A Theoretical Analysis for Assessing the Variability of Secondary Model Thermal Inactivation Kinetic Parameters
Abstract
:1. Introduction
2. Materials and Methods
Monte Carlo Simulation
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taoukis, P.S.; Tsironi, T.S.; Giannakourou, M.C. Reaction Kinetics. In Handbook of Food Processing and Engineering, Volume I: Food Engineering Fundamentals; Tzia, K., Varzakas, T., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 529–570. [Google Scholar]
- Van Boekel, M.A.J.S.; Tijskens, L.M.M. Kinetic modeling. In Food Process Modeling; Tijskens, L.M.M., Hertog, M.L.A.T.M., Nicolai, B.M., Eds.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Villota, R.; Hawkes, J.G. Reaction kinetics in food systems. In Handbook of Food Engineering, 2nd ed.; Heldman, D.R., Lund, D.B., Eds.; CRC Press: New York, NY, USA, 2007; pp. 125–287. [Google Scholar]
- Van Boekel, M.A.J.S. Statistical aspects of kinetic modeling for food science problems. J. Food Sci. 1996, 61, 477–486. [Google Scholar] [CrossRef]
- Angelidis, A.S.; Papageorgiou, D.K.; Tyrovouzis, N.A.; Stoforos, N.G. Kinetics of Listeria monocytogenes cell reduction in processed cheese during storage. Food Control 2013, 29, 18–21. [Google Scholar] [CrossRef]
- Dermesonluoglu, E.; Katsaros, G.; Tsevdou, M.; Giannakourou, M.; Taoukis, P. Kinetic study of quality indices and shelf life modelling of frozen spinach under dynamic conditions of the cold chain. J. Food Eng. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Huang, L. Dynamic computer simulation of Clostridium perfringens growth in cooked ground beef. Int. J. Food Microbiol. 2003, 87, 217–227. [Google Scholar] [CrossRef]
- Huang, L. Numerical analysis of the growth of Clostridium perfringens in cooked beef under isothermal and dynamic conditions. J. Food Saf. 2004, 24, 53–70. [Google Scholar] [CrossRef]
- Huang, L.; Vinyard, B.T. Direct dynamic kinetic analysis and computer simulation of growth of Clostridium perfringens in cooked turkey during cooling. J. Food Sci. 2016, 81, M692–M701. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V.M. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.L.; Farid, M.; Silva, F.V.M. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Kinetic modelling of vitamin C loss in frozen green vegetables under variable storage conditions. Food Chem. 2003, 83, 33–41. [Google Scholar] [CrossRef]
- Ávila, I.M.L.B.; Silva, C.L.M. Modelling kinetics of thermal degradation of colour in peach puree. J. Food Eng. 1999, 39, 161–166. [Google Scholar] [CrossRef]
- Bai, J.W.; Gao, Z.J.; Xiao, H.W.; Wang, X.T.; Zhang, Q. Polyphenol oxidase inactivation and vitamin C degradation kinetics of Fuji apple quarters by high humidity air impingement blanching. Int. J. Food Sci. Technol. 2013, 48, 1135–1141. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Changes in quality attributes during storage of high-pressure and thermally processed pineapple puree. Food Bioprocess Technol. 2016, 9, 768–791. [Google Scholar] [CrossRef]
- Colle, I.J.P.; Lemmens, L.; Tolesa, G.N.; Van Buggenhout, S.; De Vleeschouwer, K.; Van Loey, A.M.; Hendrickx, M.E. Lycopene degradation and isomerization kinetics during thermal processing of an olive oil/tomato emulsion. J. Agric. Food Chem. 2010, 58, 12784–12789. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, L.F.; Fernandes, F.A.N.; Magalhães, M.M.A.; Brito, E.S. Non-enzymatic browning in clarified cashew apple juice during thermal treatment: Kinetics and process control. Food Chem. 2008, 106, 172–179. [Google Scholar] [CrossRef]
