Saffron Samples of Different Origin: An NMR Study of Microwave-Assisted Extracts
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Sample Code | Country (region) | Producer |
|---|---|---|
| AB | Italy (Abruzzi) PDO* | Agricultural company “Matergia”, Barisciano (Aquila) |
| GR | Greece | Local market |
| LA | Italy (Latium) | Agricultural company “Roncaglia”, Ronciglione (Viterbo) |
| SA1 | Italy (Sardinia) | Local market |
| SA2 | Italy (Sardinia) | “Zafferano Monreale snc” San Gavino Monreale, (Cagliari) |
| SA3 | Italy (Sardinia) | Agricultural company “Curreli Franco” San Gavino Monreale, (Cagliari) |
| SA4 | Italy (Sardinia) PDO | Agricultural company “Itria”, Turri (VS) |
| SP1 | Spain | “Don Jerez” |
| SP2 | Spain | “Cameo Zafferano Red” |
| TK | Turkey | Local market |
| HU | Hungary | “Nehéz Gyula” |
2.2. Microwave-Assisted and Conventional Extractions
2.3. High-Resolution NMR Measurements
2.4. Measurement of the Metabolic Content and Statistical Analysis
3. Results and Discussion
3.1. Microwave-Assisted Extraction
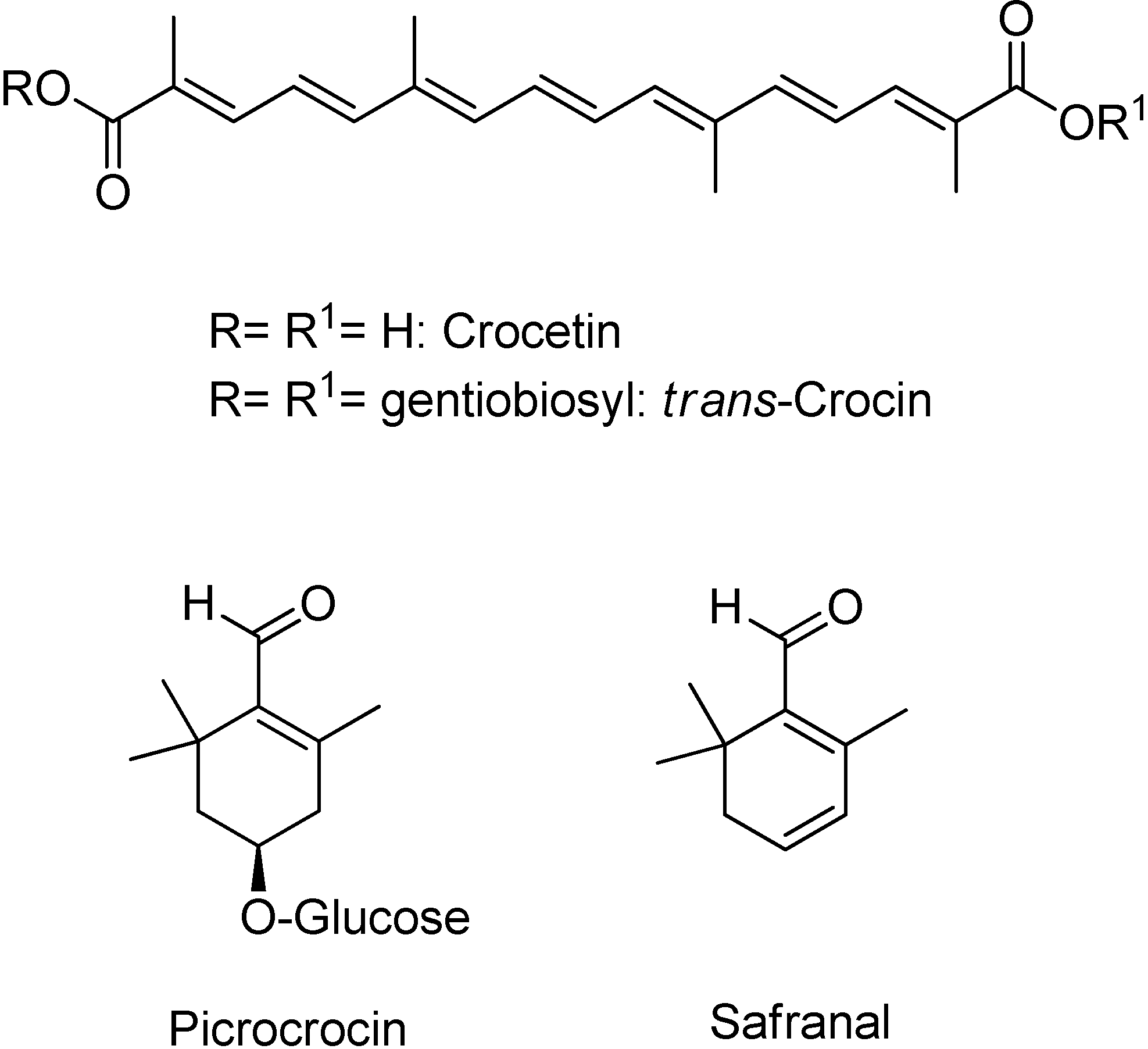
3.2. NMR Analysis of Saffron Extracts
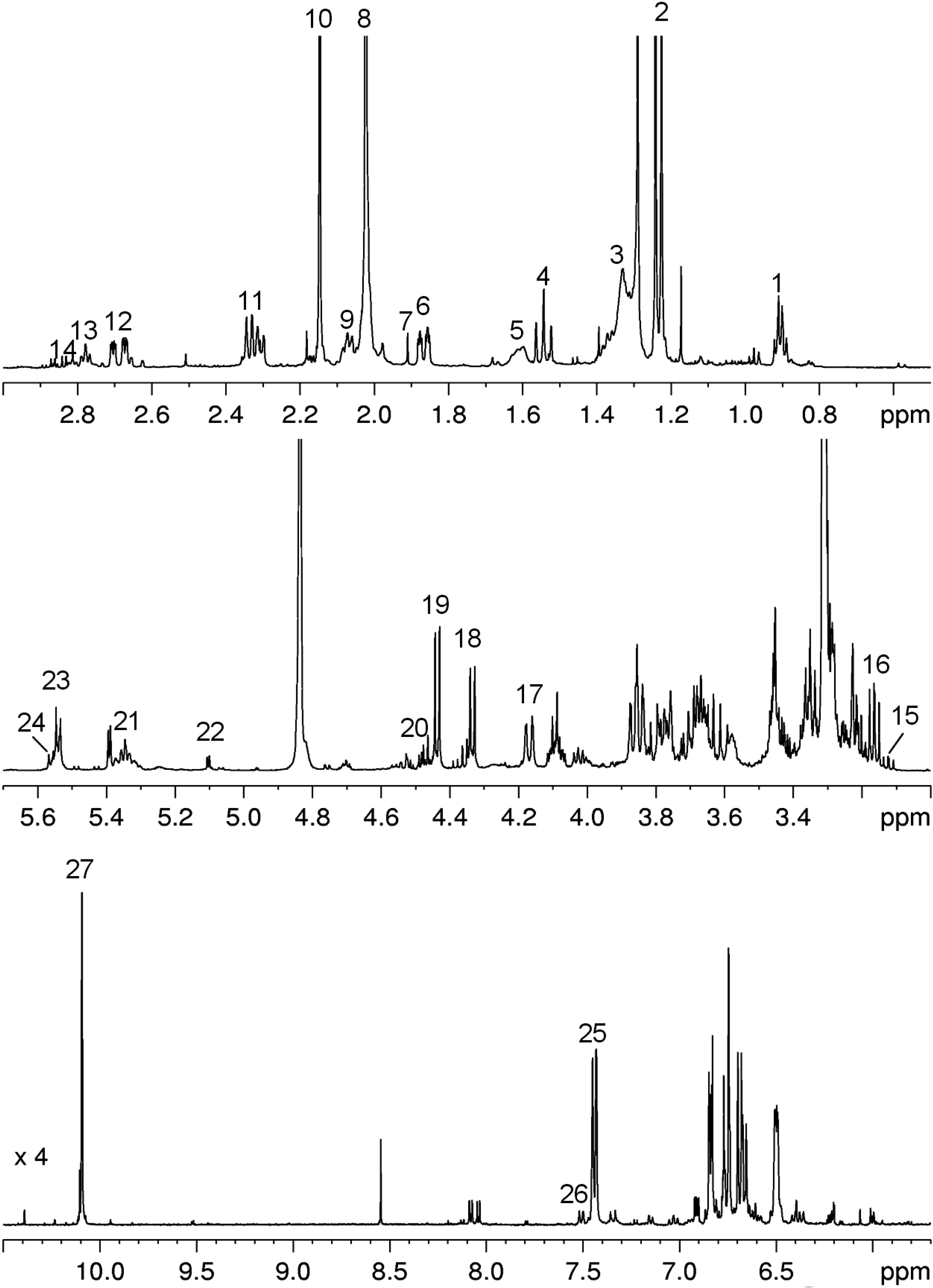
| Compound | Assignment | ¹H, ppm | Multiplicity, JH–H, Hz | ¹³C, ppm |
|---|---|---|---|---|
| Linoleic acid C18:2 | CH2-2 | 2.33 | ||
| CH2-3 | 1.61 | |||
| CH2-4 | 1.34 | |||
| CH2-8,14 | 2.07 | t: 7.4 | ||
| CH-9,10,12,13 | 5.34 * | |||
| CH2-11 | 2.78 * | t: 6.8 | ||
| CH2-15,16,17 | 1.32 | |||
| CH3-18 | 0.91 | t: 7.0 | ||
| Linolenic acid C18:3 | CH2-1,4,5,6,7 | 1.34 | ||
| CH2-2 | 2.33 | |||
| CH2-3 | 1.61 | |||
| CH2-8 | 2.08 | t: 7.4 | ||
| CH-9,10,12,13 | 5.34 | |||
| CH2-11,14 | 2.81 * | t: 6.8 | ||
| CH-15 | 5.30 * | |||
| CH-16 | 5.38 * | |||
