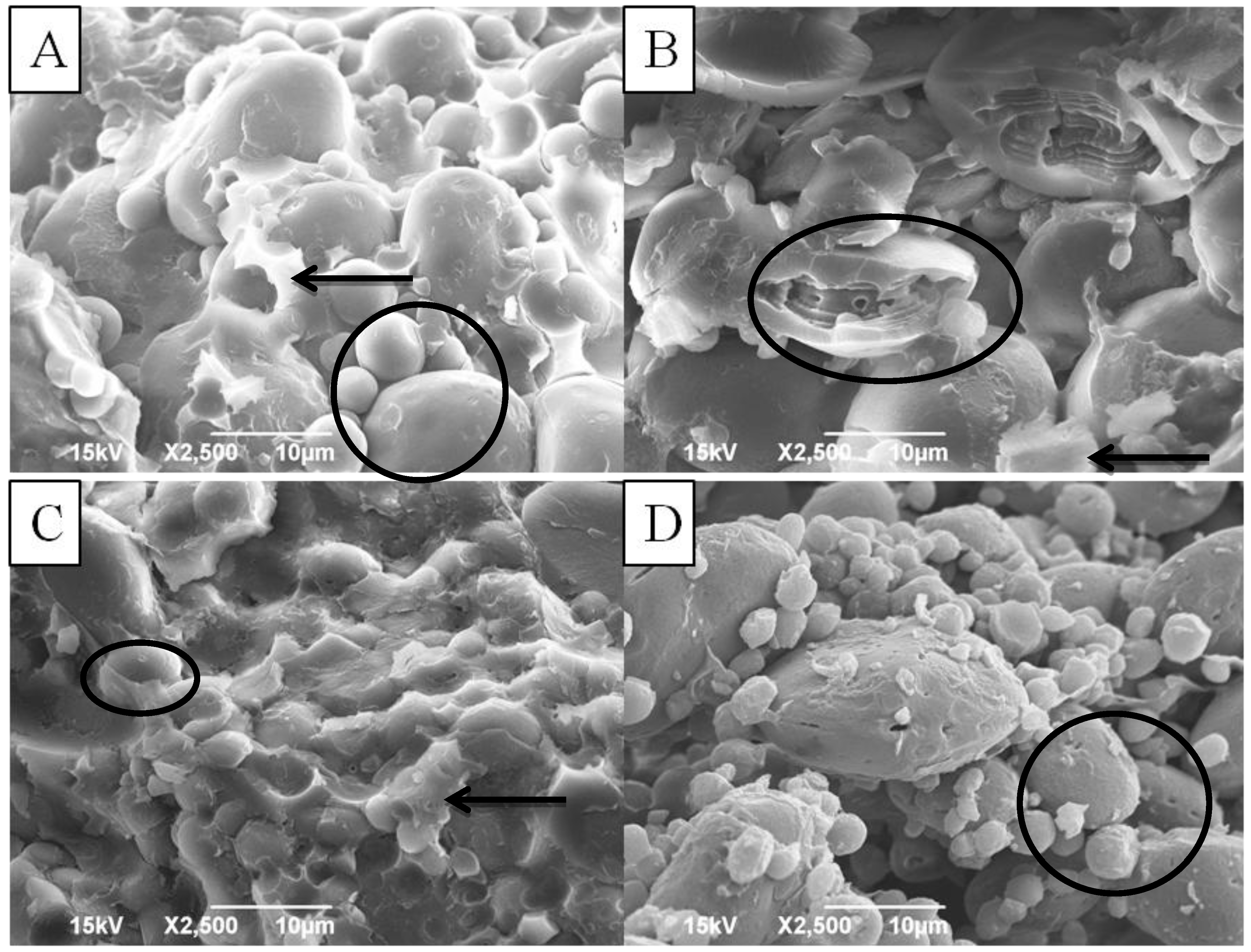
3.4. HPSEC of Starch in Non-sprouted and Sprouted HRS and HWS Wheat
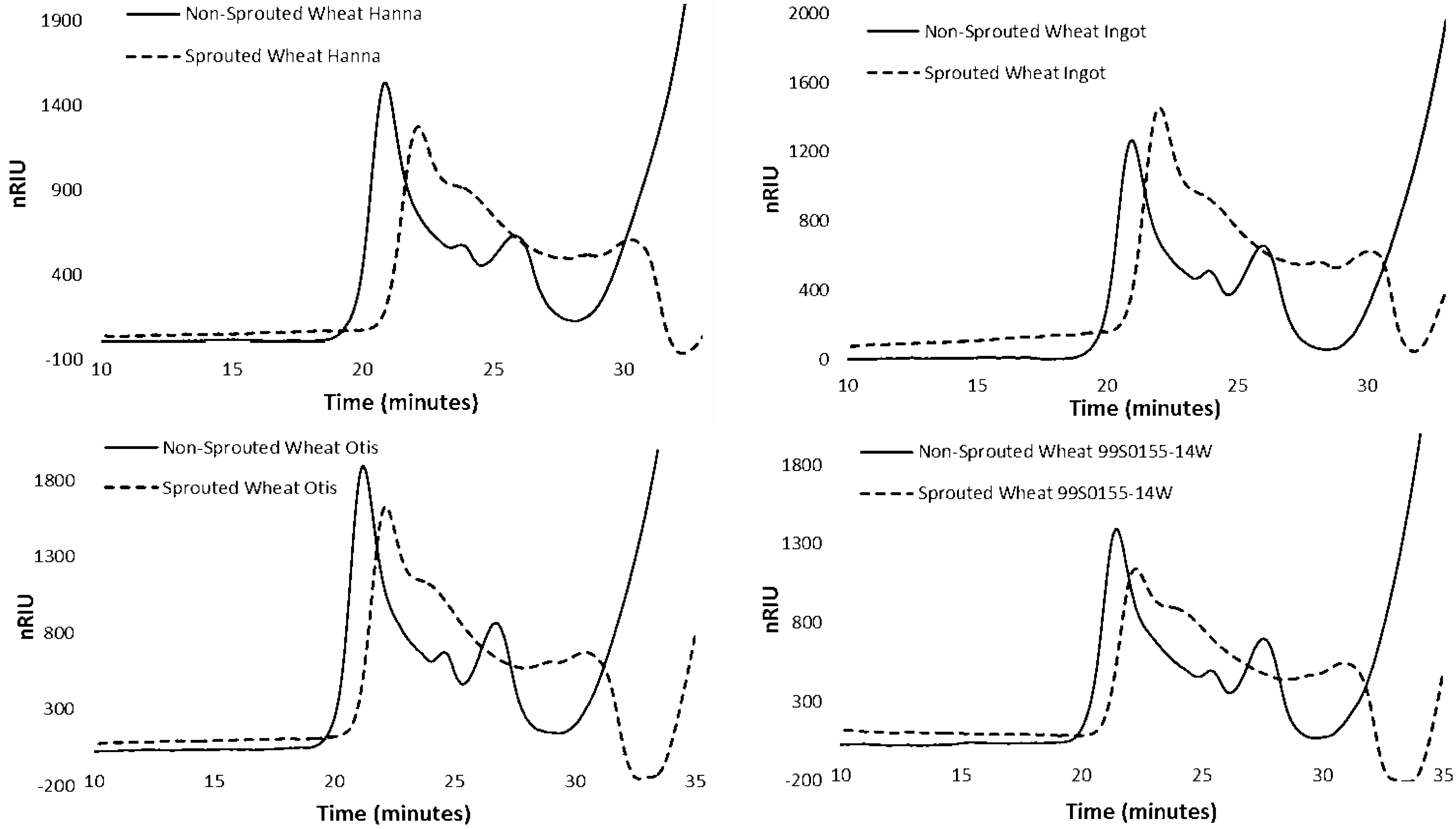
| Treatment | Genotype | HMW-AP | LMW-AP | AM | |||
|---|---|---|---|---|---|---|---|
| (%) | MWT | (%) | MWT | (%) | MWT | ||
| Non-Sprouted | Hanna | 61.5 | 1.68 × 107 | 11.8 | 4.61 × 106 | 26.8 | 1.82 × 106 |
| Ingot | 60.6 | 1.57 × 107 | 12.8 | 4.08 × 106 | 26.6 | 1.60 × 106 | |
| Otis | 62.8 | 1.41 × 107 | 11.1 | 2.99 × 106 | 26.1 | 1.15 × 106 | |
| 99S0155-14W | 64.5 | 1.26 × 107 | 11.0 | 2.10 × 106 | 24.6 | 0.80 × 106 | |
| LSD | 1.2 | 1.03 × 106 | 1.1 | 6.25 × 105 | 0.1 | 1.71 × 105 | |
| Sprouted | Hanna | 31.6 | 0.95 × 107 | 40.8 | 5.21 × 106 | 27.5 | 0.24 × 106 |
| Ingot | 34.0 | 1.01 × 107 | 38.2 | 5.46 × 106 | 27.8 | 0.27×106 | |
| Otis | 32.9 | 0.95 × 107 | 37.5 | 4.90 × 106 | 29.7 | 0.24 × 106 | |
