Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Bacteriocin Preparations
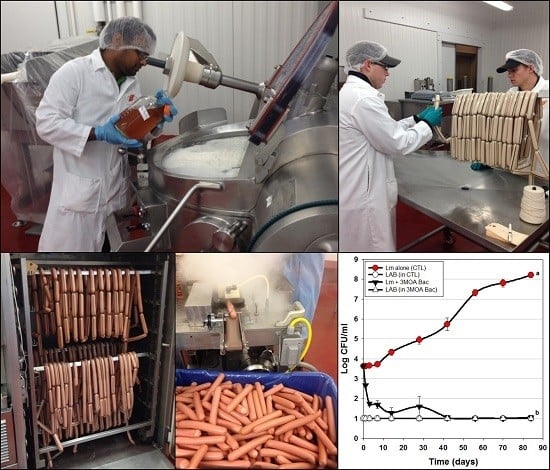
2.3. Manufacture of Hotdogs for Bacteriocin Applications
2.4. Hotdog Challenge Studies
2.4.1. Preliminary Treatment of Hotdogs Prior to Challenge Studies
2.4.2. Trial #1: Application of Mixed Mode-of-Action (MOA) Bac+ LAB Co-Inoculated with L. monocytogenes in Shelf Life Challenge Studies
2.4.3. Trial #2: Application of Mixed MOA Bacteriocin Preparations Added during the Manufacture of Hotdogs
2.4.4. Trial #3: Application of Mixed Mode-of-Action Bac+ CFS on the Surface of RTE Meats (Hotdogs)
2.4.5. Trials #4 and #5: Surface Application of Filter vs. Pasteurized Bac+ CFS and Neutralized vs. Non-Neutralized CFS in L. monocytogenes Challenge Studies on Hotdogs
2.5. Statistical Analysis
3. Results and Discussion
3.1. Trial #1: Application of Mixed MOA Bac+ LAB vs. L. monocytogenes on Hotdogs
