Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Bacterial Strains and Growth Conditions
2.3. Determination of Extracellular Phosphate Concentration
2.4. Determination of Intra- and Extra-Cellular ATP Concentration
2.5. Absorbance Scans of Culture Supernatant after Antimicrobial Treatment
2.6. Monolayer Studies
2.7. Analysis of Isotherms
2.8. Data Analysis
3. Results
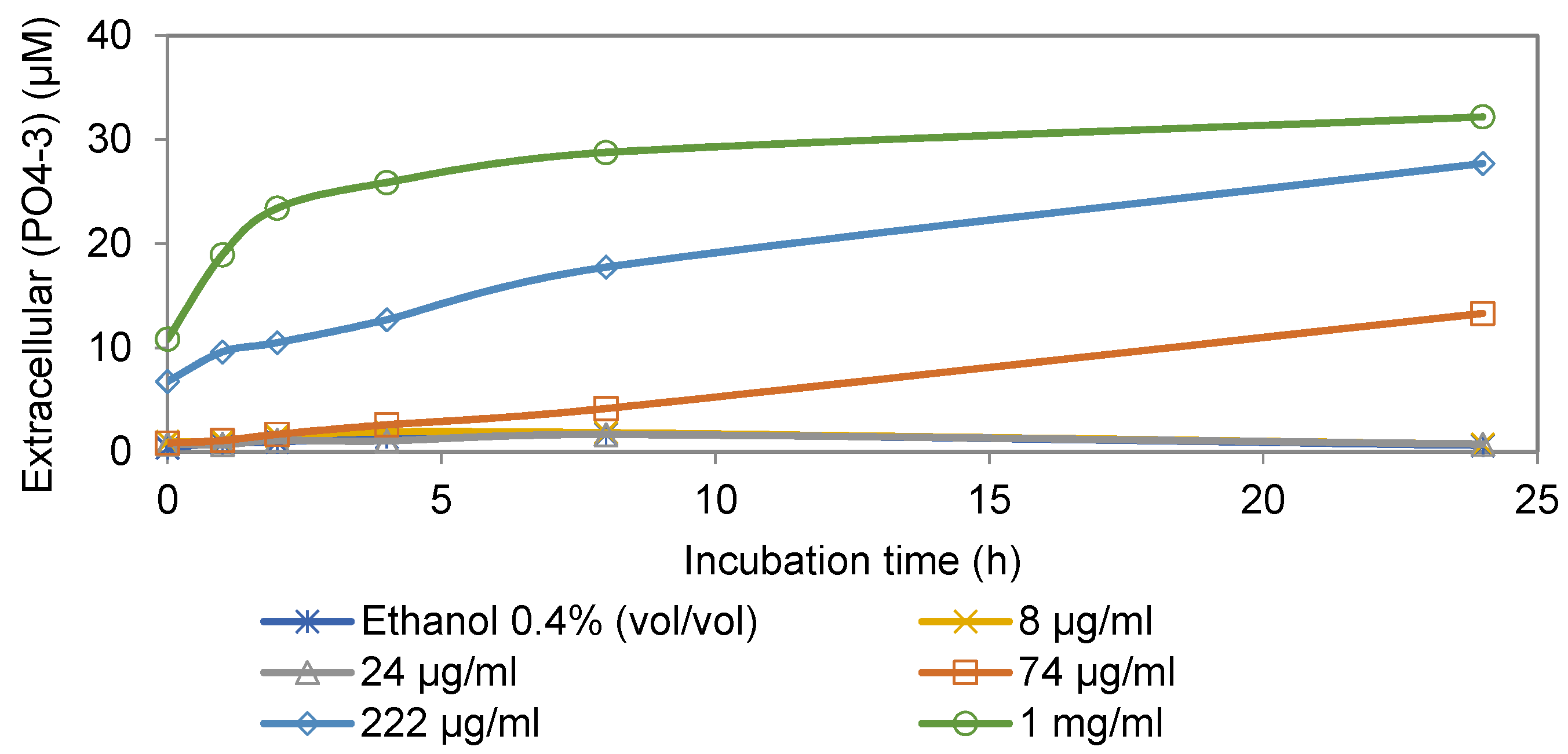
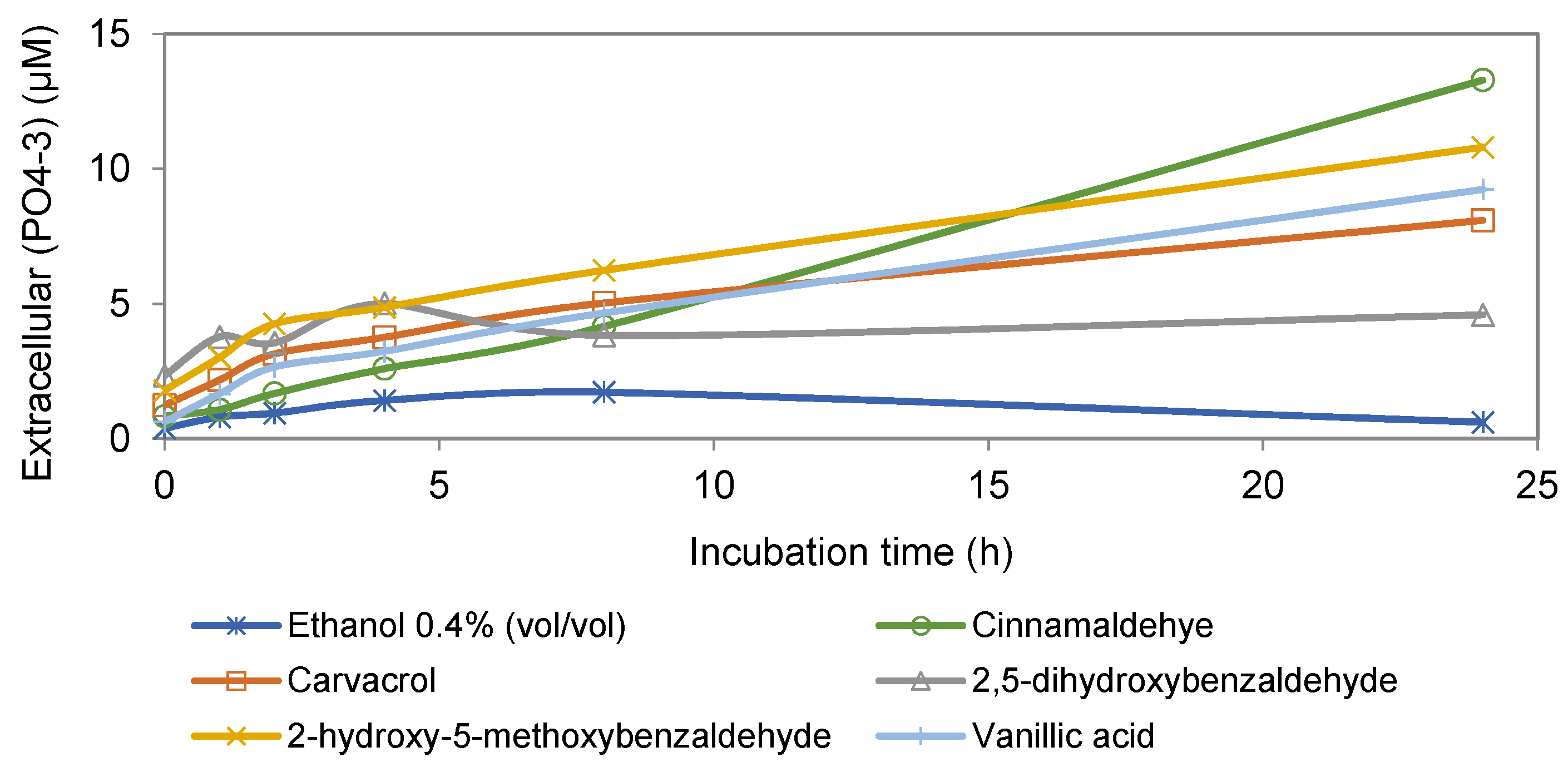
3.1. Extracellular Phosphate Assay
3.2. Intracellular and Extracellular ATP Assays
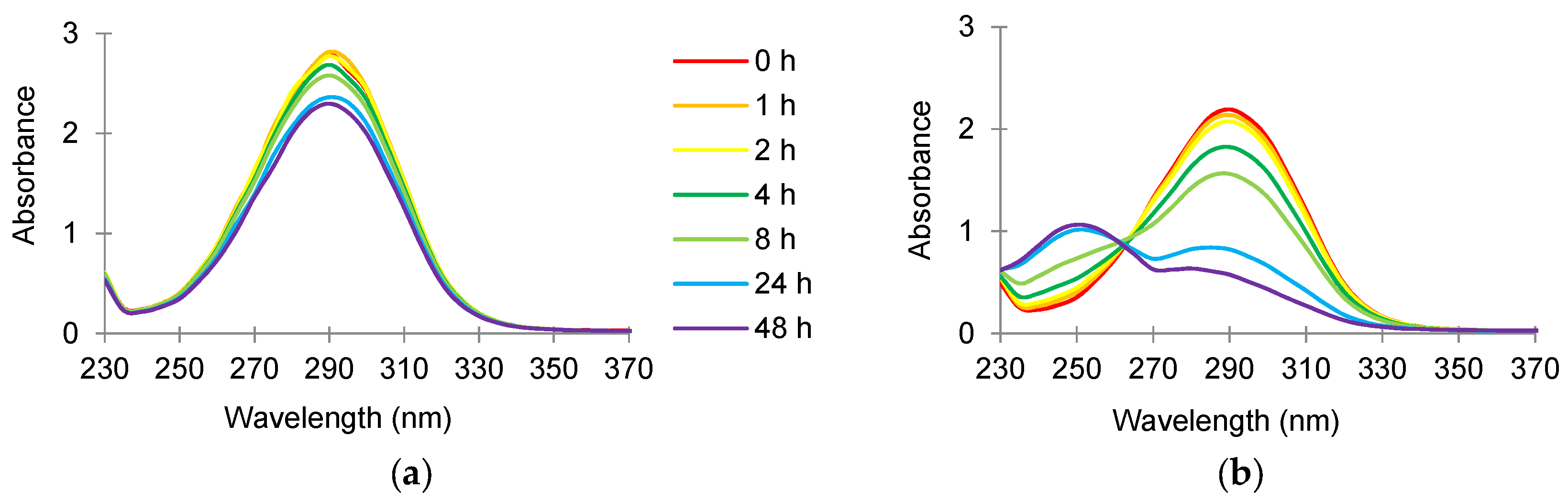
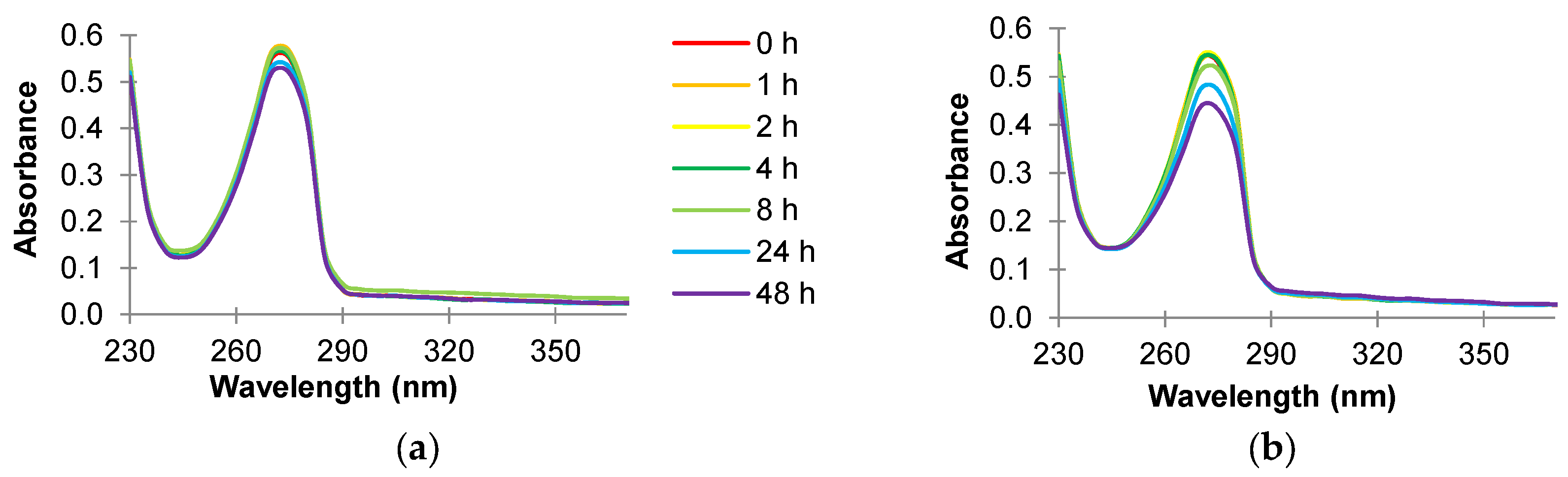
3.3. Absorbance Scans of Culture Supernatant after Antimicrobial Treatment
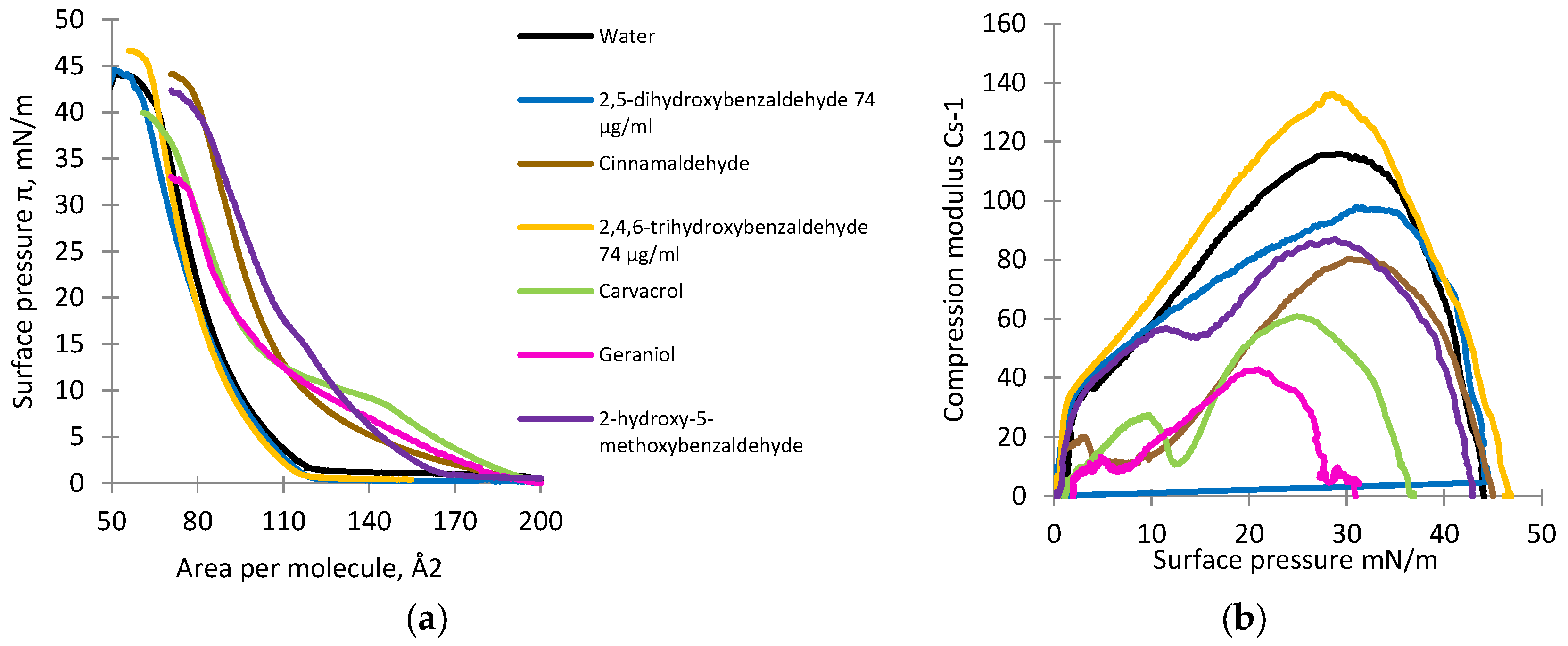
3.4. Monolayer Studies
4. Discussion
Mechanisms of Antimicrobial Effects against Map
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Friedman, M.; Henika, P.R.; Levin, C.E. Bactericidal activities of health-promoting, food-derived powders against the foodborne pathogens Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. J. Food Sci. 2013, 78, M270–M275. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antimicrobial activities of plant essential oils and their components against antibiotic-susceptible and antibiotic-resistant foodborne pathogens. In Essential Oils and Nanotechnology for Treatment of Microbial Diseases; Rai, M., Zachino, S., Derita, M.D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 14–38. [Google Scholar]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multi-beneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Friedman, M.; Patel, J.; Jaroni, D.; Ravishankar, S. The antimicrobial effects of cinnamon leaf oil against multi-drug resistant Salmonella Newport on organic leafy greens. Int. J. Food Microbiol. 2013, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Moore-Neibel, K.; Gerber, C.; Patel, J.; Friedman, M.; Jaroni, D.; Ravishankar, S. Antimicrobial activity of oregano oil against antibiotic-resistant Salmonella enterica on organic leafy greens at varying exposure times and storage temperatures. Food Microbiol. 2013, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Gonzales-Barron, U.; Butler, F.; Yadav, A.S.; Friedman, M. Predictive thermal inactivation model for the combined effect of temperature, cinnamaldehyde and carvacrol on starvation-stressed multiple Salmonella serotypes in ground chicken. Int. J. Food Microbiol. 