- Isleroglu, H.; Kemerli, T.; Sakin-Yilmazer, M.; Guven, G.; Ozdestan, O.; Uren, A.; Kaymak-Ertekin, F. Effect of steam baking on acrylamide formation and browning kinetics of cookies. J. Food Sci. 2012, 77, E257–E263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, H.; Lu, H. Use of linear and weibull functions to model ascorbic acid degradation in Chinese winter jujube during postharvest storage in light and dark conditions. J. Food Process. Preserv. 2014, 38, 856–863. [Google Scholar] [CrossRef]
- Kreyenschmidt, J.; Hübner, A.; Beierle, E.; Chonsch, L.; Scherer, A.; Petersen, B. Determination of the shelf life of sliced cooked ham based on the growth of lactic acid bacteria in different steps of the chain. J. Appl. Microbiol. 2010, 108, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L.; De Vleeschouwer, K.; Moelants, K.R.N.; Colle, I.J.P.; Van Loey, A.M.; Hendrickx, M.E. β-Carotene isomerization kinetics during thermal treatments of carrot puree. J. Agric. Food Chem. 2010, 58, 6816–6824. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Drosinos, E.H.; Vaidanis, A.; Metaxopoulos, I. Development of a predictive model for spoilage of cooked cured meat products and its validation under constant and dynamic temperature storage conditions. J. Food Sci. 2006, 71, M157–M167. [Google Scholar] [CrossRef]
- Mohebbi, M.; Hasanpour, N.; Ansarifar, E.; Amiryousefi, M.R. Physicochemical properties of bell pepper and kinetics of its color change influenced by Aloe vera and gum tragacanth coatings during storage at different temperatures. J. Food Process. Preserv. 2014, 38, 684–693. [Google Scholar] [CrossRef]
- Ndoye, F.T.; Alvarez, G. Characterization of ice recrystallization in ice cream during storage using the focused beam reflectance measurement. J. Food Eng. 2015, 148, 24–34. [Google Scholar] [CrossRef]
- Nisha, P.; Singhal, R.S.; Pandit, A.B. Kinetic modelling of texture development in potato cubes (Solanum tuberosum L.), green gram whole (Vigna radiate L.) and red gram splits (Cajanus cajan L.). J. Food Eng. 2006, 76, 524–530. [Google Scholar] [CrossRef]
- Roberts, J.S.; Tong, C.H. Drying kinetics of hygroscopic porous materials under isothermal conditions and the use of a first-order reaction kinetic model for predicting drying. Int. J. Food Prop. 2003, 6, 355–367. [Google Scholar] [CrossRef]
- Schmitz-Schug, I.; Kulozik, U.; Foerst, P. Reaction kinetics of lysine loss in a model dairy formulation as related to the physical state. Food Bioprocess Technol. 2014, 7, 877–886. [Google Scholar] [CrossRef]
- Tsironi, T.; Dermesonlouoglou, E.; Giannakourou, M.; Taoukis, P. Shelf life modelling of frozen shrimp at variable temperature conditions. LWT-Food Sci. Technol. 2009, 42, 664–671. [Google Scholar] [CrossRef]
- Aspridou, Z.; Koutsoumanis, K.P. Individual cell heterogeneity as variability source in population dynamics of microbial inactivation. Food Microbiol. 2015, 45, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Dynamic determination of kinetic parameters, computer simulation, and probabilistic analysis of growth of Clostridium perfringens in cooked beef during cooling. Int. J. Food Microbiol. 2015, 195, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Angelidis, A.S. Probabilistic modeling approach for evaluating the compliance of ready-to-eat foods with new European union safety criteria for Listeria monocytogenes. Appl. Environ. Microb. 2007, 73, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Lianou, A.; Koutsoumanis, K.P. A stochastic approach for integrating strain variability in modeling Salmonella enterica growth as a function of pH and water activity. Int. J. Food Microbiol. 