Rapid Prediction of Moisture Content in Intact Green Coffee Beans Using Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Near-Infrared Spectroscopy
2.3. Moisture Content Determination
2.4. Data Processing
3. Results
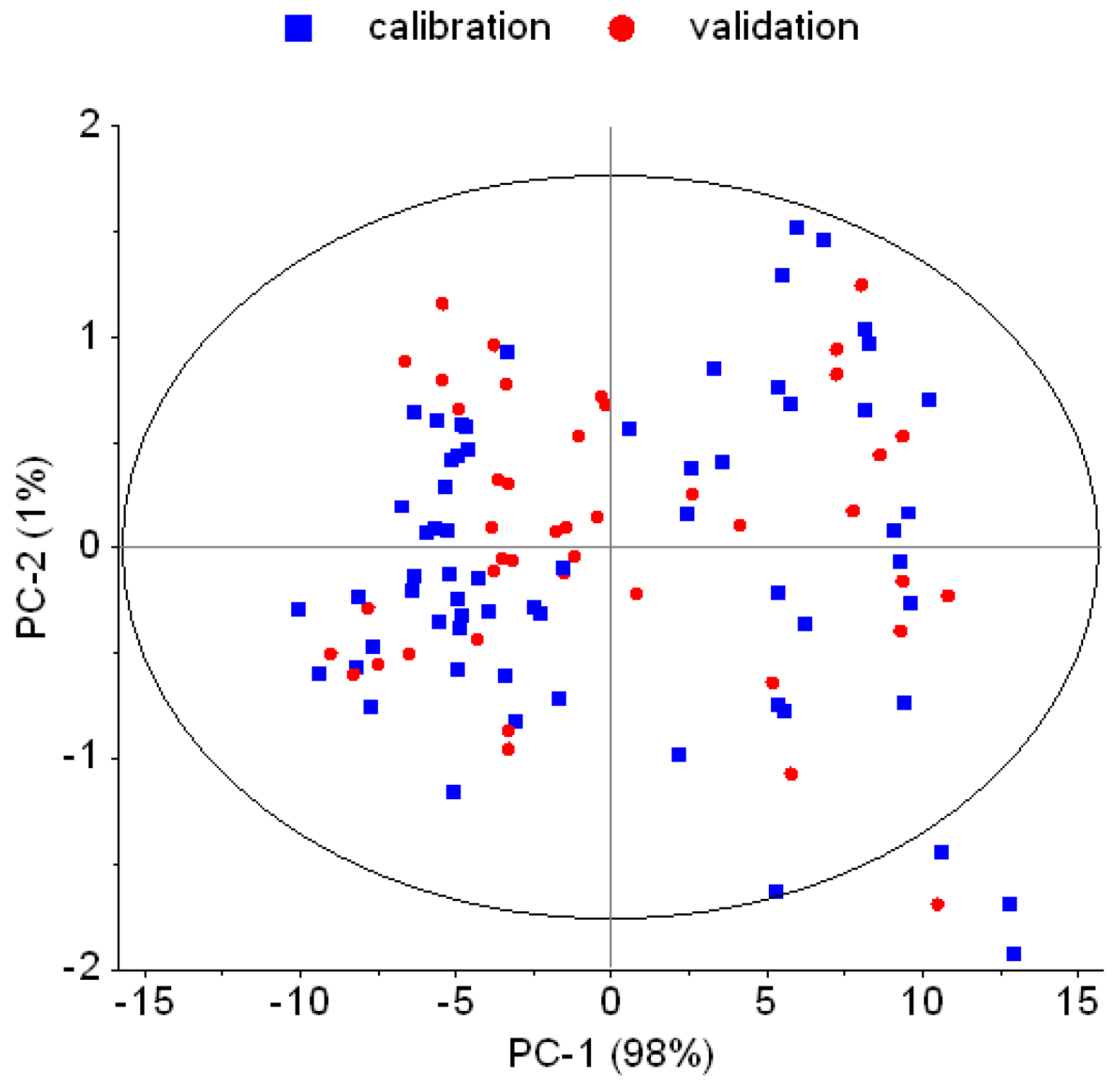
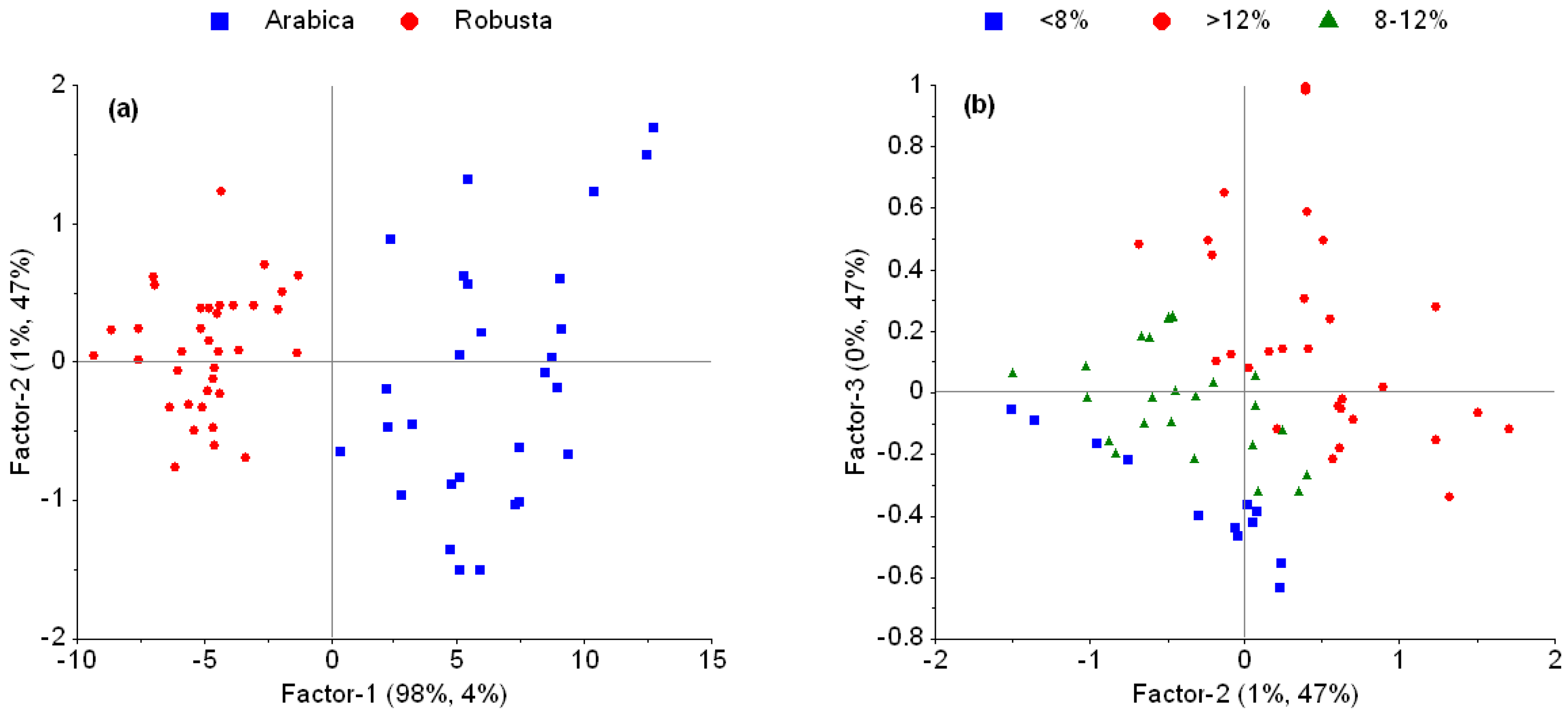