| CH2-17 | 2.08 | t: 7.4 | ||
| CH3-18 | 0.97 | t: 7.0 | ||
| Phosphatidylcholine | (CH3)3N | 3.21 | s | 54.6 |
| Acetic acid AcOH | CH3 | 1.91 * | s | |
| All fatty acids except C18:3, FA | CH3 | 0.90 * | t: 6.7 | 14.6–14.4 |
| All-trans-crocin DBtCROC (aglycone) | C-8,8′ | 168.6 | ||
| C-9,9′ | 126.5 | |||
| CH-10,10′ | 7.44 * | dm: 11.3 | 141.9 | |
| CH-11,11′ | 6.68 | dd: 15.0; 11.4 | 124.7 | |
| CH-12,12′ | 6.76 | d: 15.0 | 146.2 | |
| C-13,13′ | 138.1 | |||
| CH-14,14′ | 6.50 | dm: 7.7 | 137.4 | |
| CH-15,15′ | 6.84 | dd: 7.8; 2.9 | 133.0 | |
| CH3-19,20 | 2.02 | s | 12.6; 12.9 | |
| 13-cis-crocin DBcCROC (aglycone) | CH-10 | 7.51 * | d: 11.8 | |
| CH-10′ | 7.44 | |||
| CH-11,11′ | 6.68 | |||
| CH-12 | 7.34 | d: 14.8 | ||
| CH-12′ | 6.76 | |||
| CH-14 | 6.37 | d: 12.2 | ||
| CH-14′ | 6.50 | |||
| CH-15 | 7.03 | dd: 13.4; 12.8 | ||
| CH-15′ | 6.74 | |||
| (β-d-gentiobiosyl) (GB-CROC) | CH-1 | 5.54 * | d: 7.7 | 95.9 |
| CH-2 | 3.45 | 77.8 | ||
| CH-3,4,5 | 3.56–3.39 | |||
| CH2-6 | 4.17 | dd: 11.5; 2 | 69.5 | |
| 3.78 | 69.5 | |||
| CH-1′ | 4.33 | d: 7.8 | 104.5 | |
| CH-2′ | 3.23 | 75.1 | ||
| CH-3′ | 3.34 | |||
| CH2-6′,6′ | 3.85 | m | 62.7 | |
| 3.66 | m | 62.7 | ||
| (β-d-glucosyl) | CH-1 | 5.56 * | d: 7.7 | 96.0 |
| (βGLC-CROC) | CH2-6, 6 | 3.84; 3.69 | 62.7 | |
| Picrocrocin (PCROC) (aglycone) | C-1 | 141.1 | ||
| C-2 | 155.6 | |||
| CH2-3,3 | 2.69 | ddd: 18.7; 5.5; 2.2 | 42.4 | |
| 2.31 | ddm: 18.7; 9 | 42.4 | ||
| CH-4 | 4.09 | m | 72.0 | |
| CH2-5,5 | 1.87 | ddd: 12.6; 3.3; 2.2 | 48.3 | |
| 1.55 | t: 12.2 | 48.3 | ||
| C-6 | 36.6 | |||
| CH3-7,8 | 1.23 | s | 27.9 | |
| CH3-7,8 | 1.24 | s | 29.3 | |
| CH3-9 | 2.15 * | d < 1 Hz | 19.3 | |
| CHO-10 | 10.09 | s | ||
| (β-d-glucosyl) | CH-1′ | 4.44 | d: 7.8 | 102.6 |