| 99S0155-14W | 28.9 | 0.92 × 107 | 42.2 | 4.88 × 106 | 28.9 | 0.19 × 106 | |
| LSD | 0.8 | 7.60 × 104 | 1.7 | 1.89 × 105 | 1.1 | 7.71 × 103 | |
| ΔD | Hanna | 29.9 | 0.73 × 107 | 29.0 | 0.60 × 106 | 0.7 | 1.58 × 106 |
| Ingot | 26.6 | 0.56 × 107 | 25.4 | 1.38 × 106 | 1.2 | 1.33 × 106 | |
| Otis | 29.9 | 0.46 × 107 | 26.4 | 2.09 × 106 | 3.6 | 0.91 × 106 | |
| 99S0155-14W | 35.6 | 0.34 × 107 | 31.2 | 2.78 × 106 | 4.3 | 0.61 × 106 | |
| LSD | 1.5 | 1.01 × 106 | 2.0 | 4.80 × 105 | 1.1 | 1.66 × 105 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Groos, C.; Gay, G.; Perretant, M.R.; Gervais, L.; Bernard, M.; Dedryver, F.; Charmet, G. Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor. Appl. Genet. 2002, 104, 39–47. [Google Scholar] [CrossRef]
- Mares, D.J. Temperature dependence of germinability of wheat (Triticum aestivum L.) grain in relation to pre-harvest sprouting. Crop Pasture Sci. 1984, 35, 115–128. [Google Scholar] [CrossRef]
- Wahl, T.I.; O’Rourke, A.D. The Economics of Sprout Damage in Wheat. In Pre-harvest Sprouting in Cereals; Walker-Simmons, M.K., Ried, J.L., Eds.; AACC International Press: St. Paul, MN, USA, 1992; pp. 10–17. [Google Scholar]
- Rugg, M.O.P. Evaluating Hard Red and White Spring Wheat (Triticum Aestivum L.) Genotypes for Tolerance to Pre-harvest Sprouting. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2011. [Google Scholar]
- Sorenson, B.; Wiersma, J. Sprout damaged wheat, crop insurance and quality concerns. Minn. Crop News Arch. 2004. Available online: http://web.archive.org/web/20130205033819/ http://www. extension.umn.edu/cropEnews/2004/04MNCN31.htm (accessed on 2 April 2014).
