Effect of Technological Treatments on Human-Like Leptin Level in Bovine Milk for Human Consumption
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples and Analyses
2.2. Statistical Analysis
3. Results
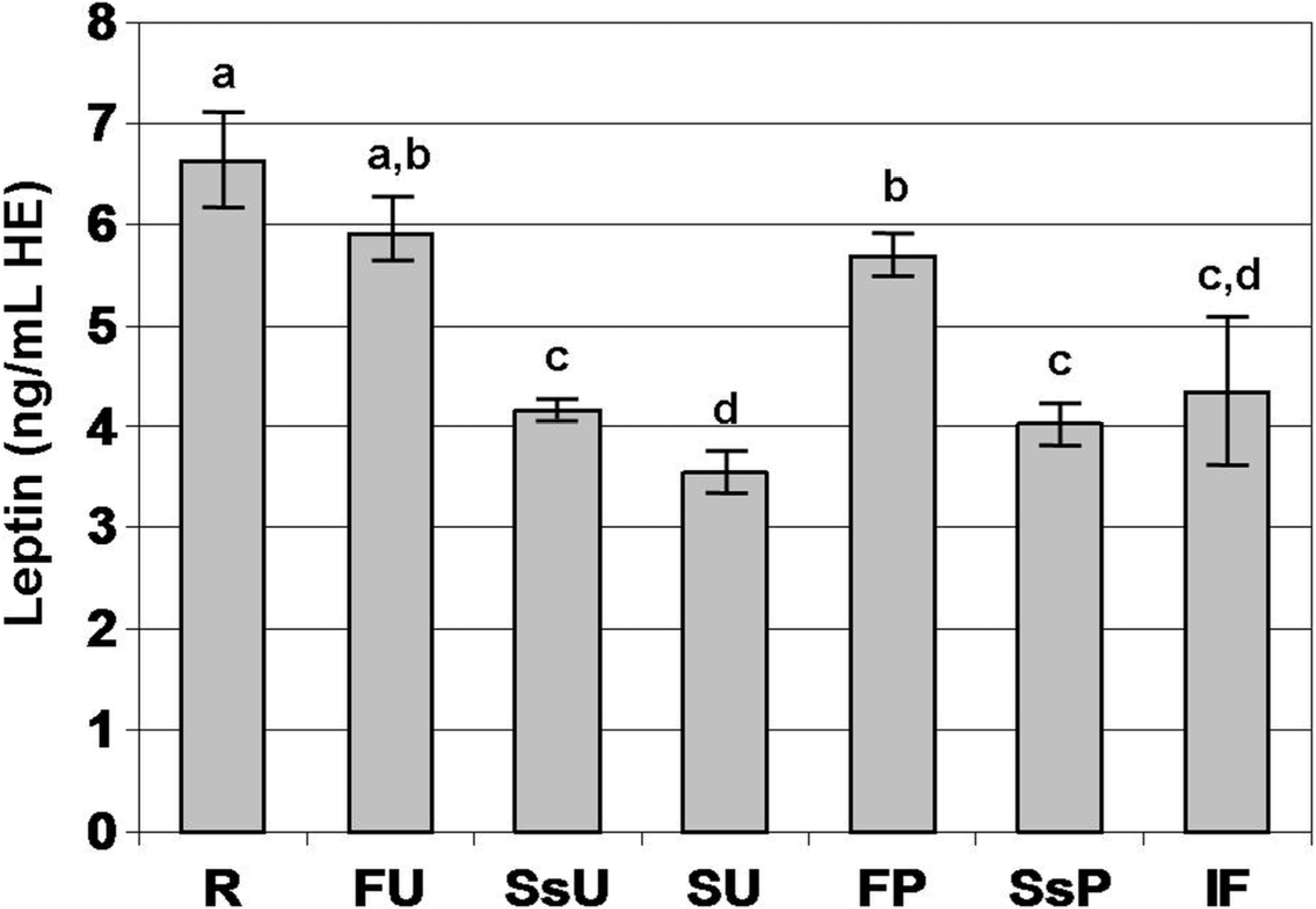
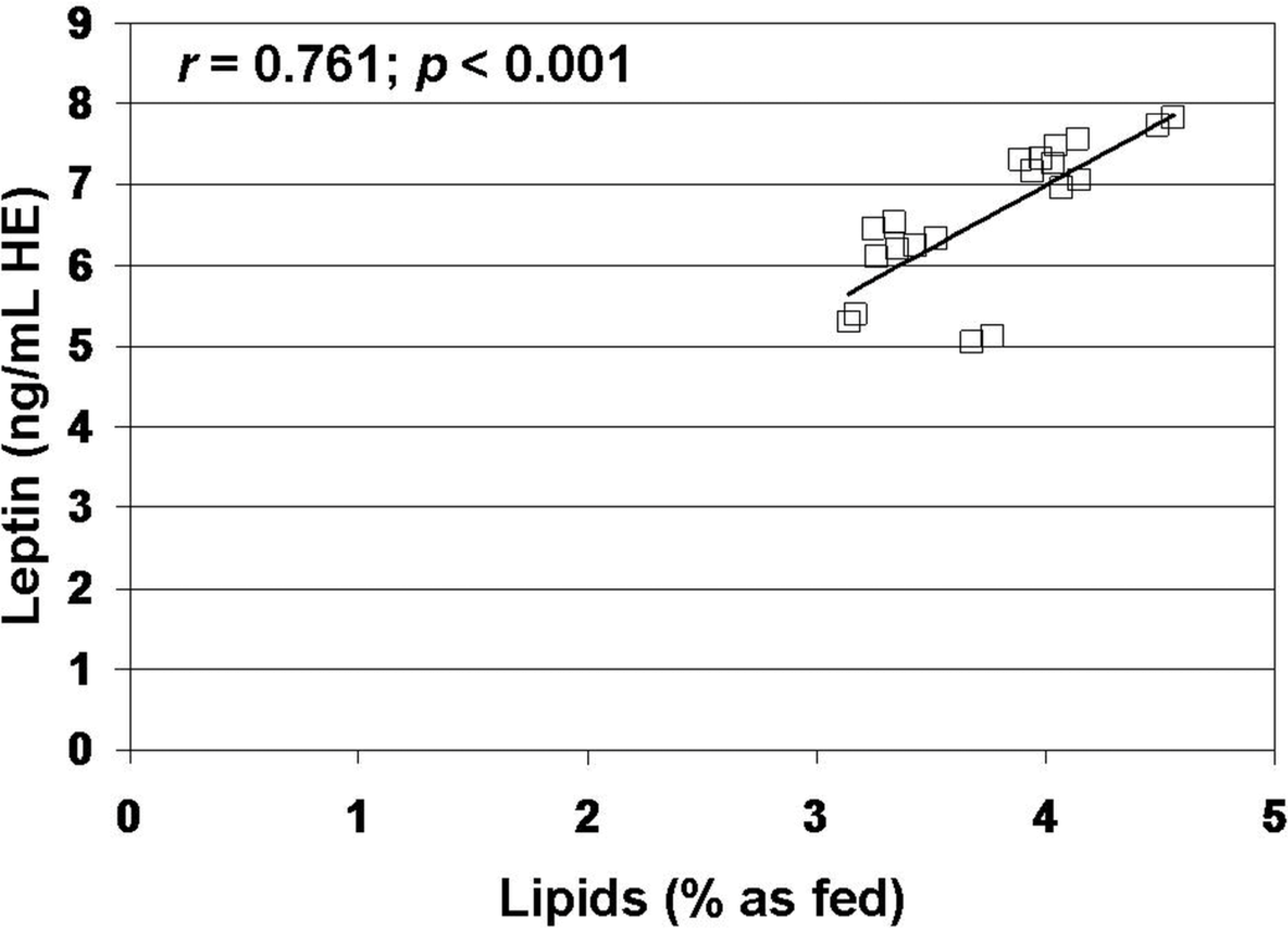
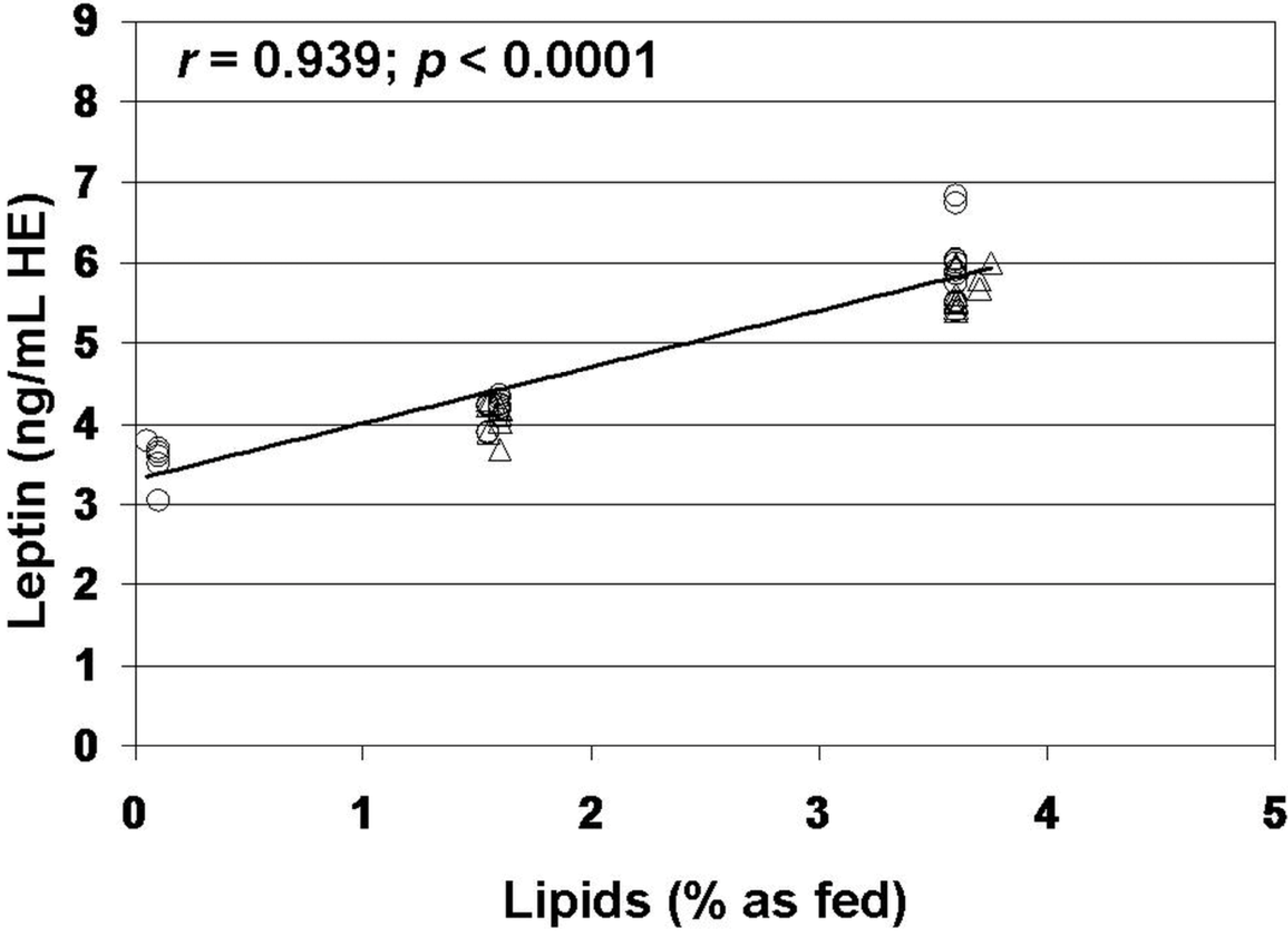
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ahima, R.S.; Flier, J.S. Leptin. Ann. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Vitali, A.; Magistrelli, D.; Azevedo, J.; Bernabucci, U.; Ronchi, B.; Rosi, F. Leptin and puberty in goat. Ital. J. Anim. Sci. 2005, 4, 383–385. [Google Scholar]
- Caprio, M.; Fabbrini, E.; Isidori, A.M.; Aversa, A.; Fabbri, A. Leptin in reproduction. Trends Endocrinol. Metab. 2001, 12, 65–72. [Google Scholar] [CrossRef]
- Casabiell, X.; Piñeiro, V.; Tomé, M.A.; Peinó, R.; Diéguez, C.; Casanueva, F.F. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metab. 1997, 82, 4270–4273. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; McGuire, M.K.; Portocarrero, C.P.; McGuire, M.A.; Beerman, K. Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem. Biophys. Res. Commun. 1997, 240, 742–747. [Google Scholar] [CrossRef]