3.1. Spectral Properties, Outliers, and Effect of Pre-Processing
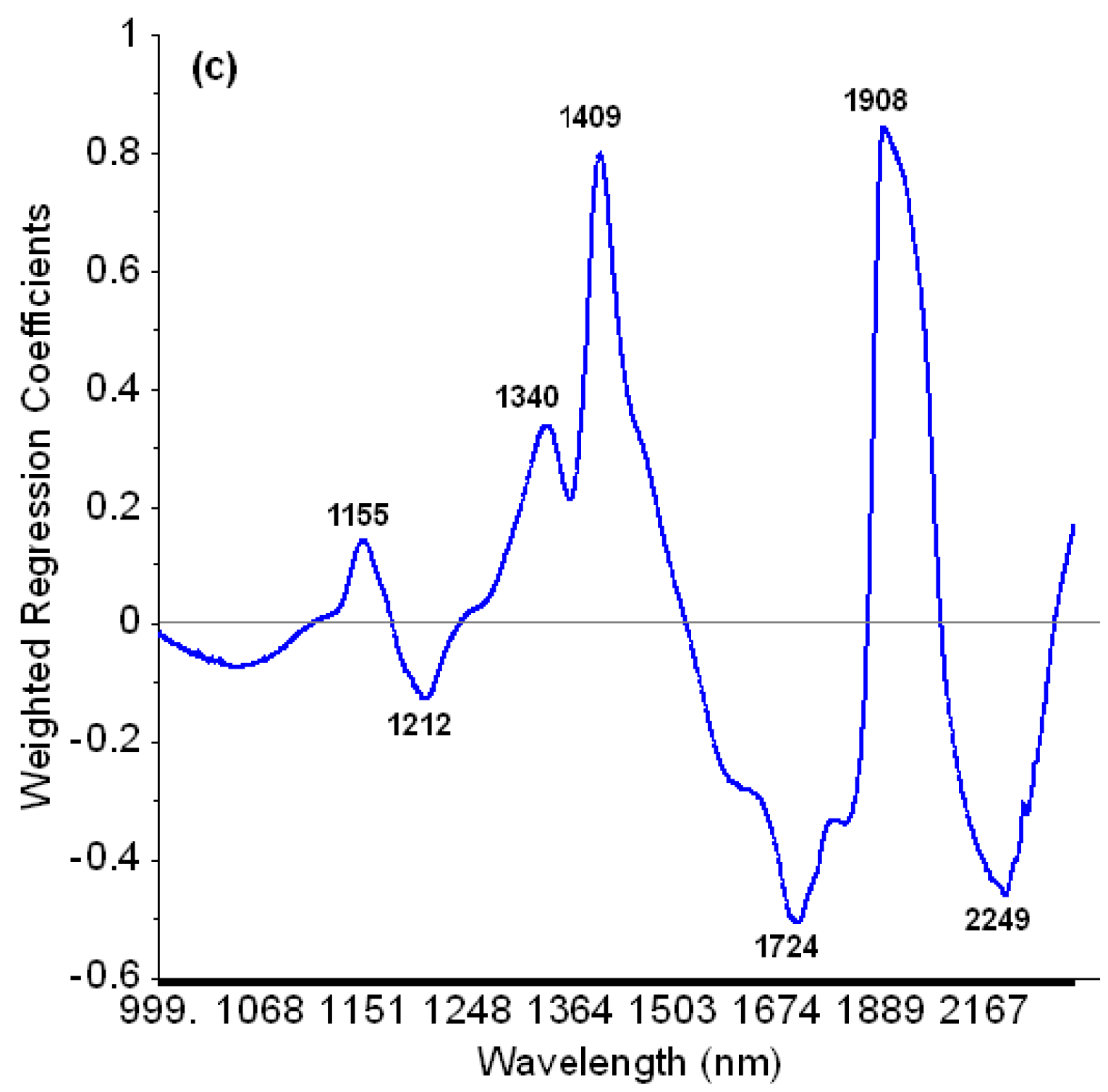
3.2. Prediction of Moisture Content from NIR Reflectance Spectra
4. Discussion
4.1. Outliers and Effect of Pre-Processing
4.2. Prediction of Moisture Content Using NIR Infrared Spectra
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Coffee Organization (ICO). National quality standards. In Proceedings of the Promotion and Market Development Committee 6th Meeting, Belo Horizonte, Brazil, 9 September 2013; Available online: http://www.ico.org/documents/cy2012-13/pm-29e-quality-standards.pdf (accessed on 1 February 2017).