| CH-2′ | 3.16 | dd: 9.2; 7.8 | 75.2 | |
| CH-3′ | 3.35 | m | 78.1 | |
| CH-4′ | 3.28 | m | 78.0 | |
| CH-5′ | 3.29 | m | 71.7 | |
| CH2-6′,6′ | 3.67 | m | 62.7 | |
| 3.86 | t: 11.2 | |||
| α-Glucose αGLC | CH-1 | 5.10 * | d: 3.7 | 94.0 |
| CH-2 | 3.35 | dd: 9.6; 3.7 | 73.9 | |
| CH-3 | 3.67 | dd: 9.6; 9.0 | 74.9 | |
| CH-4 | 3.30 | m | 71.9 | |
| CH-5 | 3.77 | 73.0 | ||
| CH2-6, 6 | 3.78; 3.69 | 62.8 | ||
| β-Glucose βGLC | CH-1 | 4.47 | d: 7.8 | 98.3 |
| CH-2 | 3.12 * | dd: 9.2; 7.8 | 76.3 | |
| CH-3 | 3.33 | 78.2 | ||
| CH-5 | 3.27 | 78.1 | ||
| CH2-6,6 | 3.85; 3.65 | 62.9 |

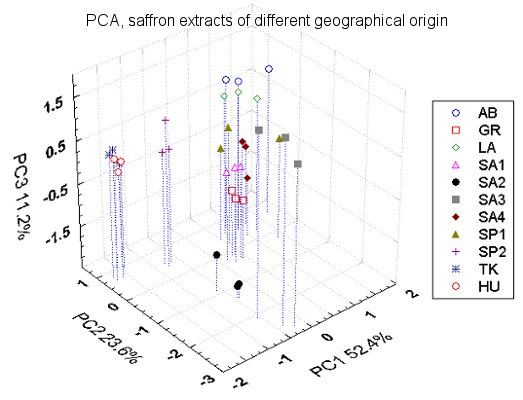
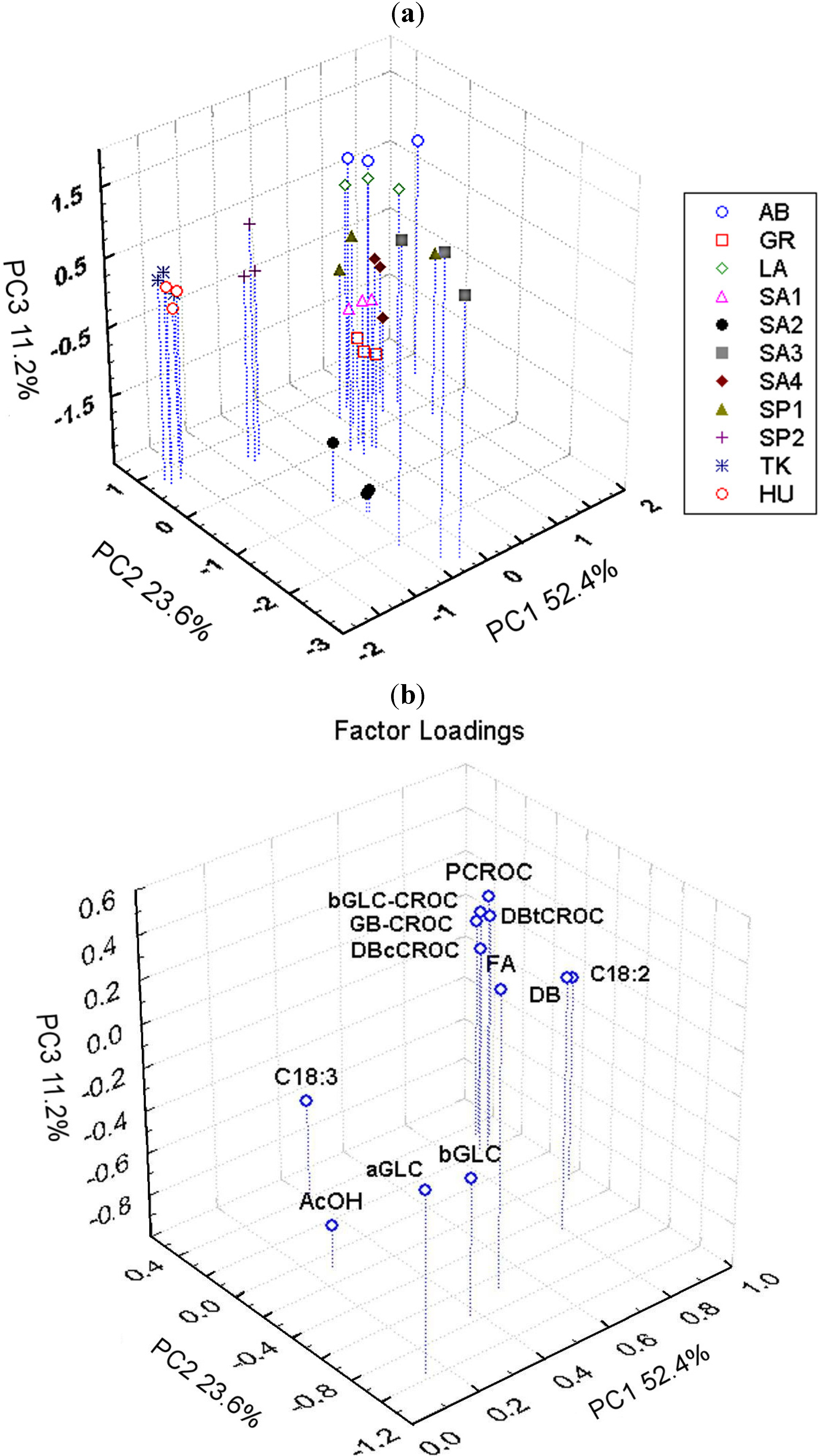
3.3. Comparison between Saffron Samples of Different Geographical Origin

| Sample code | DBtCROC/DBcCROC |
|---|---|
| AB | 16.0 |
| GR | 18.7 |
| LA | 54.2 |
| SA1 | 22.8 |
| SA2 | 19.8 |
| SA3 | 12.8 |
| SA4 | 17.3 |
| SP1 | 17.3 |
| SP2 | 30.2 |
| TK | n.d. |
| HU | n.d. |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; N’Da, C.I.; Gidaro, M.C.; Costa, G.; et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical applications of Saffron (Crocus sativus) and its constituents: A review. Drug Res. (Stuttg.) 2014, in press. [Google Scholar]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, in press. [Google Scholar]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014, 6, 99–107. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Kim, S.C.; Park, C.B.; Lee, P.C. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem. Cell Biol. 2014, 92, 105–111. [Google Scholar]
- Maggi, L.; Carmona, M.; del Campo, C.P.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Worldwide market screening of saffron volatile composition. J. Sci. Food Agric. 2009, 89, 1950–1954. [Google Scholar] [CrossRef]