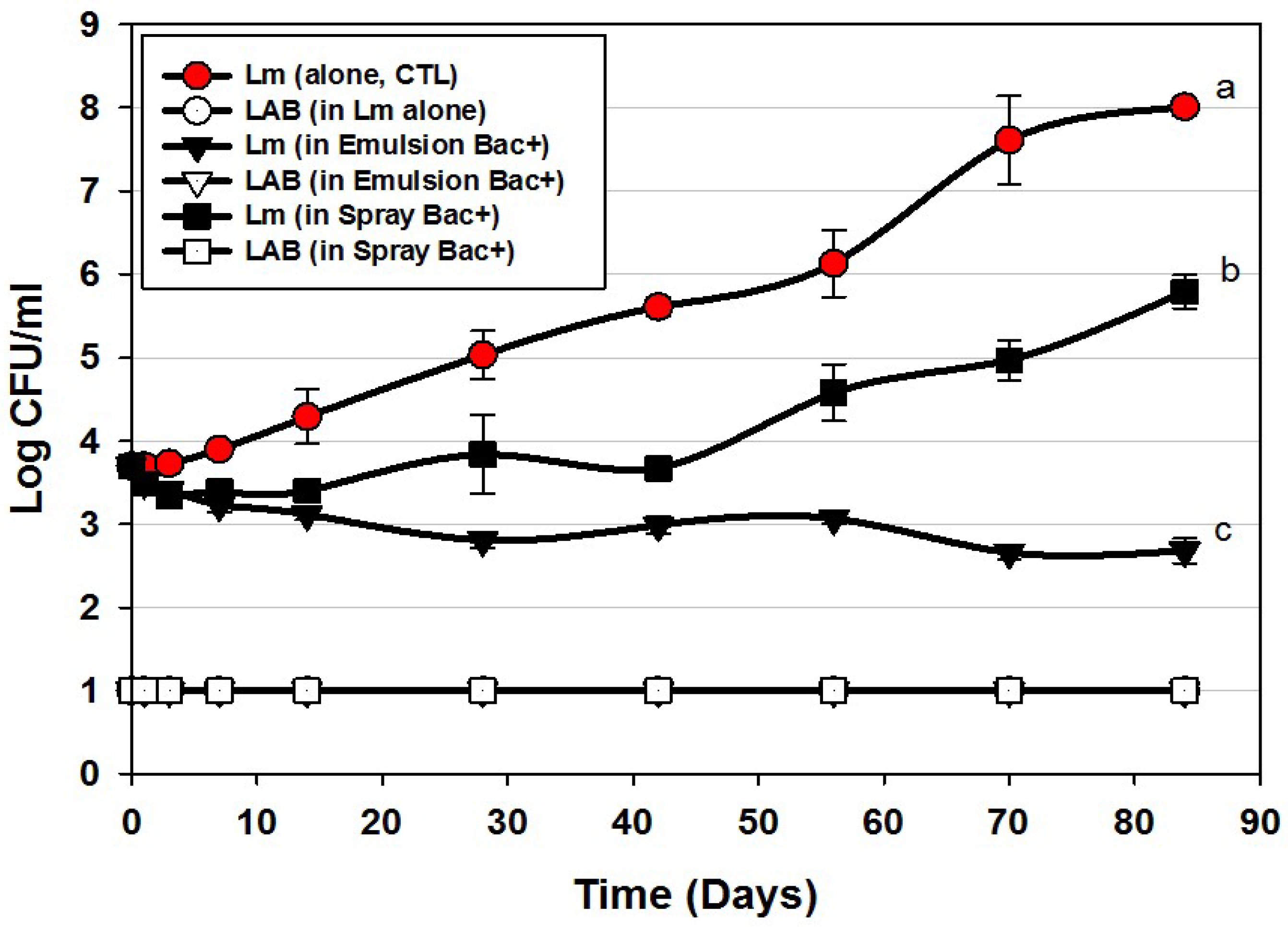
3.2. Trial #2: Listeria Monocytogenes Challenge Studies Using Hotdogs Made with Bacteriocin Extracts Added during Manufacture or Sprayed Post-Cook onto Encased Products
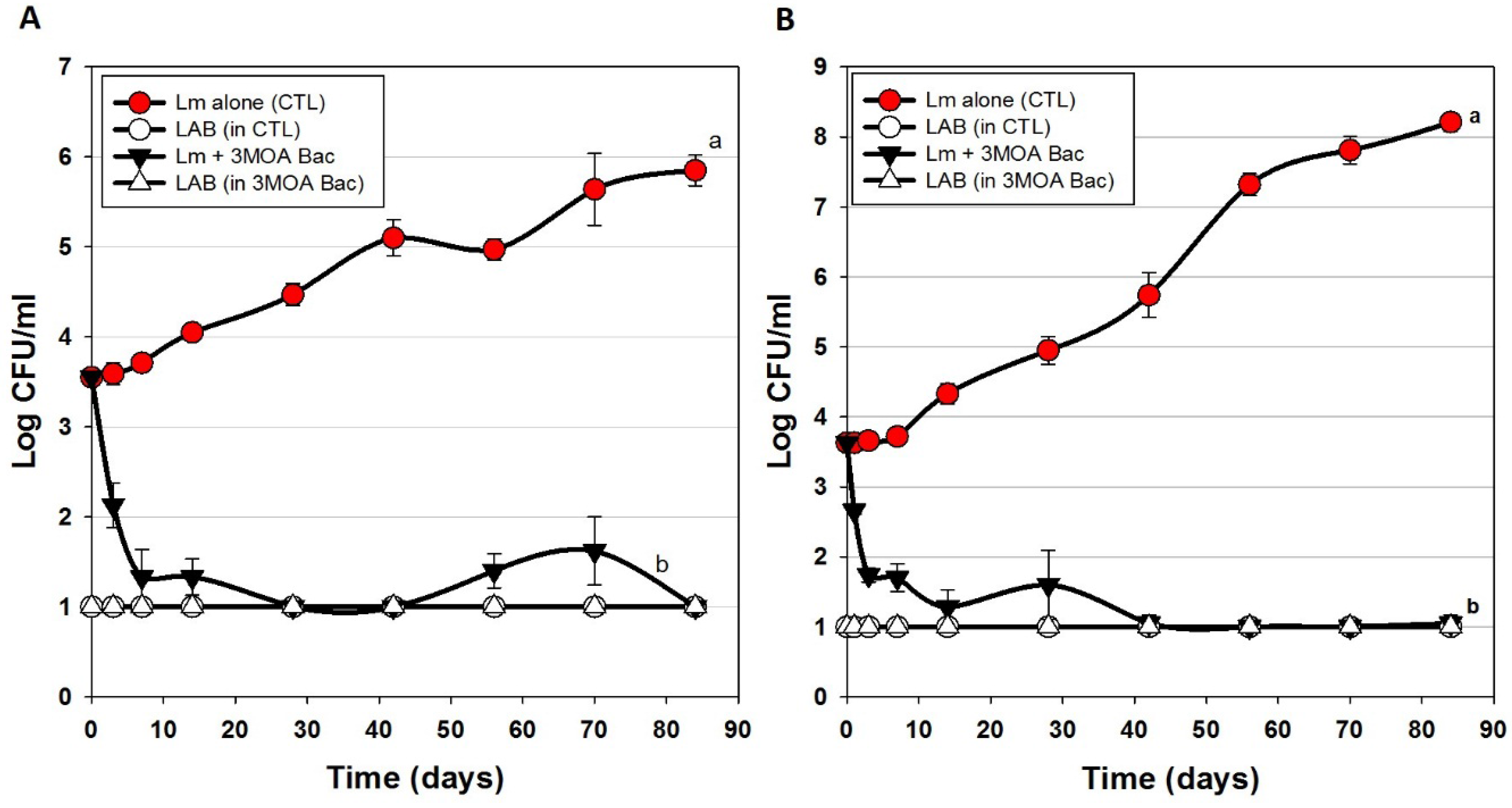
3.3. Trials #3, #4, and #5: Listeria monocytogenes Challenge Studies with Multiple-MOA Bacteriocin Extracts Added after Peeling (During Packaging)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez-Milian, A.; Payeras-Cifre, A. What Is new in listeriosis? BioMed Res. Int. 2014, 2014, 358051. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, A. Chapter 2.9.7, Listeria Monocytogenes, 2015th ed.; The World Organisation for Animal Health (OIE): Paris, France, 2015; pp. 1–18. [Google Scholar]
- Levine, P.; Rose, B.; Green, S.; Ransom, G.; Hill, W. Pathogen testing of ready-to-eat meat and poultry products collected at federally inspected establishments in the United States, 1990 to 1999. J. Food Prot. 2001, 64, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Gamble, R.; Muriana, P.M. Microplate fluorescence assay for measurement of the ability of strains of Listeria monocytogenes from meat and meat-processing plants to adhere to abiotic surfaces. Appl. Environ. Microbiol. 2007, 73, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Muriana, P.M. Adherence characteristics of Listeria strains isolated from three ready-to-eat meat processing plants. J. Food Prot. 2009, 72, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition (FDA-CFSAN); USDA Food Safety and Inspection Service (USDA-FSIS). Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria monocytogenes among Selected Categories of Ready-to-Eat Foods; U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2003.