- Pittia, P.; Nicoli, M.C.; Sacchetti, G. Effect of moisture and water activity on textural properties of raw and roasted coffee beans. J. Texture Stud. 2007, 38, 116–134. [Google Scholar] [CrossRef]
- Reh, C.; Gerber, A.; Prodolliet, J.; Vuataz, G. Water content determination in green coffee—Method comparison to study specificity and accuracy. Food Chem. 2006, 96, 423–430. [Google Scholar] [CrossRef]
- Van Hilten, H.J.; Fisher, P.J.; Wheeler, M.A. The Coffee Exporter’s Guide, 3rd ed.; International Trade Centre UNCTAD/GATT: Geneva, Switzerland, 2011. [Google Scholar]
- Gautz, L.D.; Smith, V.E.; Bittenbender, H.C. Measuring coffee bean moisture content. Eng. Noteb. 2008, 3, 1–3. [Google Scholar]
- Palacios-Cabrera, H.; Taniwaki, M.H.; Menezes, H.C.; Iamanaka, B.T. The production of ochratoxin A by Aspergillus ochraceus in raw coffee at different equilibrium relative humidity and under alternating temperatures. Food Control 2004, 15, 531–535. [Google Scholar] [CrossRef]
- Pardo, E.; Ramos, A.; Sanchis, V.; Marin, S. Modelling of effects of water activity and temperature on germination and growth of ochratoxigenic isolates of on a green coffee-based medium. Int. J. Food Microbiol. 2005, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Finzer, J.R.D.; Limaverde, J.R.; Freitas, A.O.; Júnior, J.L.; Sfredo, M.A. Drying of coffee berries in a vibrated tray dryer operated with solids recycle and single-stage. J. Food Process Eng. 2003, 26, 207–222. [Google Scholar] [CrossRef]
- Subedi, R.N. Comparative analysis of dry and wet processing of coffee with respect to quality and cost in kavre district, Nepal: A case of panchkhal village. Int. Res. J. Appl. Basic Sci. 2011, 2, 181–193. [Google Scholar]
- Yani, A. Fungal infection at coffee beans during primary processing (case study in Bengkulu Province). Akta Agrosia 2008, 11, 87–95. [Google Scholar]
- Aklimawati, L.; Mawardi, S. Characteristics of Quality Profile and Agribusiness of Robusta Coffee in Tambora Mountainside, Sumbawa. Pelita Perkeb. Coffee Cocoa Res. J. 2014, 30, 159–180. [Google Scholar]
- Bucheli, P.; Meyer, I.; Pittet, A.; Vuataz, G.; Viani, R. Industrial storage of green Robusta coffee under tropical conditions and its impact on raw material quality and ochratoxin A content. J. Agric. Food Chem. 1998, 46, 4507–4511. [Google Scholar] [CrossRef]
- Baggenstoss, J.; Poisson, L.; Kaegi, R.; Perren, R.; Escher, F. Roasting and aroma formation: Effect of initial moisture content and steam treatment. J. Agric. Food Chem. 2008, 56, 5847–5851. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.C.F.; Franca, A.S.; Oliveira, L.S. A comparative evaluation of methodologies for water content determination in green coffee. LWT - Food Sci. Technol. 2007, 40, 1300–1303. [Google Scholar] [CrossRef]
- Munawar, A.A.; von Hörsten, D.; Wegener, J.K.; Pawelzik, E.; Mörlein, D. Rapid and non-destructive prediction of mango quality attributes using Fourier transform near infrared spectroscopy and chemometrics. Eng. Agric. Environ. Food 2016, 9, 208–215. [Google Scholar] [CrossRef]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Isengard, H.D. Rapid water determination in foodstuffs. Trends Food Sci. Technol. 1995, 6, 155–162. [Google Scholar] [CrossRef]
- Isengard, H.-D. Water content, one of the most important properties of food. Food Control 2001, 12, 395–400. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.d.S.M.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Morgano, M.A.; Faria, C.G.; Ferrão, M.F.; Bragagnolo, N.; Ferreira, M.M.d.C. Determination of moisture in raw coffee by near infra-red reflectance spectroscopy and multivariate regression. Food Sci. Technol. Camp. 2008, 28, 12–17. [Google Scholar]