- Zalacain, A.; Ordoudi, S.A.; Díaz-Plaza, E.M.; Carmona, M.; Blázquez, I.; Tsimidou, M.Z.; Alonzo, G.L. Near-Infrared spectroscopy in saffron quality control: Determination of chemical composition and geographical origin. J. Agric. Food Chem. 2005, 53, 9337–9341. [Google Scholar]
- Zougagh, M.; Ríos, A.; Valcárcel, M. Determination of total safranal by in situ acid hydrolysis in supercritical fluid media: Application to the quality control of commercial saffron. Anal. Chim. Acta 2006, 578, 117–121. [Google Scholar] [CrossRef]
- Zougagh, M.; Simonet, B.M.; Ríos, A.; Valcárcel, M. Use of non-aqueous capillary electrophoresis for the quality control of commercial saffron samples. J. Chromatogr. A 2005, 1085, 293–298. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Kelly, S.D.; Marigheto, N.; Alonso, G.L. Geographical origin differentiation of saffron spice (Crocus sativus L. stigmas)—Preliminary investigation using chemical and multi-element (H, C, N) stable isotope analysis. Food Chem. 2011, 128, 543–548. [Google Scholar]
- Yilmaz, A.; Nyberg, N.T.; Mølgaard, P.; Asili, J.; Jaroszewski, J.W. 1H NMR metabolic fingerprinting of saffron extracts. Metabolomics 2010, 6, 511–517. [Google Scholar]
- International Organization for Standardization (ISO). Saffron (Crocus sativus Linnaeus). Pt 1: Specifications. Pt 2: Test Methods; ISO: Geneva, Switzerland, 1993. [Google Scholar]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar]