2013, 165, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Yadav, A.S.; Hwang, C.-A.; Sheen, S.; Mukhopadhyay, S.; Friedman, M. Kinetics of thermal destruction of Salmonella in ground chicken containing trans-cinnamaldehyde and carvacrol. J. Food Prot. 2012, 75, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.P.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [PubMed]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E.; Kozukue, N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 2006, 69, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, S.; Jaroni, D.; Zhu, L.; Olsen, C.W.; McHugh, T.H.; Friedman, M. Inactivation of Listeria monocytogenes on ham and bologna using pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde. J. Food Sci. 2012, 77, M377–M382. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Kim, S.-K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J. Agric. Food Chem. 2006, 54, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Zhu, L.; Feinstein, Y.; Ravishankar, S. Carvacrol facilitates heat-induced inactivation of Escherichia coli O157:H7 and inhibits formation of heterocyclic amines in grilled ground beef patties. J. Agric. Food Chem. 2009, 57, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, S.; Zhu, L.; Law, B.; Joens, L.; Friedman, M. Plant-derived compounds inactivate antibiotic-resistant Campylobacter jejuni strains. J. Food Prot. 2008, 71, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Mild, R.M.; Joens, L.A.; Friedman, M.; Olsen, C.W.; McHugh, T.H.; Law, B.; Ravishankar, S. Antimicrobial edible apple films inactivate antibiotic resistant and susceptible Campylobacter jejuni strains on chicken breast. J. Food Sci. 2011, 76, M163–M168. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Buick, R.; Elliott, C.T. Antibacterial activities of naturally occurring compounds against antibiotic-resistant Bacillus cereus vegetative cells and spores, Escherichia coli, and Staphylococcus aureus. J. Food Prot. 2004, 67, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, S.; Zhu, L.; Olsen, C.W.; McHugh, T.H.; Friedman, M. Edible apple film wraps containing plant antimicrobials inactivate foodborne pathogens on meat and poultry products. J. Food Sci. 2009, 74, M440–M445. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Lombard, J.E.; Gardner, I.A.; Jafarzadeh, S.R.; Fossler, C.P.; Harris, B.; Capsel, R.T.; Wagner, B.A.; Johnson, W.O. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013, 108, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W.; Collins, M.T.; Koets, A.P.; McGuirk, S.M.; Roussel, A.J. Paratuberculosis (Johne’s disease) in cattle and other susceptible species. J. Vet. Intern. Med. 2012, 26, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Pithua, P.; Kollias, N.S. Estimated prevalence of caprine paratuberculosis in boer goat herds in Missouri, USA. Vet. Med. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Arrazuria, R.; Juste, R.A.; Elguezabal, N. Mycobacterial infections in rabbits: From the wild to the laboratory. Transbound. Emerg. Dis. 2017, 64, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Shitaye, J.E.; Matlova, L.; Horvathova, A.; Moravkova, M.; Dvorska-Bartosova, L.; Treml, F.; Lamka, J.; Pavlik, I. Mycobacterium avium subsp. avium distribution studied in a naturally infected hen flock and in the environment by culture, serotyping and IS901 RFLP methods. Vet. Microbiol. 2008, 127, 155–164. [Google Scholar] [CrossRef]
- Gaukler, S.M.; Linz, G.M.; Sherwood, J.S.; Dyer, N.W.; Bleier, W.J.; Wannemuehler, Y.M.; Nolan, L.K.; Logue, C.M. Escherichia coli, Salmonella, and Mycobacterium avium subsp. paratuberculosis in Wild European starlings at a Kansas cattle feedlot. Avian Dis. 2009, 53, 544–551. [Google Scholar] [CrossRef]
- Carta, T.; Álvarez, J.; Pérez de la Lastra, J.M.; Gortázar, C. Wildlife and paratuberculosis: A review. Res. Vet. Sci. 2013, 94, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Whyte, P.; More, S.J.; Green, M.J.; O’Grady, L.; Garcia, A.; Doherty, M.L. The effect of paratuberculosis on milk yield—A systematic review and meta-analysis. J. Dairy Sci. 2016, 99, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, C.; Graesboll, K.; Nielsen, S.S.; Toft, N.; Halasa, T. Epidemiological and economic consequences of purchasing livestock infected with Mycobacterium avium subsp. paratuberculosis. BMC Vet. Res. 2017, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Click, R.E. Successful