Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Gas-Liquid Chromatography-Mass Spectrometry (GLC-MS)
2.3. Antioxidant Activity
2.4. Cytotoxicity
2.5. Hemolytic Activity
2.6. Hierarchical Cluster Analysis
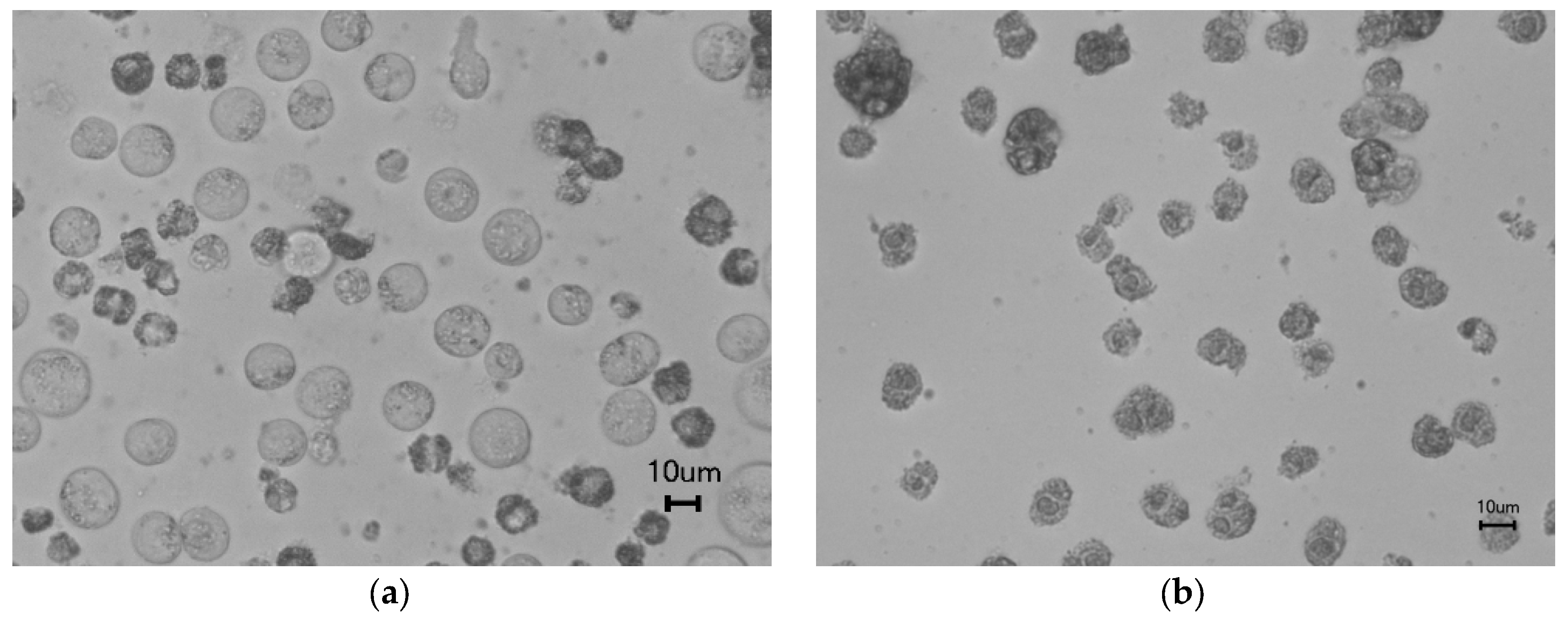
2.7. Microscopic Observation
2.8. Data Analysis
3. Results and Discussion
3.1. Chemical Composition
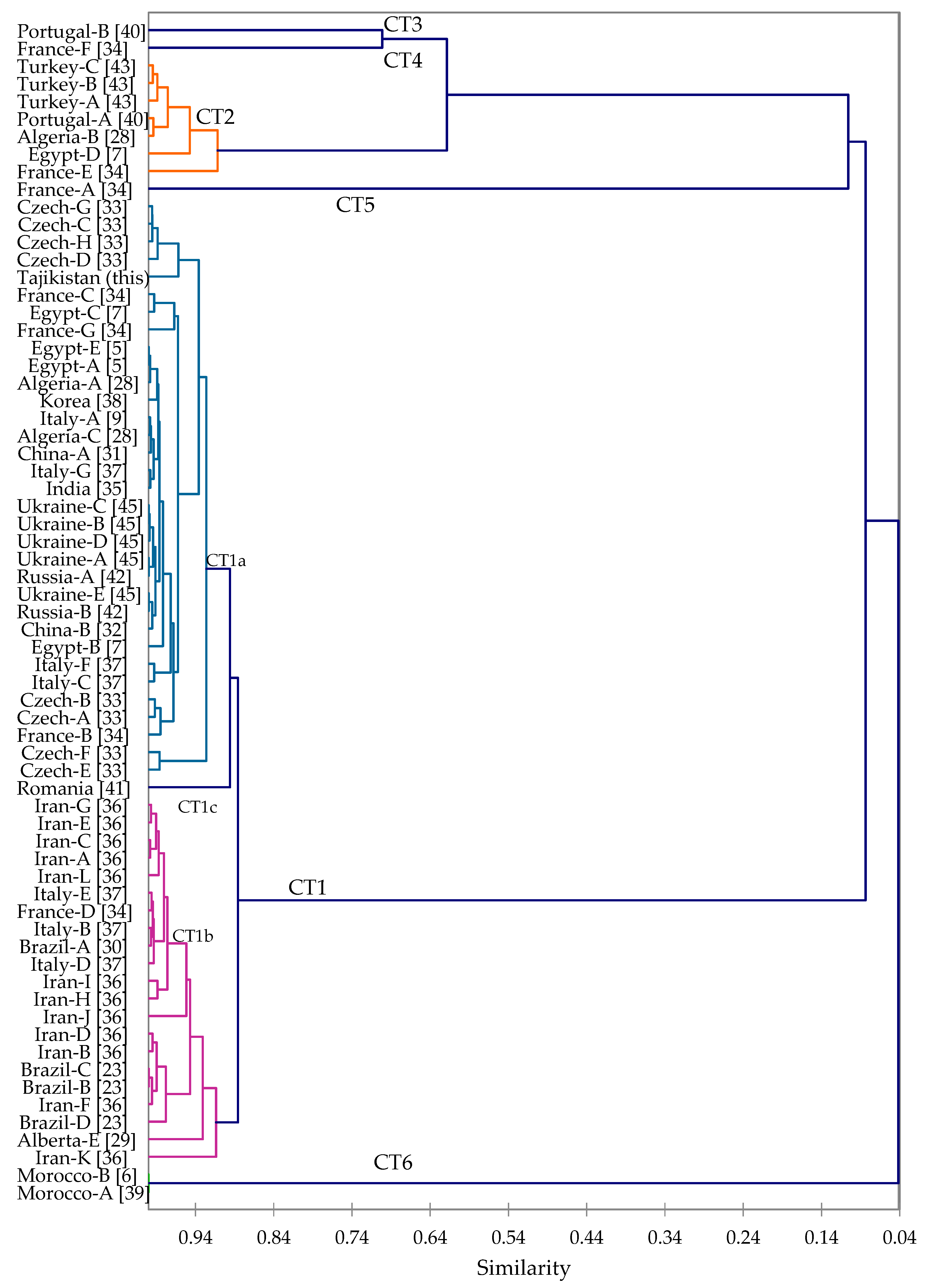
3.2. Cluster Analysis
3.3. Antioxidant Activity
3.4. Cytotoxicity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Mimica-Dukic, N.; Kujundyic, S.; Sokovic, M.; Couladis, M. Essential oil composition and antifungal activity of Foeniculum vulgare Mill. Obtained by different distillation conditions. Phytother. Res. 2003, 17, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2012, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World; Briza: Pretoria, Africa, 2004. [Google Scholar]
- Roby, M.H.; Sarhan, M.A.; Selim, K.A.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Ouariachi, E.E.; Lahhit, N.; Bouyanzer, A.; Hammouti, B.; Paolini, J.; Majidi, L.; Desjobert, J.M.; Costa, J. Chemical composition and antioxidant activity of essential oils and solvent extracts of Foeniculum vulgare Mill. from Morocco. J. Chem. Pharm. Res. 2014, 6, 743–748. [Google Scholar]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, I.; Audeh, A.; Alnaser, A.; Tayoub, G. Chemical constituents and insecticidal activity of the essential oil from fruits of Foeniculum vulgare Miller on larvae of khapra beetle (Trogoderma granarium Everts). Herba Polonica 2013, 59, 86–96. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Pagiotti, R.; Dominici, L.; Fatigoni, C.; Vannini, S.; Levorato, S.; Moretti, M. Investigation of the cytotoxic, genotoxic, and apoptosis-inducing effects of estragole isolated from fennel (Foeniculum vulgare). J. Nat. Prod. 2014, 77, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Alok, U.; Khanna, A. In vitro cytotoxicity of essensial oils: A review. Int. J. Res. Pharm. Chem. 2013, 3, 675–681. [Google Scholar]