- Koulakiotis, N.S.; Pittenauer, E.; Halabalaki, M.; Tsarbopoulos, A.; Allmaier, G. Comparison of different tandem mass spectrometric techniques (ESI-IT, ESI- and IP-MALDI-QRTOF and vMALDI-TOF/RTOF) for the analysis of crocins and picrocrocin from the stigmas of Crocus sativus L. Rapid Commun. Mass Spectrom. 2012, 26, 670–678. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Progr. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; di Cocco, M.; Capuani, G.; de Salvador, F.R.; Delfini, M. Monitoring of metabolic profiling and water status of Hayward kiwifruits by nuclear magnetic resonance. Talanta 2010, 82, 1826–1838. [Google Scholar] [CrossRef]
- Capitani, D.; Sobolev, A.P.; Tomassini, A.; Sciubba, F.; de Salvador, F.R.; Mannina, L.; Delfini, M. Peach fruit: Metabolic comparative analysis of two varieties with different resistances to insect attacks by NMR spectroscopy. J. Agric. Food Chem. 2013, 61, 1718–1726. [Google Scholar] [CrossRef]
- Capitani, D.; Sobolev, A.P.; Delfini, M.; Vista, S.; Antiochia, R.; Proietti, N.; Bubici, S.; Ferrante, G.; Carradori, S.; de Salvador, F.R.; Mannina, L. NMR methodologies in the analysis of blueberries. Electrophoresis 2014, 35, 1615–1626. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P. High resolution NMR characterization of olive oils in terms of quality, authenticity and geographical origin. Magn. Reson. Chem. 2011, 49, S3–S11. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444. [Google Scholar] [CrossRef]
- Savorani, F.; Picone, G.; Badiani, A.; Fagioli, P.; Capozzi, F.; Engelsen, S.B. Metabolic profiling and aquaculture differentiation of gilthead sea bream by 1H NMR metabonomics. Food Chem. 2010, 120, 907–914. [Google Scholar] [CrossRef]
- Straubinger, M.; Bau, B.; Eckstein, S.; Fink, M.; Winterhalter, P. Identification of novel glycosidic aroma precursors in saffron (Crocus sativus L.). J. Agric. Food Chem. 1998, 46, 3238–3243. [Google Scholar] [CrossRef]