treatment of asymptomatic or clinically terminal bovine Mycobacterium avium subspecies paratuberculosis infection (Johne’s disease) with the bacterium Dietzia used as a probiotic alone or in combination with dexamethasone: Adaption to chronic human diarrheal diseases. Virulence 2011, 2, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Wells, S.; Donahue, M.; Stabel, J.; Oakes, J.M.; Sreevatsan, S.; Fetrow, J. Effect of feeding heat-treated colostrum on risk for infection with Mycobacterium avium ssp. paratuberculosis, milk production, and longevity in Holstein dairy cows. J. Dairy Sci. 2015, 98, 5630–5641. [Google Scholar] [CrossRef] [PubMed]
- Verhegghe, M.; Rasschaert, G.; Herman, L.; Goossens, K.; Vandaele, L.; De Bleecker, K.; Vlaemynck, G.; Heyndrickx, M.; De Block, J. Reduction of Mycobacterium avium ssp. paratuberculosis in colostrum: Development and validation of 2 methods, one based on curdling and one based on centrifugation. J. Dairy Sci. 2017, 100, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Tauer, L.W.; Schukken, Y.H.; Gómez, M.I.; Smith, R.L.; Lu, Z.; Grohn, Y.T. Economic analysis of Mycobacterium avium subspecies paratuberculosis vaccines in dairy herds. J. Dairy Sci. 2012, 95, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Schukken, Y.H.; Smith, R.L.; Mitchell, R.M.; Gröhn, Y.T. Impact of imperfect Mycobacterium avium subsp. paratuberculosis vaccines in dairy herds: A mathematical modeling approach. Prev. Vet. Med. 2013, 108, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Lemke, J.J.; Berry, A.; Nelson, K.; Hines, M.E.; Talaat, A.M. Superior protection from live-attenuated vaccines directed against Johne’s Disease. Clin. Vaccine Immunol. 2017, 24, e00478-16. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, K.K.; Gupta, R.D.; Gupta, S.; Singh, S.V.; Bhatia, A.K.; Jayaraman, S.; Kumar, N.; Goel, A.; Rathore, A.S.; Sahzad; et al. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet. Q. 2016, 36, 203–227. [Google Scholar] [CrossRef] [PubMed]