- Moberg, L. Good manufacturing practices for refrigerated foods. J. Food Prot. 1989, 52, 363–367. [Google Scholar] [CrossRef]
- NACMCF. Hazard analysis and critical control point principles and application guidelines. National Advisory Committee on Microbiological Criteria for Foods. J. Food Prot. 1998, 61, 762–775. [Google Scholar]
- El-Ziney, M.; Debevere, J.; Jakobsen, M.; Reuterin, N.A. Natural Food Antimicrobial Systems; CRC Press: London, UK, 2000; pp. 567–587. [Google Scholar]
- Holzapfel, W.; Geisen, R.; Schillinger, U. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol. 1995, 24, 343–362. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Papagianni, M.; Anastasiadou, S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Factories 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Balciunas, E.M.; Castillo Martinez, F.A.; Todorov, S.D.; Franco, B.D.G.D.M.; Converti, A.; Oliveira, R.P.D.S. Novel biotechnological applications of bacteriocins: A review. Food Control 2013, 32, 134–142. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Daeschel, M. Applications of Bacteriocins in Food Systems; Butterworth-Heinemann: Boston, MA, USA, 1990; pp. 91–115. [Google Scholar]
- Muriana, P.M. Bacteriocins for control of Listeria spp in food. J. Food Prot. 1996, 54–63. [Google Scholar]
- Anonymous. Nisin preparation: Affirmation of GRAS status as a direct human food ingredient. Fed. Regist. 1988, 54, 11247–11251. [Google Scholar]
- Lemay, M.J.; Choquette, J.; Delaquis, P.J.; Claude, G.; Rodrigue, N.; Saucier, L. Antimicrobial effect of natural preservatives in a cooked and acidified chicken meat model. Int. J. Food Microbiol. 2002, 78, 217–226. [Google Scholar] [CrossRef]
- Macwana, S.; Muriana, P.M. Spontaneous bacteriocin resistance in Listeria monocytogenes as a susceptibility screen for identifying different mechanisms of resistance and modes of action by bacteriocins of lactic acid bacteria. J. Microbiol. Methods 2012, 88, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Gautam, D.; Muriana, P. Identification of multiple bacteriocins in Enterococcus spp. using an Enterococcus-specific bacteriocin PCR array. Microorganisms 2015, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, P.P.; Muriana, P.M. A microplate growth inhibition assay for screening bacteriocins against Listeria monocytogenes to differentiate their mode-of-action. Biomolecules 2015, 5, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Chunhua, W.; Muriana, P.M. Incidence of Listeria monocytogenes in packages of retail franks. J. Food Prot. 1994, 57, 382–386. [Google Scholar]
- Bouchard, D.S.; Seridan, B.; Saraoui, T.; Rault, L.; Germon, P.; Gonzalez-Moreno, C.; Nader-Macias, F.M.E.; Baud, D.; François, P.; Chuat, V.; et al. Lactic acid bacteria isolated from bovine mammary microbiota: Potential allies against bovine mastitis. PLoS ONE 2016, 10, e0144831. [Google Scholar] [CrossRef] [PubMed]
- Garver, K.I.; Muriana, P.M. Detection, identification and characterization of bacteriocin-producing lactic acid bacteria from retail food products. Int. J. Food Microbiol. 1993, 20, 241–258. [Google Scholar] [CrossRef]
- Henning, C.; Vijayakumar, P.; Adhikari, R.; Jagannathan, B.; Gautam, D.; Muriana, P.M. Isolation and taxonomic identity of bacteriocin-producing lactic acid bacteria from retail foods and animal sources. Microorganisms 2015, 3, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Amezquita, A.; Brashears, M.M. Competitive inhibition of Listeria monocytogenes in ready-to-eat meat products by lactic acid bacteria. J. Food Prot. 2002, 65, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.; Berg, J. Inhibition of Listeria monocytogenes in cooked ham by virulent bacteriophages and protective cultures. Appl. Environ. Microbiol. 2009, 75, 6944–6946. [Google Scholar] [CrossRef] [PubMed]
- Chollet, E.; Sebti, I.; Martial-Gros, A.; Degraeve, P. Nisin preliminary study as a potential preservative for sliced ripened cheese: NaCl, fat and enzymes influence on nisin concentration and its antimicrobial activity. Food Control 2008, 19, 982–989. [Google Scholar] [CrossRef]
- Jung, D.-S.; Bodyfelt, F.W.; Daeschel, M.A. Influence of Fat and Emulsifiers on the Efficacy of Nisin in Inhibiting Listeria monocytogenes in Fluid Milk1. J. Dairy Sci. 1992, 75, 387–393. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Garriga, M.; Aymerich, M.T. Functionalty of enterococci in meat products. Int. J. Food Microbiol. 2003, 88, 223–233. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Holzapfel, W.H.; Stiles, M.E. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 1999, 47, 1–24. [Google Scholar] [CrossRef]
- USDA Food Safety and Inspection Service (USDA-FSIS). Compliance Guidelines to Control Listeria monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products; USDA-FSIS: Washington, DC, USA, 2012.