- Assimiadis, M.A.; Tarantilis, P.A.; Polissiou, M.G. UV-Vis, FT-Raman, and 1H NMR spectroscopies of cis-trans carotenoids from saffron (Crocus sativus L.). Appl. Spectros. 1998, 52, 519–522. [Google Scholar] [CrossRef]
- Van Calsteren, M.R.; Bissonnette, M.C.; Cormier, F.; Dufresne, C.; Ichi, T.; LeBlanc, J.C.Y.; Perreault, D.; Roewer, I. Spectroscopic characterization of crocetin derivatives from Crocussativus and Gardenia jasminoides. J. Agric. Food Chem. 1997, 45, 1055–1061. [Google Scholar]
- Braun, S.; Kalinowski, H.O.; Berger, S. 150 and More Basic NMR Experiments, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Mannina, L.; Cristinzio, M.; Sobolev, A.P.; Ragni, P.; Segre, A.L. High-field NMR study of truffles (Tuber aestivum vittadini). J. Agric. Food Chem. 2004, 52, 7988–7996. [Google Scholar] [CrossRef]
- Johnson, C.S., Jr. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Progr. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sobolev, A.P.; Carradori, S.; Capitani, D.; Vista, S.; Trella, A.; Marini, F.; Mannina, L. Saffron Samples of Different Origin: An NMR Study of Microwave-Assisted Extracts. Foods 2014, 3, 403-419. https://doi.org/10.3390/foods3030403
Sobolev AP, Carradori S, Capitani D, Vista S, Trella A, Marini F, Mannina L. Saffron Samples of Different Origin: An NMR Study of Microwave-Assisted Extracts. Foods. 2014; 3(3):403-419. https://doi.org/10.3390/foods3030403
Chicago/Turabian StyleSobolev, Anatoly P., Simone Carradori, Donatella Capitani, Silvia Vista, Agata Trella, Federico Marini, and Luisa Mannina. 2014. "Saffron Samples of Different Origin: An NMR Study of Microwave-Assisted Extracts" Foods 3, no. 3: 403-419. https://doi.org/10.3390/foods3030403