- McNees, A.L.; Markesich, D.; Zayyani, N.R.; Graham, D.Y. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.A.; Rajic, A.; Stark, K.D.; Mc, E.S. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: A systematic review and meta-analyses of the evidence. Epidemiol. Infect. 2015, 143, 3135–3157. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; Thanigachalam, S.; Dow, C.T.; Collins, M.T. Exploring the role of Mycobacterium avium subspecies paratuberculosis in the pathogenesis of type 1 diabetes mellitus: A pilot study. Gut Pathog. 2013, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Hesam Shariati, S.; Alaei, A.; Keshavarz, R.; Mosavari, N.; Rabbani, A.; Niegowska, M.; Sechi, L.A.; Feizabadi, M.M. Detection of Mycobacterium avium subsp. paratuberculosis in Iranian patients with type 1 diabetes mellitus by PCR and ELISA. J. Infect. Dev. Ctries. 2016, 10, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Songini, M.; Mannu, C.; Targhetta, C.; Bruno, G. Type 1 diabetes in Sardinia: Facts and hypotheses in the context of worldwide epidemiological data. Acta Diabetol. 2017, 54, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Yokoyama, K.; Hattori, N. Conflicting role of Mycobacterium species in multiple sclerosis. Front. Neurol. 2017, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chauhan, D.S.; Singh, S.V.; Kumar, V.; Singh, A.; Yadav, A.; Yadav, V.S. Current status of Mycobacterium avium subspecies paratuberculosis infection in animals & humans in India: What needs to be done? Indian J. Med. Res. 2016, 144, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Corbett, C.S.; De Buck, J.; Orsel, K.; Barkema, H.W. Fecal shedding and tissue infections demonstrate transmission of Mycobacterium avium subsp. paratuberculosis in group-housed dairy calves. Vet. Res. 2017, 48, 27. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Paccagnini, D.; Salza, S.; Pacifico, A.; Ahmed, N.; Zanetti, S. Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: An infectious trigger? Clin. Infect. Dis. 2008, 46, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.; Rosu, V.; Paccagnini, D.; Cossu, D.; Pacifico, A.; Sechi, L.A. MAP3738c and MptD are specific tags of Mycobacterium avium subsp. paratuberculosis infection in type I diabetes mellitus. Clin. Immunol. 2011, 141, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.; Rosenfeld, G.; Bressler, B. Treatment of Crohn’s disease patients with infliximab is detrimental for the survival of Mycobacterium avium ssp. paratuberculosis within macrophages and shows a remarkable decrease in the immunogenicity of mycobacterial proteins. J. Crohn’s Colitis 2012, 6, 628–629. [Google Scholar] [CrossRef]