2011, 149, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Channon, H.A.; Hamilton, A.J.; D’Souza, D.N.; Dunshea, F.R. Estimating the impact of various pathway parameters on tenderness, flavour and juiciness of pork using Monte Carlo simulation methods. Meat Sci. 2016, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Evrendilek, G.A.; Avsar, Y.K.; Evrendilek, F. Modelling stochastic variability and uncertainty in aroma active compounds of PEF-treated peach nectar as a function of physical and sensory properties, and treatment time. Food Chem. 2016, 190, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.C.; Koutsoumanis, K.; Dermesonlouoglou, E.; Taoukis, P.S. Applicability of the shelf life decision system (slds) for control of nutritional quality of frozen vegetables. Acta Hortic. 2001, 566, 275–280. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Application of a TTI-based distribution management system for quality optimization of frozen vegetables at the consumer end. J. Food Sci. 2003, 68, 201–209. [Google Scholar] [CrossRef]
- Sui, X.; Zhou, W. Monte Carlo modelling of non-isothermal degradation of two cyanidin-based anthocyanins in aqueous system at high temperatures and its impact on antioxidant capacities. Food Chem. 2014, 148, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wesolek, N.; Roudot, A.C. Assessing aflatoxin B1 distribution and variability in pistachios: Validation of a Monte Carlo modeling method and comparison to the Codex method. Food Control 2016, 59, 553–560. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall/CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Poschet, F.; Bernaerts, K.; Geeraerd, A.H.; Scheerlinck, N.; Nicolai, B.M.; Van Impe, J.F. Sensitivity analysis of microbial growth parameter distributions with respect to data quality and quantity by using Monte Carlo analysis. Math. Comput. Simul. 2004, 65, 231–243. [Google Scholar] [CrossRef]
- Poschet, F.; Geeraerd, A.H.; Van Loey, A.M.; Hendrickx, M.E.; Van Impe, J.F. Assessing the optimal experiment setup for first order kinetic studies by Monte Carlo analysis. Food Control 2005, 16, 873–882. [Google Scholar] [CrossRef]
- Poschet, F.; Geeraerd, A.H.; Scheerlinck, N.; Nicolai, M.B.; Van Impe, J.F. Monte Carlo analysis as a tool to incorporate variation on experimental data in predictive microbiology. Food Microbiol. 2003, 20, 285–295. [Google Scholar] [CrossRef]
- Jiménez, N.; Bohuon, P.; Lima, J.; Dornier, M.; Vaillant, F.; Pérez, A.M. Kinetics of anthocyanin degradation and browning in reconstituted blackberry juice treated at high temperatures (100–180 °C). J. Agric. Food Chem. 2010, 58, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.R.; Elizondo, N.J.; Bohuon, P. Kinetics of ascorbic acid degradation and colour change in ground cashew apples treated at high temperatures (100–180 °C). Int. J. Food Sci. Technol. 2010, 45, 1724–1731. [Google Scholar] [CrossRef]
- Bigelow, W.D.; Bohart, G.S.; Richardson, A.C.; Ball, C.O. Heat Penetration in Processing Canned Foods; Bulletin 16-L; Research Laboratory, National. Canners Association: Washington, DC, USA, 1920. [Google Scholar]
- Ball, C.O. Bulletin of the National Research Council No. 37; National Research Council: Washington, DC, USA, 1923; Volume 7, Part 1. [Google Scholar]
- Lammerding, A.M.; Fazil, A. Hazard identification and exposure assessment for microbial food safety risk assessment. Int. J. Food Microbiol. 2000, 58, 147–157. [Google Scholar] [CrossRef]
- Taoukis, P.S. Modeling the use of time-temperature indicators in distribution and stock rotation. In Food Process Modeling; Tijkskens, L.M.M., Hertog, M.L.A.T.M., Nicolai, B.M., Eds.; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Prokopiou, P.; Goula, A.M.; Stoforos, N.G. Thermal inactivation kinetics of l-carnitine. In Proceedings of the 11th International Congress on Engineering and Food (ICEF 11), Athens, Greece, 22–26 May 2011; Taoukis, P.S., Stoforos, N.G., Karathanos, V.T., Saravacos, G.D., Eds.; Volume III, pp. 1809–1810.