- Heydarzade, A.; Moravvej, G. Contact toxicity and persistence of essential oils from Foeniculum vulgare, Teucrium polium and Satureja hortensis against Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) adults. Turk. J. Entomol. 2012, 36, 507–518. [Google Scholar]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Bassi, A.M.; Penco, S.; Canuto, R.A.; Muzioa, G.; Ferro, M. Comparative evaluation of cytotoxicity and metabolism of four aldehydes in two hepatoma cell lines. Drug Chem. Toxicol. 1997, 20, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Sonboli, A.; Esmaeili, M.A.; Gholipour, A.; Kanani, M.R. Composition, cytotoxicity and antioxidant activity of the essential oil of Dracocephalum surmandinum from Iran. Nat. Prod. Commun. 2010, 5, 341–344. [Google Scholar]
- Sharopov, F.S.; Wink, M.; Khalifaev, D.R.; Zhang, H.; Dosoky, N.S.; Setzer, W.N. Chemical composition and antiproliferative activity of the essential oil of Galagania fragrantissima Lipsky (Apiaceae). Am. J. Essent. Oils Nat. Prod. 2013, 1, 11–13. [Google Scholar]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Zellagui, A.; Gherraf, N.; Elkhateeb, A.; Hegazy, M.F.; Mohamed, T.A.; Touil, A.; Shahat, A.; Rhouati, S. Chemical constitutents from Algerian Foeniculum vulgare aerial parts and evaluation of antimicrobial activity. J. Chil. Chem. Soc. 2011, 56, 759–763. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Mobarak, H.; Bhuiyan, N.I.; Nandi, N.C. Constituents of essential oils from leaves and seeds of Foeniculum vulgare Mill. cultivated in Bangladesh. Bangladesh J. Bot. 2009, 38, 181–183. [Google Scholar] [CrossRef]
- Dadaliogylu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Spac, A.; Hancianu, M.; Miron, A.; Tanasescu, V.F.; Dorneanu, V.; Stanescu, U. The chemical profile of essensial oils obtained from fennel fruits (Foeniculum vulgare Mill.). Farmacia 2010, 58, 46–53. [Google Scholar]
- Radulovic, N.S.; Blagojevic, P.D. A note on the volatile secondary metabolites of Foeniculum vulgare Mill. (Apiaceae). Facta Univ. 2010, 8, 25–37. [Google Scholar] [CrossRef]
- Stefanini, M.B.; Ming, L.C.; Marques, M.O.; Facanali, R.; Meireles, M.A.; Moura, L.S.; Marchese, J.A.; Sousa, L.A. Essential oil constituents of different organs of fennel (Foeniculum vulgare var. vulgare). Braz. J. Med. Plants 2006, 8, 193–198. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Co. Carol Stream: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components—An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [PubMed]