- Elguezabal, N.; Chamorro, S.; Molina, E.; Garrido, J.M.; Izeta, A.; Rodrigo, L.; Juste, R.A. Lactase persistence, NOD2 status and Mycobacterium avium subsp. paratuberculosis infection associations to Inflammatory Bowel Disease. Gut Pathog. 2012, 4. [Google Scholar] [CrossRef]
- Gill, C.O.; Saucier, L.; Meadus, W.J. Mycobacterium avium subsp. paratuberculosis in dairy products, meat, and drinking water. J. Food Prot. 2011, 74, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Eltholth, M.M.; Marsh, V.R.; Van Winden, S.; Guitian, F.J. Contamination of food products with Mycobacterium avium paratuberculosis: A systematic review. J. Appl. Microbiol. 2009, 107, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.A.; Pietralonga, P.A.G.; Schwarz, D.G.G.; Faria, A.C.S.; Moreira, M.A.S. Short communication: Recovery of viable Mycobacterium avium subspecies paratuberculosis from retail pasteurized whole milk in Brazil. J. Dairy Sci. 2012, 95, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Serraino, A.; Finazzi, G.; Daminelli, P.; Losio, M.N.; Arrigoni, N.; Piva, S.; Florio, D.; Riu, R.; Zanoni, R.G. Sale of raw milk in Northern Italy: Food safety implications and comparison of different analytical methodologies for detection of foodborne pathogens. Foodborne Pathog. Dis. 2012, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Mundo, S.L.; Gilardoni, L.R.; Hoffman, F.J.; Lopez, O.J. Rapid and sensitive method to identify Mycobacterium avium subsp. paratuberculosis in cow milk using DNA-methylase Genotyping. Appl. Environ. Microbiol. 2012, 79, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Klanicova, B.; Slana, I.; Roubal, P.; Pavlik, I.; Kralik, P. Mycobacterium avium subsp. paratuberculosis survival during fermentation of soured milk products detected by culture and quantitative real time PCR methods. Int. J. Food Microbiol. 2012, 157, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.Y.; Grant, I.R.; Friedman, M.; Elliott, C.T.; Situ, C. Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 2008, 74, 5986–5990. [Google Scholar] [CrossRef] [PubMed]