- Pénicaud, C.; Bohuon, P.; Peyron, S.; Gontard, N.; Guillard, V. Influence of the experimental errors and their propagation on the accuracy of identified kinetics parameters: Oxygen and temperature effects on ascorbic acid oxidation during storage. Ind. Eng. Chem. Res. 2012, 51, 1131–1142. [Google Scholar] [CrossRef]
- Smid, J.H.; Verloo, D.; Barker, G.C.; Havelaar, A.H. Strengths and weaknesses of Monte Carlo simulation models and Bayesian belief networks in microbial risk assessment. Int. J. Food Microbiol. 2010, 139, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R. An Introduction to Error Analysis, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997. [Google Scholar]
- Rabinovich, S.G. Measurement Errors and Uncertainties-Theory and Practice, 3rd ed.; Springer Science and Media, Inc.: New York, NY, USA, 2005. [Google Scholar]
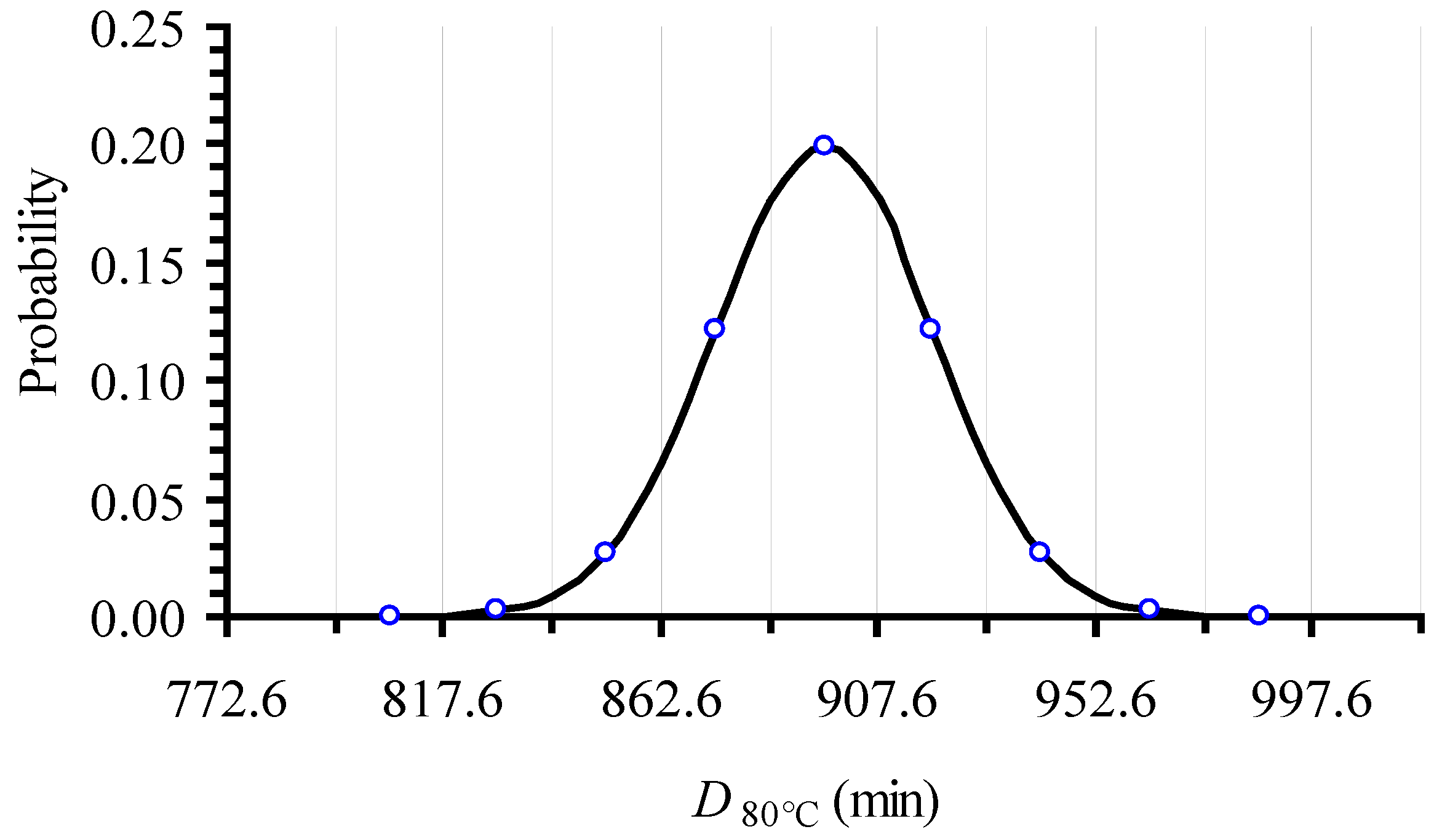
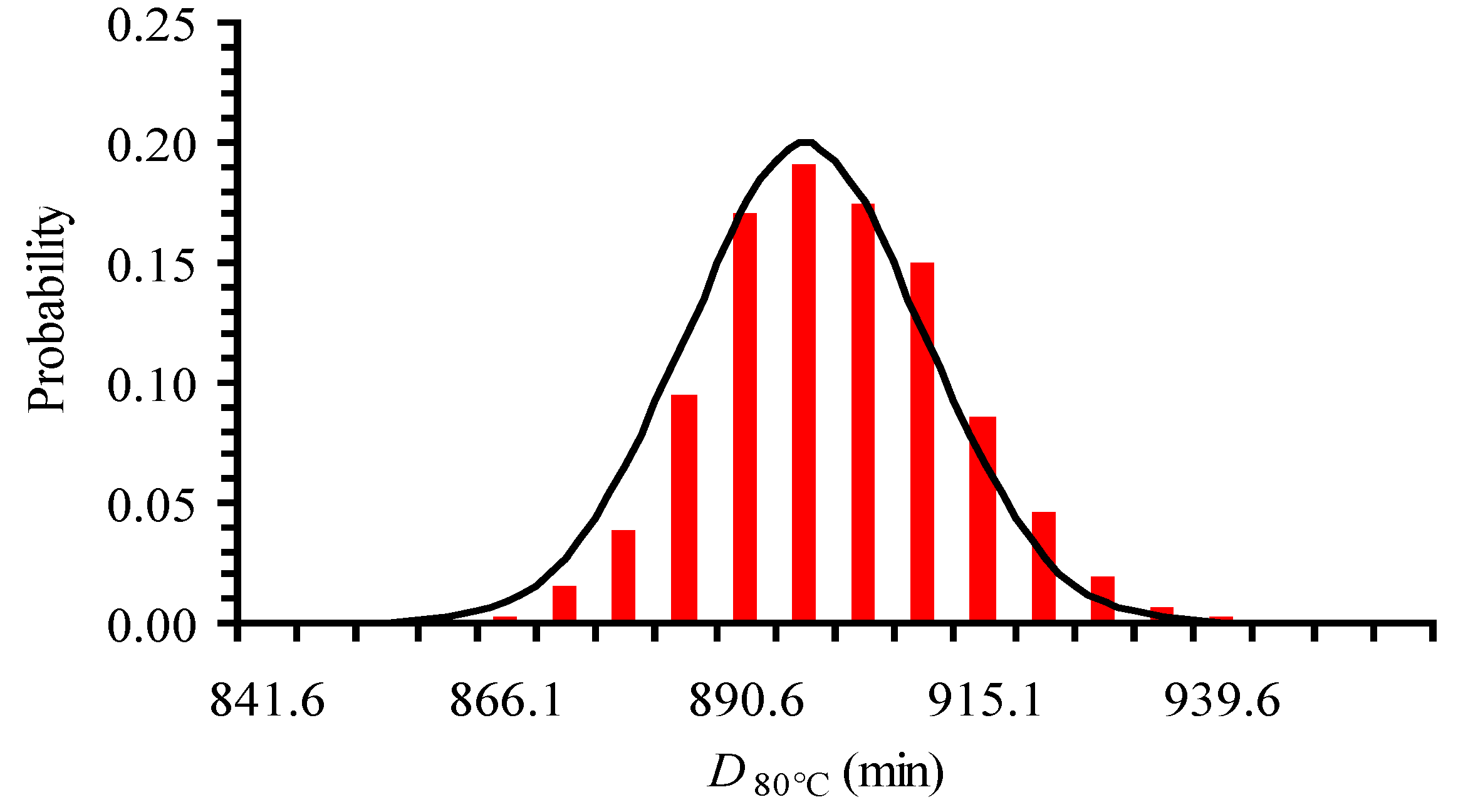

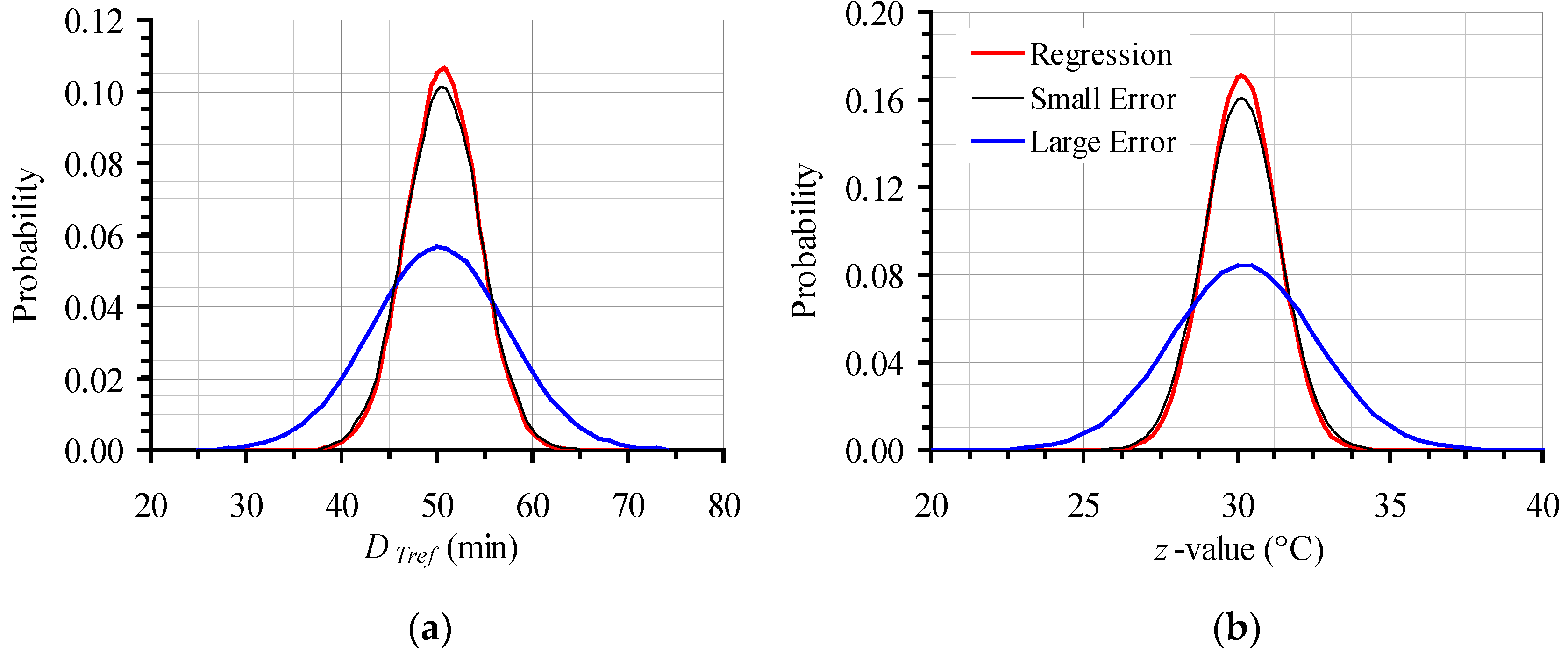
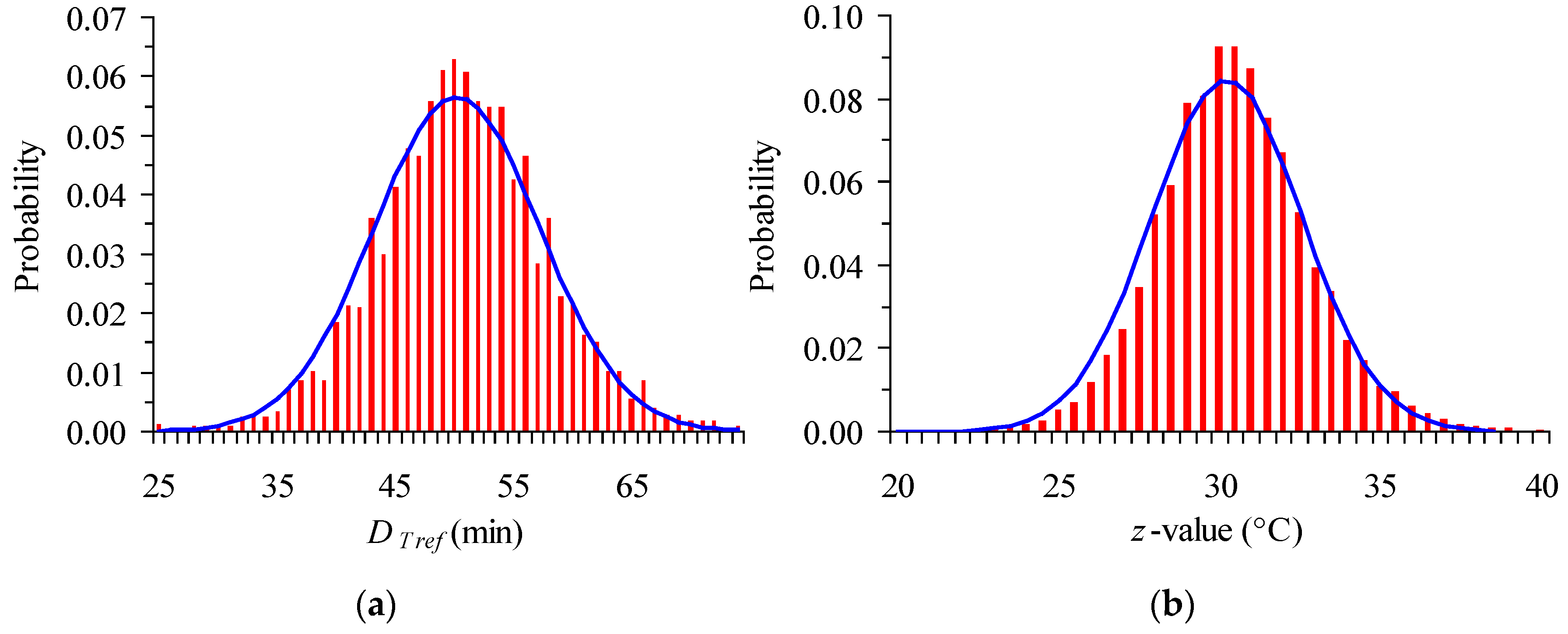

| T (°C) | From Original Data | Intentionally Expanded Error | From 500 Random Points (with the Expanded CI) | ||