- Ünlü, G.; Nielsen, B.; Ionita, C. Inhibition of Listeria monocytogenes in hot dogs by surface application of freeze-dried bacteriocin-containing powders from lactic acid bacteria. Probiotics Antimicrob. Proteins 2016, 8, 102–110. [Google Scholar] [CrossRef] [PubMed]

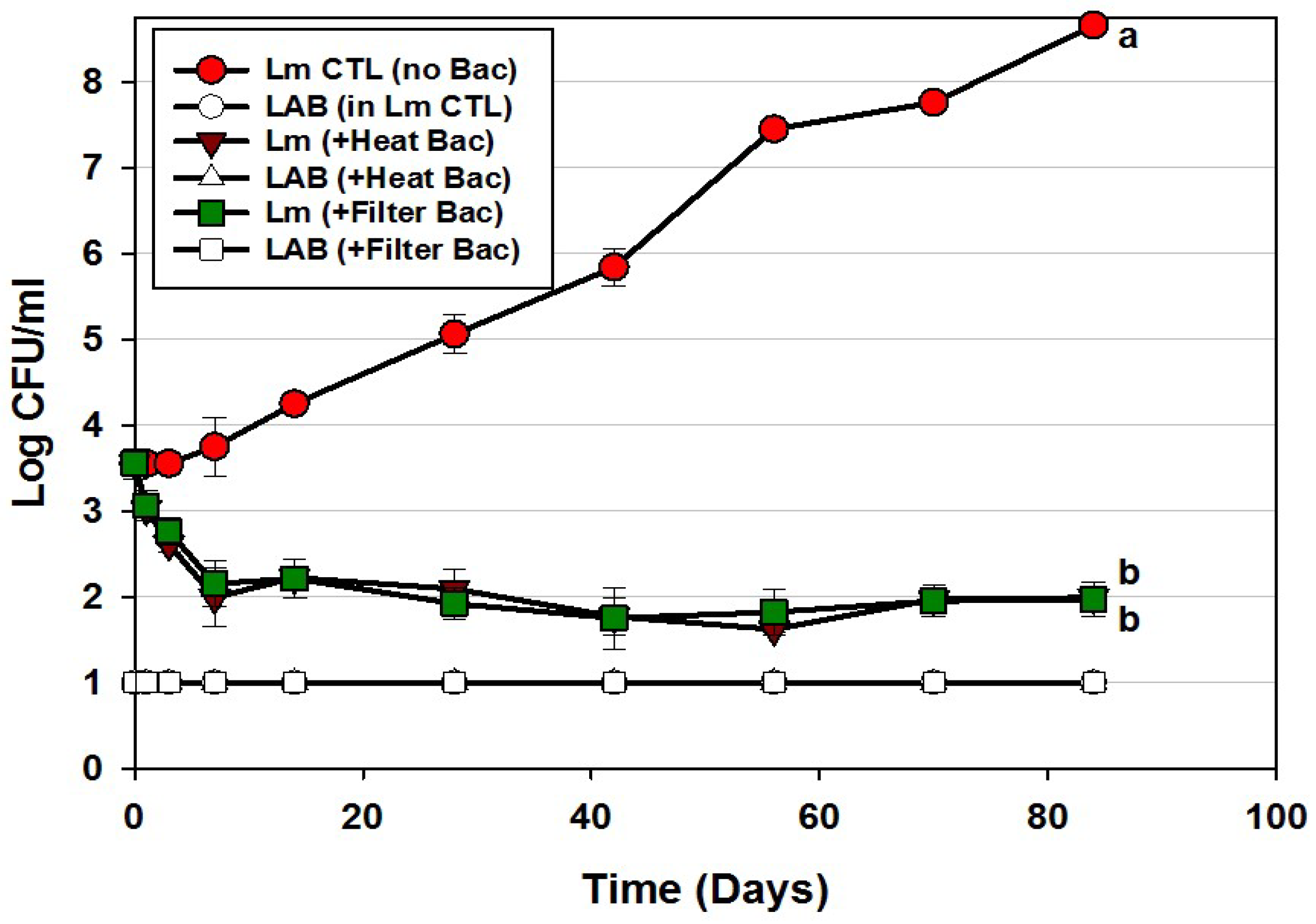
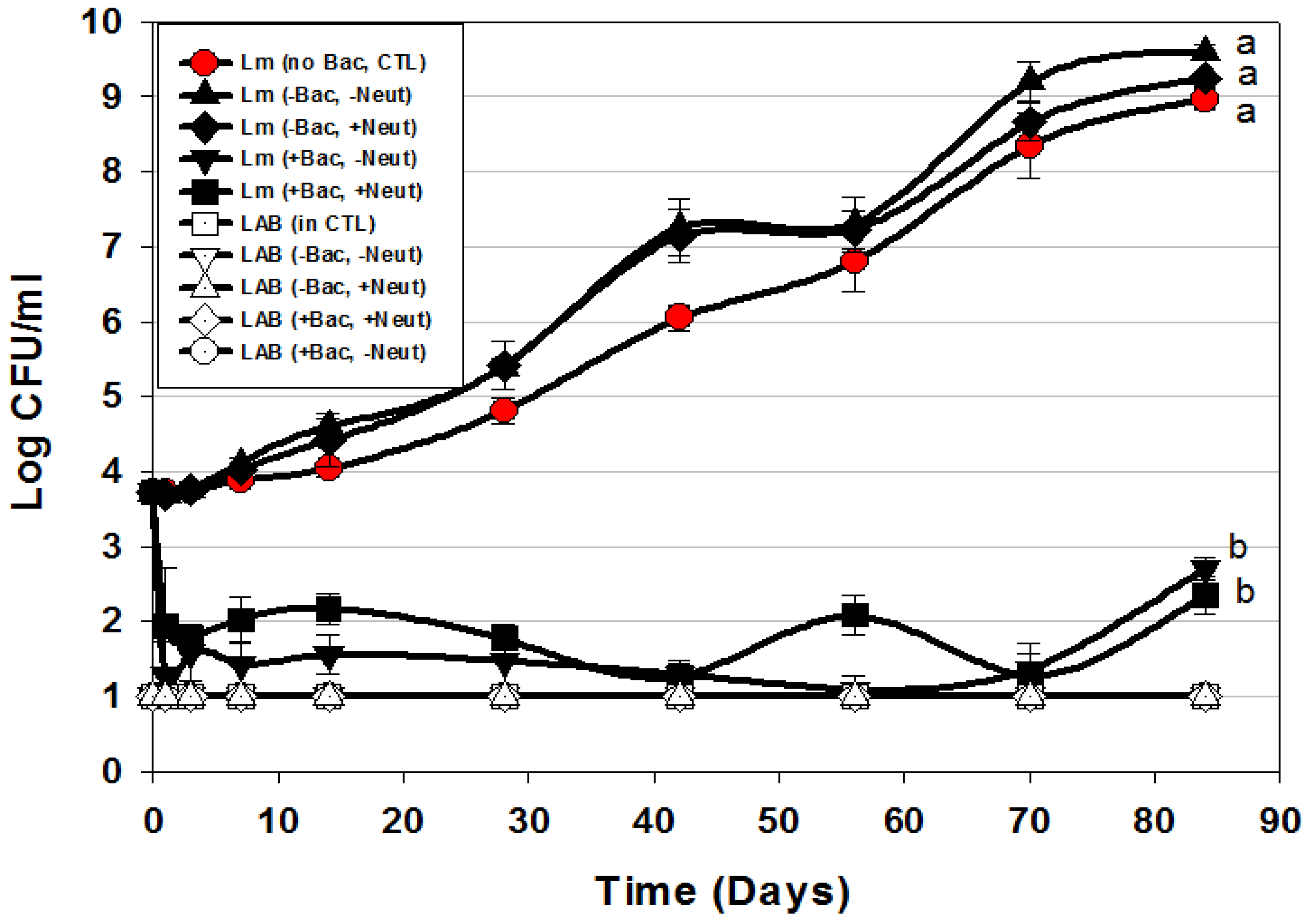
 ) or in combination (Lm+LAB; ○) with 5 Bac+ LAB (En. faecium FS56-1, En. thailandicus FS92, En. faecium FS97-2, En. thailandicus RP-1, and Pe. acidilactici Bac 3). All trials were performed in triplicate replication; data points represent the means and error bars represent standard deviation from the mean. Treatments with different letters are significantly different (repeated measures, p < 0.05); those with the same letters are not significantly different (p > 0.05).