- Sharopov, F.S. Phytochemistry and Bioactivities of Selected Plant Species with Volatile Secondary Metabolites. Ph.D. Thesis, Ruperto-Carola University of Heidelberg, Heidelberg, Germany, 2015. [Google Scholar]
- Sharopov, F.; Valiev, A.; Satyal, P.; Setzer, W.N.; Wink, M. Chemical composition and anti-proliferative activity of the essential oil of Coriandrum sativum L. Am. J. Essent. Oils Nat. Prod. 2017, 5, 11–15. [Google Scholar]
- Zoubiri, S.; Baaliouamer, A. Chemical composition and insecticidal properties of some aromatic herbs essential oils from Algeria. Food Chem. 2011, 129, 179–182. [Google Scholar] [CrossRef]
- Embong, M.B.; Hadziyev, D.; Molnar, S. Essential oils from spices grown in Alberta. Fennel oil (Foeniculum vulgare var. dulce). Can. J. Plant Sci. 1977, 57, 829–837. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.M.; Dias, H.J.; Crotti, A.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhou, L.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, X.; Liang, J. In vitro antifungal activity and mechanism of essential oil from fennel (Foeniculum vulgare l.) on dermatophyte species. J. Med. Microbiol. 2015, 64, 93–103. [Google Scholar] [CrossRef]
- Pavela, R.; Zabka, M.; Bednar, J.; Tríska, J.; Vrchotova, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Muckensturm, B.; Foechterlen, D.; Reduron, J.P.; Dantont, T.P.; Hildenbrand, M. Phytochemical and chemotaxonomic studies of Foeniculum vulgare. Biochem. Syst. Ecol. 1997, 25, 353–358. [Google Scholar] [CrossRef]
- Hashmi, N.; Khan, M.M.; Moinuddin; Idrees, M.; Khan, Z.H.; Ali, A.; Varshney, L. Depolymerized carrageenan ameliorates growth, physiological attributes, essential oil yield and active constituents of Foeniculum vulgare Mill. Carbohydr. Polym. 2012, 90, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Rahimmalek, M.; Maghsoudi, H.; Sabzalian, M.R.; Ghasemi Pirbalouti, A. Variability of essential oil content and composition of different Iranian fennel (Foeniculum vulgare Mill.) accessions in relation to some morphological and climatic factors. J. Agric. Sci. Technol. 2014, 16, 1365–1374. [Google Scholar]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; Falco, E.D. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [PubMed]