|---|---|---|---|---|---|
| Mean Value | ±95% CI | ±95% CI | Mean Value | ±95% CI | |
| 80 | 896.3 | 45.0 | 267.7 | 890.8 | 262.0 |
| 85 | 728.8 | 57.2 | 230.2 | 735.8 | 223.6 |
| 90 | 573.8 | 65.0 | 196.2 | 569.1 | 192.5 |
| 95 | 384.1 | 41.0 | 123.4 | 387.4 | 121.5 |
| 100 | 238.2 | 7.6 | 59.0 | 236.4 | 57.8 |
| 110 | 105.8 | 2.4 | 24.3 | 106.3 | 24.4 |
| 120 | 47.3 | 2.2 | 13.5 | 47.1 | 13.4 |
| 130 | 23.6 | 0.6 | 4.9 | 23.5 | 5.0 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakourou, M.C.; Stoforos, N.G. A Theoretical Analysis for Assessing the Variability of Secondary Model Thermal Inactivation Kinetic Parameters. Foods 2017, 6, 7. https://doi.org/10.3390/foods6010007
Giannakourou MC, Stoforos NG. A Theoretical Analysis for Assessing the Variability of Secondary Model Thermal Inactivation Kinetic Parameters. Foods. 2017; 6(1):7. https://doi.org/10.3390/foods6010007
Chicago/Turabian StyleGiannakourou, Maria C., and Nikolaos G. Stoforos. 2017. "A Theoretical Analysis for Assessing the Variability of Secondary Model Thermal Inactivation Kinetic Parameters" Foods 6, no. 1: 7. https://doi.org/10.3390/foods6010007