- Smith-Kirwin, S.M.; O’Connor, D.M.; de Johnston, J.; Lancey, E.D.; Hassink, S.G.; Funanage, V.L. Leptin expression in human mammary epithelial cells and breast milk. J. Clin. Endocrinol. Metab. 1998, 83, 1810–1813. [Google Scholar] [CrossRef]
- Bonnet, M.; Delavaud, C.; Laud, K.; Gourdou, I.; Leroux, C.; Djiane, J.; Chilliard, Y. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod. Nutr. Dev. 2002, 42, 399–413. [Google Scholar] [CrossRef]
- Salimei, E.; Varisco, G.; Rosi, F. Major constituents, leptin, and non-protein nitrogen compounds in mares’ colostrum and millk. Reprod. Nutr. Dev. 2002, 42, 65–72. [Google Scholar] [CrossRef]
- Pinotti, L.; Rosi, F. Leptin in bovine colostrum and milk. Horm. Metab. Res. 2006, 38, 89–93. [Google Scholar] [CrossRef]
- Salimei, E.; Rosi, F.; Maglieri, C.; Magistrelli, D.; Fantuz, F. Leptin in milk and plasma of dairy asses. Ital. J. Anim. Sci. 2007, 6, 654–656. [Google Scholar]
- Magistrelli, D.; Polo Dimel, G.; Rosi, F. Leptin, insulin and ghrelin levels in goat milk and in plasma of suckling kids. Small Rumin. Res. 2008, 79, 38–41. [Google Scholar] [CrossRef]