- Han, A.Y.; Lee, H.S.; Seol, G.H. Foeniculum vulgare Mill. Increases cytosolic Ca2+ concentration and inhibits store-operated Ca2+ entry in vascular endothelial cells. Biomed. Pharmacother. 2016, 84, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Lahhit, N.; Bouyanzer, A.; Desjobert, J.M.; Hammouti, B.; Salghi, R.; Costa, J.; Jama, C.; Bentiss, F.; Majidi, L. Fennel (Foeniculum vulgare) essential oil as green corrosion inhibitor of carbon steel in hydrochloric acid solution. Port. Electrochim. Acta 2011, 29, 127–138. [Google Scholar] [CrossRef]
- Sousa, R.M.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae essential oils and volatile compounds on hatchability, development, reproduction and nutrition of Pseudaletia unipuncta (Lepidoptera: Noctuidae). Ind. Crops Prod. 2015, 63, 226–237. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mircea, C.; Trifan, A.; Hritcu, L. Essential oils from Apiaceae as valuable resources in neurologicaldisorders: Foeniculi vulgare aetheroleum. Ind. Crops Prod. 2016, 88, 51–57. [Google Scholar] [CrossRef]
- Gorbunova, E.V. Obosnovanie Osnovnich Elementov Technologii Kompleksnoy Pererabotki Sirya Fenchelya Obiknovennogo (Foeniculum vulgare Mill.). Ph.D. Thesis, Michurinsk State Agricultural University, Michurinsk, Russia, 2015. [Google Scholar]
- Oezcan, M.M.; Chalchat, J.C.; Arslan, D.; Ateş, A.; Uenver, A. Comparative essential oil composition and antifungal effect of bitter fennel (Foeniculum vulgare ssp. piperitum) fruit oils obtained during different vegetation. J. Med. Food 2006, 9, 552–561. [Google Scholar]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. [Google Scholar] [CrossRef]
- Timasheva, L.A.; Gorbunova, E.V. A promising trend in the processing of fennel (Foeniculum vulgare Mill.) whole plants. Foods Raw Mater. 2014, 2, 51–57. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Lampasona, M.P.; Catalan, C. Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control 2006, 17, 745–752. [Google Scholar] [CrossRef]
- Barazani, O.; Cohen, Y.; Fait, A.; Diminshtein, S.; Dudai, N.; Ravid, U.; Putievsky, E.; Friedman, J. Chemotypic differentiation in indigenous populations of Foeniculum vulgare var. vulgare in israel. Biochem. Syst. Ecol. 2002, 30, 721–731. [Google Scholar] [CrossRef]