- Crandall, P.G.; Ricke, S.C.; O’Bryan, C.A.; Parrish, N.M. In vitro effects of citrus oils against Mycobacterium tuberculosis and non-tuberculous Mycobacteria of clinical importance. J. Environ. Sci. Health B 2012, 47, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Nowotarska, S.W.; Nowotarski, K.I.; Friedman, M.; Situ, C. Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 2014, 19, 7497–7515. [Google Scholar] [CrossRef] [PubMed]
- Blankemeyer, J.T.; White, J.B.; Stringer, B.K.; Friedman, M. Effect of α-tomatine and tomatidine on membrane potential of frog embryos and active transport of ions in frog skin. Food Chem. Toxicol. 1997, 35, 639–646. [Google Scholar] [CrossRef]
- Friedman, M.; Burns, C.F.; Butchko, C.A.; Blankemeyer, J.T. Folic acid protects against potato glycoalkaloid α-chaconine-induced disruption of frog embryo cell membranes and developmental toxicity. J. Agric. Food Chem. 1997, 45, 3991–3994. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.A.; Yu, C.B.; Park, H.D. Bacteriocidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003, 37, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 2005, 49, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Gidalevitz, D.; Ishitsuka, Y.; Muresan, A.S.; Konovalov, O.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y. Interaction of antimicrobial peptide protegrin with biomembranes. Proc. Natl. Acad. Sci. USA 2003, 100, 6302–6307. [Google Scholar] [CrossRef] [PubMed]
- Giordani, C.; Molinari, A.; Toccacieli, L.; Calcabrini, A.; Stringaro, A.; Chistolini, P.; Arancia, G.; Diociaiuti, M. Interaction of tea tree oil with model and cellular membranes. J. Med. Chem. 2006, 49, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Epand, R.F. Domains in bacterial membranes and the action of antimicrobial agents. Mol. BioSyst. 2009, 5, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Nowotarski, K.; Gruszecki, W.I.; Janas, T. The effect of hexadecaprenol on molecular organisation and transport properties of model membranes. Acta Biochim. Pol. 2000, 47, 661–673. [Google Scholar] [PubMed]