- Esteban-Dı́ez, I.; González-Sáiz, J.M.; Pizarro, C. An evaluation of orthogonal signal correction methods for the characterisation of arabica and robusta coffee varieties by NIRS. Anal. Chim. Acta 2004, 514, 57–67. [Google Scholar] [CrossRef]
- Fearn, T. Assessing Calibrations: SEP, RPD, RER and R 2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Shabbak, A.; Midi, H. An Improvement of the Hotelling T 2 Statistic in Monitoring Multivariate Quality Characteristics. Math. Probl. Eng. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Morales-Medina, G.; Guzmán, A. Prediction of density and viscosity of Colombian crude oils from chromatographic data. CTF-Cienc. Tecnol. Futuro 2012, 4, 57–73. [Google Scholar]
- Pizarro, C.; Esteban-Dı́ez, I.; Nistal, A.-J.; González-Sáiz, J.-M. Influence of data pre-processing on the quantitative determination of the ash content and lipids in roasted coffee by near infrared spectroscopy. Anal. Chim. Acta 2004, 509, 217–227. [Google Scholar] [CrossRef]
- Sørensen, K.M.; Petersen, H.; Engelsen, S.B. An On-Line Near-Infrared (NIR) Transmission Method for Determining Depth Profiles of Fatty Acid Composition and Iodine Value in Porcine Adipose Fat Tissue. Appl. Spectrosc. 2012, 66, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.-W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. Technol. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Ky, C.-L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Martı́n, M.J.; Pablos, F.; González, A.G.; Valdenebro, M.S.; León-Camacho, M. Fatty acid profiles as discriminant parameters for coffee varieties differentiation. Talanta 2001, 54, 291–297. [Google Scholar] [CrossRef]
- Pan, L.; Lu, R.; Zhu, Q.; McGrath, J.M.; Tu, K. Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2015, 102, 42–50. [Google Scholar] [CrossRef]
- Williams, P. Grains and Seeds. In Near-Infrared Spectroscopy in Food Science and Technology; Ozaki, Y., McClure, W.F., Christy, A.A., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. 165–217. [Google Scholar]


| No. | Purpose | Species | Origin |
|---|---|---|---|
| 1 | Calibration | Arabica | West Nusa Tenggara |
| 2 | South Sulawesi | ||
| 3 | Aceh | ||
| 4 | Robusta | South Sumatera | |
| 5 | Bali | ||
| 6 | East Java | ||
| 7 | North Sumatera | ||
| 8 | Validation | Arabica | West Java |
| 9 | North Sumatera | ||
| 10 | Robusta | South Sumatera | |
| 11 | East Java | ||
| 12 | Bengkulu |
| Model | Parameter | Full Spectral Range PLSR | Spectral Subset | ||
|---|---|---|---|---|---|
| Raw | EMSC | Raw (MLR) | Raw (PLS) | ||
| Calibration | LVs | 3 | 4 | n/a | 3 |
| R2 calibration | 0.9834 | 0.9850 | 0.9839 | 0.9743 | |
| R2 cross validation | 0.9802 | 0.9811 | 0.9779 | 0.9698 | |
| RMSEC (% MC) | 0.52 | 0.49 | 0.51 | 0.65 | |
| RMSECV (% MC) | 0.58 | 0.56 | 0.60 | 0.71 | |
| Prediction | R2 prediction | 0.9641 | 0.9817 | 0.9632 | 0.9669 |
| RMSEP (% MC) | 0.80 | 0.57 | 0.93 | 0.77 | |
| Bias (% MC) | 0.42 | 0.28 | 0.45 | 0.39 | |
| RPD | 6.21 | 8.53 | 3.47 | 6.39 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, A.; Hörsten, D.v.; Pawelzik, E.; Mörlein, A.D. Rapid Prediction of Moisture Content in Intact Green Coffee Beans Using Near Infrared Spectroscopy. Foods 2017, 6, 38. https://doi.org/10.3390/foods6050038
Adnan A, Hörsten Dv, Pawelzik E, Mörlein AD. Rapid Prediction of Moisture Content in Intact Green Coffee Beans Using Near Infrared Spectroscopy. Foods. 2017; 6(5):38. https://doi.org/10.3390/foods6050038
Chicago/Turabian StyleAdnan, Adnan, Dieter von Hörsten, Elke Pawelzik, and And Daniel Mörlein. 2017. "Rapid Prediction of Moisture Content in Intact Green Coffee Beans Using Near Infrared Spectroscopy" Foods 6, no. 5: 38. https://doi.org/10.3390/foods6050038