- Gross, M.; Friedman, J.; Dudai, N.; Larkov, O.; Cohen, Y.; Bar, E.; Ravid, U.; Putievsky, E.; Lewinsohn, E. Biosynthesis of estragole and t-anethole in bitter fennel (Foeniculum vulgare Mill. var. vulgare) chemotypes. Changes in sam: Phenylpropene o-methyltransferase activities during development. Plant Sci. 2002, 163, 1047–1053. [Google Scholar]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2010; p. 994. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, A.; Frosali, S.; Andreassi, M.; Stefano, A.D. Lycopene phytocomplex, but not pure lycopene, is able to trigger apoptosis and improve the efficacy of photodynamic therapy in HL60 human leukemia cells. Exp. Biol. Med. 2010, 235, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar]
- Kulbacka, J.; Daczewska, M.; Dubińska-Magiera, M.; Choromańska, A.; Rembiałkowska, N.; Surowiak, P.; Kulbacki, M.; Kotulska, M.; Saczko, J. Doxorubicin delivery enhanced by electroporation to gastrointestinal adenocarcinoma cells with P-gp overexpression. Bioelectrochemistry 2014, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, Z.M.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with abc-transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.T.S. Cytotoxic and xenoestrogenic effects via biotransformation of trans-anethole on isolated rat hepatocytes and cultured mcf-7 human breast cancer cells. Biochem. Pharmacol. 2003, 66, 63–73. [Google Scholar] [CrossRef]
- Muthukumari, D.; Padma, P.R.; Sumathi, S. In vitro analysis of anethole as an anticancerous agent for triple negative breast cancer. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 314–318. [Google Scholar]
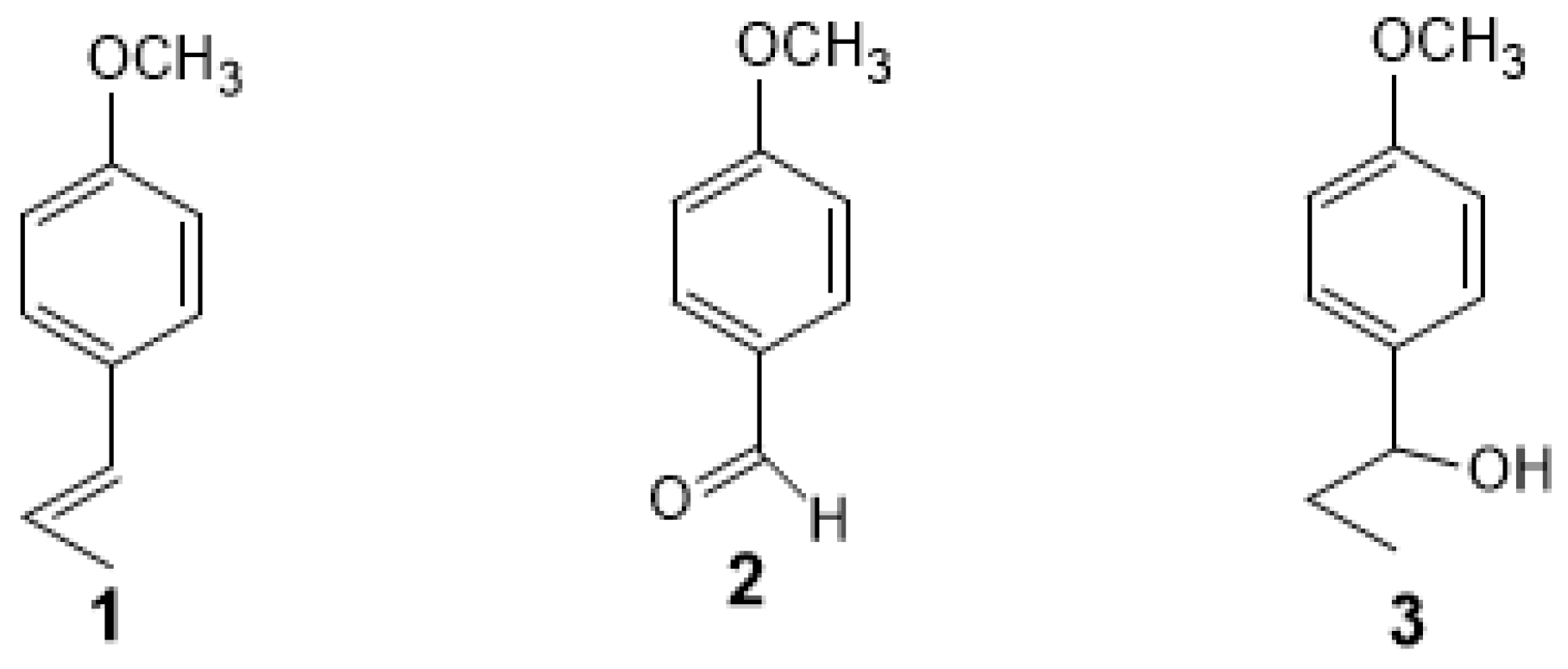
| Compounds | % * | RT ** | RI *** |
|---|---|---|---|
| trans-Anethole | 36.8 | 36.022 | 1286 |
| α-Ethyl-p-methoxybenzyl alcohol | 9.10 | 54.059 | 1569 |
| p-Anisaldehyde | 7.73 | 33.825 | 1254 |
| Carvone | 4.87 | 33.119 | 1243 |
| 1-Phenylpenta-2,4-diyne | 4.75 | 35.875 | 1283 |
| Fenchyl butanoate | 4.23 | 46.653 | 1448 |
| Neomenthol | 3.62 | 28.027 | 1170 |
| (2E)-Dodecenal | 3.44 | 47.807 | 1467 |
| β-Ethyl-p-methoxybenzyl alcohol | 3.27 | 54.498 | 1577 |
| trans-Thujone | 2.95 | 24.404 | 1118 |
| Fenchone | 2.75 | 22.408 | 1089 |
| Carvacrol | 2.15 | 36.802 | 1297 |