) or in combination (Lm+LAB; ○) with 5 Bac+ LAB (En. faecium FS56-1, En. thailandicus FS92, En. faecium FS97-2, En. thailandicus RP-1, and Pe. acidilactici Bac 3). All trials were performed in triplicate replication; data points represent the means and error bars represent standard deviation from the mean. Treatments with different letters are significantly different (repeated measures, p < 0.05); those with the same letters are not significantly different (p > 0.05).
 ) or in combination (Lm+LAB; ○) with 5 Bac+ LAB (En. faecium FS56-1, En. thailandicus FS92, En. faecium FS97-2, En. thailandicus RP-1, and Pe. acidilactici Bac 3). All trials were performed in triplicate replication; data points represent the means and error bars represent standard deviation from the mean. Treatments with different letters are significantly different (repeated measures, p < 0.05); those with the same letters are not significantly different (p > 0.05).
) or in combination (Lm+LAB; ○) with 5 Bac+ LAB (En. faecium FS56-1, En. thailandicus FS92, En. faecium FS97-2, En. thailandicus RP-1, and Pe. acidilactici Bac 3). All trials were performed in triplicate replication; data points represent the means and error bars represent standard deviation from the mean. Treatments with different letters are significantly different (repeated measures, p < 0.05); those with the same letters are not significantly different (p > 0.05).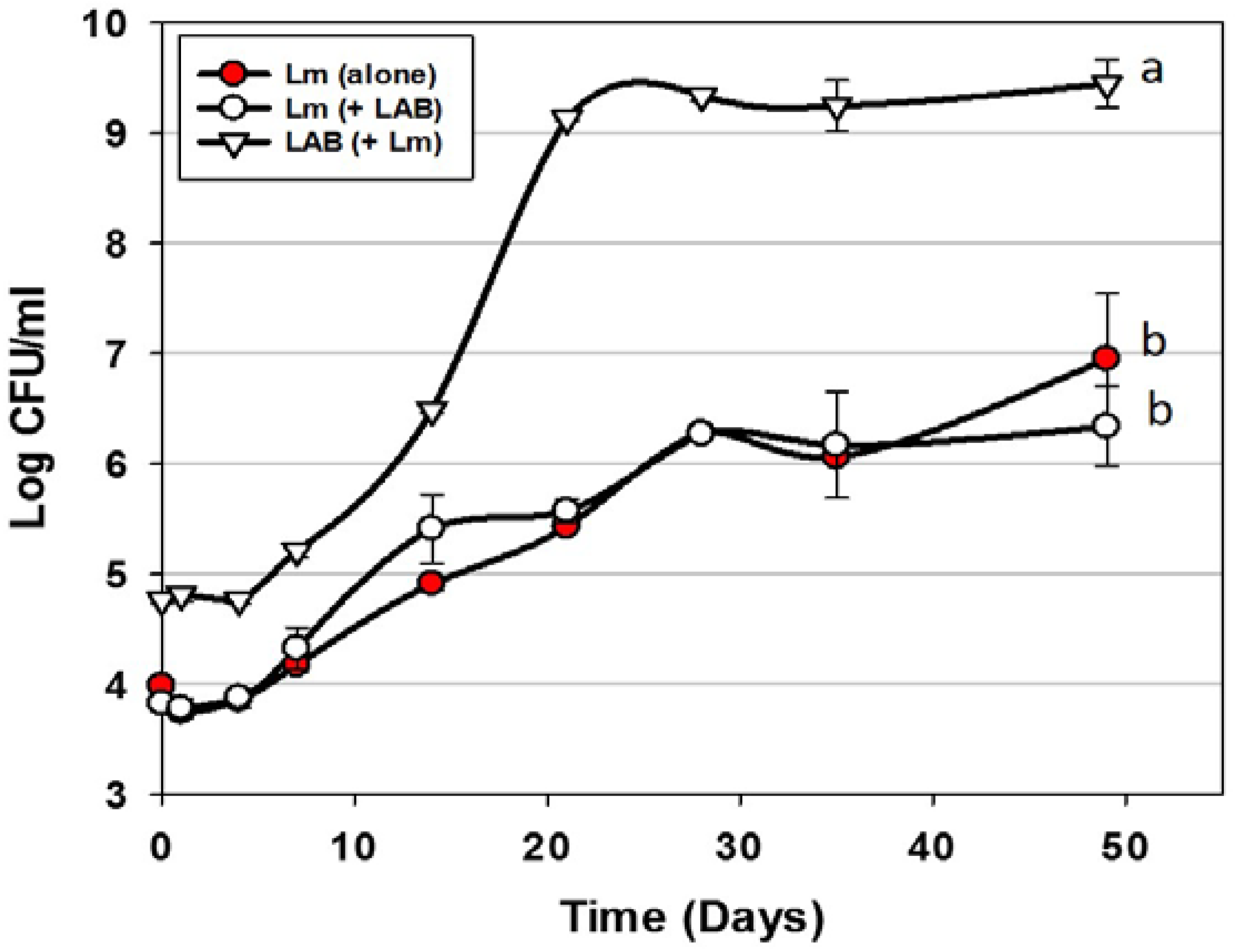
| Microorganism | Strain Designation | Source/Reference |
|---|---|---|
| Lactobacillus delbrueckii | 4797-2 | Muriana culture collection |