- Flintham, J.E. Different genetic components control coat-imposed and embryo-imposeddormancy in wheat. Seed Sci. Res. 2000, 10, 43–50. [Google Scholar] [CrossRef]
- Flintham, J.; Adlam, R.; Bassoi, M.; Holdsworth, M.; Gale, M. Mapping genes for resistance to sprouting damage in wheat. Euphytica 2002, 126, 39–45. [Google Scholar] [CrossRef]
- Mares, D.; Mrva, K.; Cheong, J.; Williams, K.; Watson, B.; Storlie, E.; Sutherland, M.; Zou, Y. A QTL located on chromosome 4A associated with dormancy in white- and red-grained wheats of diverse origin. Theor. Appl. Genet. 2005, 111, 1357–1364. [Google Scholar] [CrossRef]
- Mares, D.; Mrva, K.; Tan, M.K.; Sharp, P. Dormancy in white-grained wheat: Progress towards identification of genes and molecular markers. Euphytica 2002, 126, 47–53. [Google Scholar] [CrossRef]
- Himi, E.; Mares, D.J.; Yanagisawa, A.; Noda, K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J. Exp. Bot. 2002, 53, 1569–1574. [Google Scholar] [CrossRef]
- Anderson, J.A.; Sorrells, M.E.; Tanksley, S.D. RFLP analysis of genomic regions associated with resistance to preharvest sprouting in wheat. Crop Sci. 1993, 33, 453–459. [Google Scholar] [CrossRef]
- Morad, M.M.; Rubenthaler, G.L. Germination of soft white wheat and its effect on flour fractions, breadmaking, and crumb firmness. Cereal Chem. 1983, 60, 413–417. [Google Scholar]
- Ichinose, Y.; Takata, K.; Kuwabara, T.; Iriki, N.; Abiko, T.; Yamauchi, H. Effects of increase in alpha-amylase and endo-protease activities during germination on the breadmaking quality of wheat. Food Sci. Technol. Res. 2001, 7, 214–219. [Google Scholar] [CrossRef]
- Bean, M.M.; Keagy, P.M.; Fullington, J.G.; Jones, F.T.; Mecham, D.K. Dried Japanese noodles I. Properties of laboratory prepared noodle doughs from sound and damaged wheat flours. Cereal Chem. 1974, 51, 416–426. [Google Scholar]
- Whistler, R.L.; BeMiller, J.N. Starch. In Carbohydrate Chemistry for Food Scientists; Whistler, R.L., BeMiller, J.N., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1997; pp. 117–152. [Google Scholar]
- Jane, J.; Chen, J. Effects of amylose molecular size and amylopectin branch chain length on paste properties of starch. Cereal Chem. 1992, 69, 60–65. [Google Scholar]
- AACC. General Pasting Method for Wheat or Rye Flour or Starch Using the Rapid Visco Analyzer. In International Approved Methods of Analysis, 11th ed.; Method 76-21.01; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- MacGregor, A.W.; Matsuo, R.R. Starch degradation in endosperm of barley and wheat kernels during initial stages of germination. Cereal Chem. 1972, 59, 210–216. [Google Scholar]
- Simsek, S.; Whitney, K.; Ohm, J.B. Analysis of cereal starches by high-performance size exclusion chromatography. Food Anal. Methods 2013, 6, 181–190. [Google Scholar] [CrossRef]
- Rani, K.U.; Prasada Rao, U.J.S.; Leelavathi, K.; Haridas Rao, P. Distribution of enzymes in wheat flour mill streams. J. Cereal Sci. 2001, 34, 233–242. [Google Scholar]
- Huang, G.R. A Study of Alpha Amylase Activity in Kansas Hard White Wheats. Master’s Thesis, Kansas State University, Manhattan, NY, USA, 1979. [Google Scholar]
- Tsai, M.L.; Li, C.F.; Lii, C.Y. Effects of granular structures on the pasting behaviors of starches. Cereal Chem. 1997, 74, 750–757. [Google Scholar]
- Cornell, H. The Functionality of Wheat Starch. In Starch in Food; Eliasson, A.C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 221–238. [Google Scholar]
- Lorenz, K.; Kulp, K. Sprouting of cereal grains—Effects on starch characteristics. Starch 1981, 33, 183–187. [Google Scholar] [CrossRef]
- Dronzec, B.L.; Hwang, P.; Bushuk, W. Scanning electron microscopy of starch from sprouted wheat. Cereal Chem. 1972, 49, 232–239. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simsek, S.; Ohm, J.-B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of Pre-Harvest Sprouting on Physicochemical Properties of Starch in Wheat. Foods 2014, 3, 194-207. https://doi.org/10.3390/foods3020194
Simsek S, Ohm J-B, Lu H, Rugg M, Berzonsky W, Alamri MS, Mergoum M. Effect of Pre-Harvest Sprouting on Physicochemical Properties of Starch in Wheat. Foods. 2014; 3(2):194-207. https://doi.org/10.3390/foods3020194
Chicago/Turabian StyleSimsek, Senay, Jae-Bom Ohm, Haiyan Lu, Mory Rugg, William Berzonsky, Mohammed S. Alamri, and Mohamed Mergoum. 2014. "Effect of Pre-Harvest Sprouting on Physicochemical Properties of Starch in Wheat" Foods 3, no. 2: 194-207. https://doi.org/10.3390/foods3020194