- Rosi, F.; Aufy, A.A.; Magistrelli, D. Diet influences the content of bioactive peptides in goat milk. J. Endocrinol. Investig. 2009, 32, 486–490. [Google Scholar]
- Sánchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Picó, C.; Palou, A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005, 146, 2575–2582. [Google Scholar] [CrossRef]
- Wolinski, J.; Biernat, M.; Guilloteau, P.; Weström, B.R.; Zabielski, R. Exogenous leptin controls the development of the small intestine in neonatal piglets. J. Endocrinol. 2003, 177, 215–222. [Google Scholar] [CrossRef]
- Miralles, O.; Sánchez, J.; Palou, A.; Picó, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity 2006, 14, 1371–1377. [Google Scholar] [CrossRef]
- Palou, A.; Picó, C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite 2009, 52, 249–252. [Google Scholar] [CrossRef]
- Mix, H.; Widjaja, A.; Jandl, O.; Cornberg, M.; Kaul, A.; Göke, M.; Beil, W.; Kuske, M.; Brabant, G.; Manns, M.P.; Wagner, S. Expression of leptin and leptin receptor isoforms in the human stomach. Gut 2000, 47, 481–486. [Google Scholar] [CrossRef]
- Cammisotto, P.G.; Renaud, C.; Gingras, D.; Delvin, E.; Levy, E.; Bendayan, M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J. Histochem. Cytochem. 2005, 53, 851–860. [Google Scholar]
- Barrenetxe, J.; Villaro, A.C.; Guembe, L.; Pascual, I.; Muñoz-Navas, M.; Barber, A.; Lostao, M.P. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocyte. Gut 2002, 50, 797–802. [Google Scholar]
- Cammisotto, P.G.; Gingras, D.; Bendayan, M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G773–G779. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk—The promoter of chronic Western diseases. Med. Hypotheses 2009, 72, 631–639. [Google Scholar]
- Lage, M.; Baldelli, R.; Camiña, J.P.; Rodriguez-Garcia, J.; Peñalva, A.; Dieguez, C.; Casanueva, F.F. Presence of bovine leptin in edible commercial milk and infant formula. J. Endocrinol. Investig. 2002, 25, 670–674. [Google Scholar]
- Savino, F.; Benetti, S.; Liguori, S.A.; Sorrenti, M.; Cordero di Montezemolo, L. Advances on human milk hormones and protection against obesity. Cell. Mol. Biol. 2013, 59, 89–98. [Google Scholar]
- Available online: http://www.apa.cn.it/Legislazione/Latte/DPR54.pdf (accessed on 2 April 2014).