| Listeria monocytogenes | 39-2 (R0) | [20,21,22,23] |
| Lactobacillus curvatus | FS47 | [25] |
| Lactobacillus curvatus | Beef 3 | [26] |
| Pediococcus acidilactici | Bac 3 | [26] |
| Enterococcus faecium | FS56-1 | [21,25,26] |
| Lactococcus lactis | FLS-1 | [26] |
| Enterococcus thailandicus | RP-1 | [21,26] |
| Enterococcus thailandicus | FS92 | [21,25] |
| Trial | Description of Treatment | Data |
|---|---|---|
| Trial 1 | Use of bacteriocin-producing (Bac+) cultures vs. L. monocytogenes | Figure 2 |
| Trial 2 | Bac+ CFS added into meat matrix before cooking | Figure 3 |
| Bac+ CFS sprayed onto hotdogs in casings before peeling | ||
| Trial 3 | Bac+ CFS as surface treatment (includes CFS from 2 Enterococcus strains) | Figure 4A |
| Bac+ CFS as surface treatment (includes CFS from 1 Enterococcus strain) | Figure 4B | |
| Trial 4 | Bac+ CFS as surface treatment: All CFS was from traditional lactic acid bacteria; filter vs. heat-pasteurized Bac+ CFS | Figure 5 |
| Trial 5 | Bac+ CFS as surface treatment: Neutralized vs. non-neutralized CFS and Bac+ vs. Bac− CFS | Figure 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action. Foods 2017, 6, 22. https://doi.org/10.3390/foods6030022
Vijayakumar PP, Muriana PM. Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action. Foods. 2017; 6(3):22. https://doi.org/10.3390/foods6030022
Chicago/Turabian StyleVijayakumar, Paul Priyesh, and Peter M. Muriana. 2017. "Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action" Foods 6, no. 3: 22. https://doi.org/10.3390/foods6030022