- Lorite, G.S.; Nobre, T.M.; Zaniquelli, M.E.D.; de Paula, E.; Cotta, M.A. Dibucaine effects on structural and elastic properties of lipid bilayers. Biophys. Chem. 2009, 139, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta 2009, 1788, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, F.; Imura, Y.; Ohno, K.; Zendo, T.; Nakayama, J.; Matsuzaki, K.; Sonomoto, K. Peptide-lipid huge toroidal pore, a new antimicrobial mechanism mediated by a lactococcal bacteriocin, lacticin Q. Antimicrob. Agents Chemother. 2009, 53, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Smaby, J.M.; Kulkarni, V.S.; Momsen, M.; Brown, R.E. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys. J. 1996, 70, 868–877. [Google Scholar] [CrossRef]
- Hac-Wydro, K.; Wydro, P. The influence of fatty acids on model cholesterol/phospholipid membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Ultee, A.; Gorris, L.G.M.; Smid, E.J. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 1998, 85, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, K.; Mattila, J.-P.; Kinnunen, P.K.J. Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys. J. 2008, 95, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Demel, R.A.; Peelen, T.; Siezen, R.J.; De Kruijff, B.; Kuipers, O.P. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity: A monolayer study. Eur. J. Biochem. 1996, 235, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Ruspantini, I.; Giordani, C.; Bordi, F.; Chistolini, P. Distribution of GD3 in DPPC monolayers: A thermodynamic and atomic force microscopy combined study. Biophys. J. 2004, 86, 321–328. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [PubMed]
- Paul, S.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725–731. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Friedman, M.; Brown, E.F. Molecular binding of black tea theaflavins to biological membranes: Relationship to bioactivities. J. Agric. Food Chem. 2011, 59, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
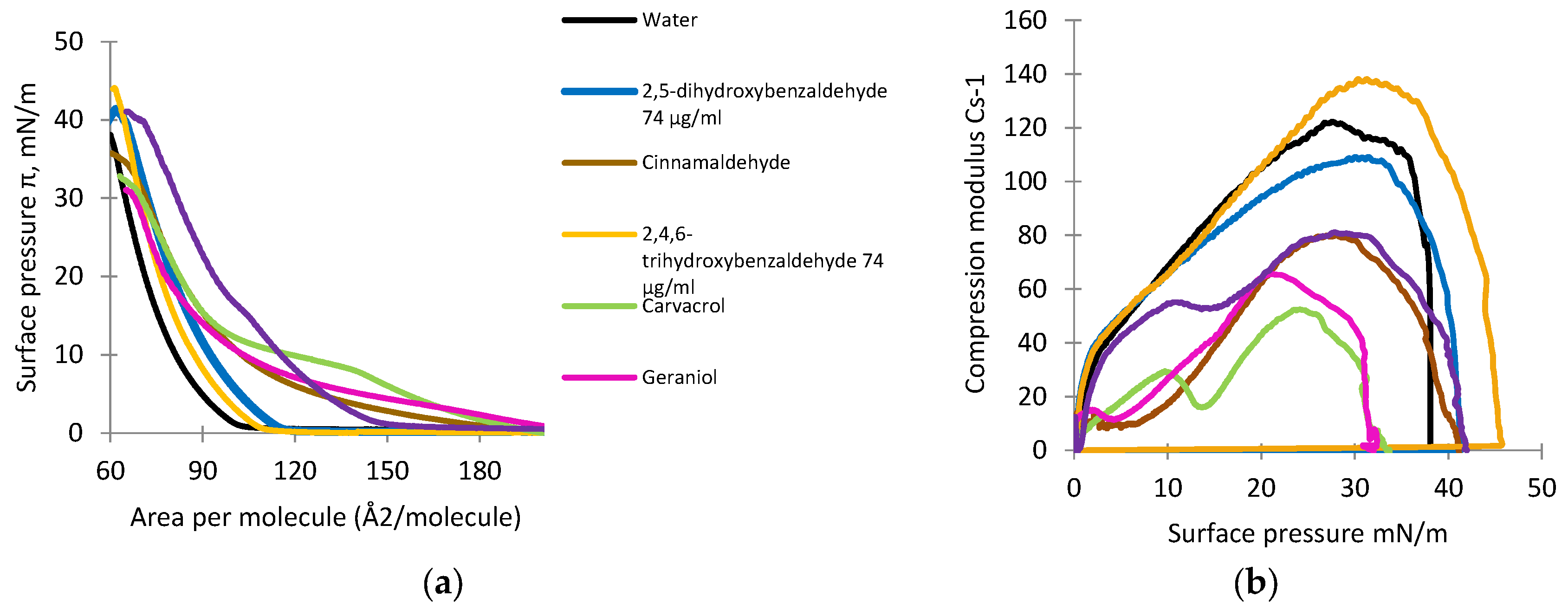
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. https://doi.org/10.3390/foods6090072
Nowotarska SW, Nowotarski K, Grant IR, Elliott CT, Friedman M, Situ C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods. 2017; 6(9):72. https://doi.org/10.3390/foods6090072
Chicago/Turabian StyleNowotarska, Stella W., Krzysztof Nowotarski, Irene R. Grant, Christopher T. Elliott, Mendel Friedman, and Chen Situ. 2017. "Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map)" Foods 6, no. 9: 72. https://doi.org/10.3390/foods6090072