- Peri, C.; Zanoni, B. Manuale di Tecnologie Alimentari; CUSL: Milan, Italy, 2003. [Google Scholar]
- Lönnerdal, B.; Havel, P.J. Serum leptin concentrations in infants: Effects of diet, sex, and adiposity. Am. J. Clin. Nutr. 2000, 72, 484–489. [Google Scholar]
- Ji, S.; Willis, G.M.; Scott, R.R.; Spurlock, M.E. Partial cloning and expression of the bovine leptin gene. Anim. Biotechnol. 1998, 9, 1–14. [Google Scholar] [CrossRef]
- Chemineau, P.; Malpaux, B.; Brillard, J.P.; Fostier, A. Seasonality of reproduction and production in farm fishes, birds and mammals. Animal 2007, 1, 419–432. [Google Scholar] [CrossRef]
- Ehrhardt, R.A.; Slepetis, R.M.; Siegal-Willott, J.; van Amburgh, M.E.; Bell, A.W.; Boisclair, Y.R. Development of a specific radioimmunoassay to measure physiological changes of circulating leptin in cattle and sheep. J. Endocrinol. 2000, 166, 519–528. [Google Scholar] [CrossRef]
- Delavaud, C.; Ferlay, A.; Faulconnier, Y.; Bocquier, F.; Kann, G.; Chilliard, Y. Plasma leptin concentration in adult cattle: Effects of breed, adiposity, feeding level, and meal intake. J. Anim. Sci. 2002, 80, 1317–1328. [Google Scholar]
- Tokuda, T.; Delavaud, C.; Chilliard, Y. Plasma leptin concentration in pre- and post-weaning lambs. Anim. Sci. 2003, 76, 221–227. [Google Scholar]
- Rosi, F.; Pinotti, L.; Campagnoli, A.; Magistrelli, D. Leptin distribution in goat milk fractions. In Book of Abstract of the 54th Annual Meeting of the European Association for Animal Production; van der Honing, Y., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2003; p. 221. [Google Scholar]
- O’Connor, D.; Funanage, V.; Locke, R.; Spear, M.; Leef, K. Leptin is not present in infant formulas. J. Endocrinol. Investig. 2003, 26, 490. [Google Scholar] [CrossRef]
- Resto, M.; O’Connor, D.; Leef, K.; Funanage, V.; Spear, M.; Locke, R. Leptin levels in preterm human breast milk and infant formula. Pediatrics 2001, 108, E15. [Google Scholar] [CrossRef]
- Manzi, P.; di Costanzo, M.G.; Mattera, M. Updating nutritional data and evaluation of technological parameters of Italian milk. Foods 2013, 2, 254–273. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Magistrelli, D.; Rosi, F. Effect of Technological Treatments on Human-Like Leptin Level in Bovine Milk for Human Consumption. Foods 2014, 3, 433-442. https://doi.org/10.3390/foods3030433
Magistrelli D, Rosi F. Effect of Technological Treatments on Human-Like Leptin Level in Bovine Milk for Human Consumption. Foods. 2014; 3(3):433-442. https://doi.org/10.3390/foods3030433
Chicago/Turabian StyleMagistrelli, Damiano, and Fabia Rosi. 2014. "Effect of Technological Treatments on Human-Like Leptin Level in Bovine Milk for Human Consumption" Foods 3, no. 3: 433-442. https://doi.org/10.3390/foods3030433