| Linalyl acetate | 1.88 | 33.503 | 1249 |
| Unidentified | 1.39 | 42.318 | 1380 |
| (E)-Chrysanthenyl acetate | 1.38 | 34.015 | 1256 |
| Thymol | 1.03 | 36.255 | 1289 |
| Fenchyl isobutanoate | 1.03 | 47.726 | 1465 |
| (E)-β-Terpineol | 1.00 | 28.642 | 1179 |
| Linalool | 0.75 | 23.139 | 1100 |
| cis-Thujone | 0.74 | 23.624 | 1107 |
| (E)-Dihydrocarvone | 0.64 | 30.422 | 1204 |
| Unidentified | 0.64 | 48.021 | 1470 |
| Geranial | 0.56 | 34.747 | 1267 |
| Myrtenyl acetate | 0.54 | 36.160 | 1288 |
| exo-Fenchyl acetate | 0.48 | 32.269 | 1231 |
| Penta-1,3-diynylbenzene | 0.46 | 40.505 | 1353 |
| Dill ether | 0.44 | 29.148 | 1186 |
| Methylchavicol (=estragole) | 0.42 | 29.973 | 1198 |
| Unidentified | 0.30 | 53.885 | 1567 |
| Caryophyllene oxide | 0.25 | 54.759 | 1581 |
| Camphor | 0.23 | 26.445 | 1147 |
| iso-Menthone | 0.10 | 27.050 | 1156 |
| 1-Hexadecene | 0.08 | 48.270 | 1474 |
| Terpene hydrocarbons: | 5.21 | ||
| Oxygenated terpenoids: | 85.78 | ||
| Others: | 6.70 | ||
| Total identified: | 97.67 |
| Sample | DPPH IC50 (g L−1) | ABTS IC50 (g L−1) | FRAP µM Fe(II)/mg of Samples |
|---|---|---|---|
| Foeniculum vulgare | 15.6 ± 1.1 ** | 10.9 ± 0.4 ** | 194 ± 18 ** |
| trans-Anethole | 23.4 ± 0.1 ** | 35.6 ± 0.1 ** | 104 ± 5.2 ** |
| Caffeic acid | 0.0017 ± 0.0002 *** | 0.0011 ± 0.0002 *** | 2380 ± 46 *** |
| Sample | HeLa | Caco-2 | MCF-7 | CCRF-CEM | CEM/ADR 5000 | RBC |
|---|---|---|---|---|---|---|
| IC50, mg L−1 * | ||||||
| Foeniculum vulgare | 207 ± 13 ** | 75 ± 4 ** | 59 ± 5 ** | 32 ± 1 ** | 165 ± 15 *** | 1100 ± 50 ** |
| Doxorubicin | 4.5 ± 0.6 ** | 1.1 ± 0.1 ** | 1.3 ± 0.3 ** | 0.25 ± 0.2 ** | 1.4 ± 0.4 ** | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.N.; Wink, M. Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan. Foods 2017, 6, 73. https://doi.org/10.3390/foods6090073
Sharopov F, Valiev A, Satyal P, Gulmurodov I, Yusufi S, Setzer WN, Wink M. Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan. Foods. 2017; 6(9):73. https://doi.org/10.3390/foods6090073
Chicago/Turabian StyleSharopov, Farukh, Abdujabbor Valiev, Prabodh Satyal, Isomiddin Gulmurodov, Salomudin Yusufi, William N. Setzer, and Michael Wink. 2017. "Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan" Foods 6, no. 9: 73. https://doi.org/10.3390/foods6090073







