The Macular Carotenoids Lutein and Zeaxanthin Are Related to Increased Bone Density in Young Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment of LZ Status: Macular Pigment & Serum LZ
2.3. Assessment of Bone Mineral Density
2.4. Assessment of Health Habits: Diet & Physical Activity
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takeda, H.; Tominari, T.; Hirata, M.; Watanabe, K.; Matsumoto, C.; Grundler, F.M.; Inada, M.; Miyaura, C. Lutein Enhances Bone Mass by Stimulating Bone Formation and Suppressing Bone Resorption in Growing Mice. Biol. Pharm. Bull. 2017, 40, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.; Inada, M.; Miyaura, C. Lutein, a carotenoid, suppresses osteoclastic bone resorption and stimulates bone formation in cultures. Biosci. Biotechnol. Biochem. 2017, 81, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Wattanapenpaiboon, N.; Lukito, W.; Wahlqvist, M.L.; Strauss, B.J. Dietary carotenoid intake as a predictor of bone mineral density. Asia Pac. J. Clin. Nutr. 2003, 12, 467–473. [Google Scholar] [PubMed]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2009, 89, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Wooten, B.R.; Snodderly, D.M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. A 1997, 14, 1187–1196. [Google Scholar] [CrossRef]
- Lawler, T.P.; Liu, Z.; Blomme, C.; Hammond, R.; Gangnon, R.; Johnson, E.; Wallace, R.; Tinker, L.; Millen, A.; Wooten, B.; et al. Lutein and Zeaxanthin Supplement Use and Macular Pigment Change Over 14 years in the Second Carotenoids and Age-Related Eye Diseases Study (CAREDS2), an Ancillary Study to the Women’s Health Initiative. FASEB J. 2017, 31 (Suppl. lb337). [Google Scholar]
- Wooten, B.R.; Hammond, B.R.; Land, R.I.; Snodderly, D.M. A practical method for measuring macular pigment optical density. IOVS 1999, 40, 2481–2489. [Google Scholar]
- Stringham, J.M.; Hammond, B.R.; Nolan, J.M.; Wooten, B.R.; Mammen, A.; Smollon, W.; Snodderly, D.M. The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Exp. Eye Res. 2008, 87, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Fletcher, L.M.; Elliott, J.G. Glare Disability, Photostress Recovery, and Chromatic Contrast: Relation to Macular Pigment and Serum Lutein and Zeaxanthin Macular Pigment and Visual Function. IOVS 2013, 54, 476–481. [Google Scholar]
- Lukaski, H.C. Soft tissue composition and bone mineral status: Evaluation by dual-energy X-ray absorptiometry. J. Nutr. 1993, 123, 438–443. [Google Scholar] [PubMed]
- Blair, S.N.; Haskell, W.L.; Ho, P.; Paffenbarger, R.S.; Vranizan, K.M.; Farquhar, J.W.; Wood, P.D. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am. J. Epidemiol. 1985, 122, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2007, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Beta-cryptoxanthin and bone metabolism: The preventive role in osteoporosis. J. Health Sci. 2008, 54, 356–369. [Google Scholar] [CrossRef]
- Maggio, D.; Polidori, M.C.; Barabani, M.; Tufi, A.; Ruggiero, C.; Cecchetti, R.; Aisa, M.C.; Stahl, W.; Cherubini, A. Low levels of carotenoids and retinol in involutional osteoporosis. Bone 2006, 38, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.L.; Cauley, J.A.; Pettinger, M.; Jackson, R.; Lacroix, A.; Leboff, M.S.; Lewis, C.E.; Nevitt, M.C.; Lewis, C.E.; Wactawski-Wade, J.; et al. Lack of relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women’s Health Initiative. Am. J. Clin. Nutr. 2005, 82, 581–588. [Google Scholar] [PubMed]
- Mares, J.A.; LaRowe, T.L.; Snodderly, D.M.; Moeller, S.M.; Gruber, M.J.; Klein, M.L.; Wooten, B.R.; Johnson, E.J.; Chappell, R.J. CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am. J. Clin. Nutr. 2006, 84, 1107–1122. [Google Scholar] [PubMed]
- Ciulla, T.A.; Curran-Celantano, J.; Cooper, D.A.; Hammond, B.R.; Danis, R.P.; Pratt, L.M.; Riccardi, K.A.; Filloon, T.G. Macular pigment optical density in a midwestern sample. Ophthalmology 2001, 108, 730–737. [Google Scholar] [CrossRef]
- Altindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2008, 28, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Khoshhal, K.I. Calcium metabolism and oxidative stress in bone fractures: Role of antioxidants. Curr. Drug Metab. 2007, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodeling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. Int. Rev. J. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]

| N = 63 | Males (N = 24) | Females (N = 39) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Macular Pigment | |||||||||
| MPOD 7.5′ | 0.57 | ± | 0.20 | 0.59 | ± | 0.20 | 0.57 | ± | 0.21 |
| MPOD 30′ | 0.46 | ± | 0.16 | 0.47 | ± | 0.15 | 0.46 | ± | 0.17 |
| MPOD 60′ | 0.30 | ± | 0.13 | 0.30 | ± | 0.11 | 0.30 | ± | 0.14 |
| MPOD 105′ | 0.12 | ± | 0.08 | 0.13 | ± | 0.08 | 0.12 | ± | 0.08 |
| Serum (μmol/L) * | |||||||||
| LZ | 0.25 | ± | 0.12 | 0.21 | ± | 0.07 | 0.28 | ± | 0.13 |
| Lutein (L) | 0.19 | ± | 0.09 | 0.14 | ± | 0.06 | 0.21 | ± | 0.10 |
| Zeaxanthin (Z) | 0.07 | ± | 0.03 | 0.06 | ± | 0.02 | 0.07 | ± | 0.03 |
| Body Fat (percentage) | |||||||||
| Total | 24.92 | ± | 6.64 | 18.70 | ± | 4.91 | 28.75 | ± | 4.23 |
| Arms | 25.25 | ± | 8.54 | 16.70 | ± | 4.85 | 30.52 | ± | 5.48 |
| Trunk | 21.60 | ± | 6.23 | 17.24 | ± | 5.88 | 24.28 | ± | 4.80 |
| Legs | 29.87 | ± | 8.62 | 20.85 | ± | 5.08 | 35.42 | ± | 4.76 |
| Bone Mineral Density (g/cm2) | |||||||||
| Proximal Femur | 1.00 | ± | 0.13 | 1.04 | ± | 0.15 | 0.97 | ± | 0.10 |
| Lumbar Spine | 1.03 | ± | 0.12 | 1.03 | ± | 0.13 | 1.04 | ± | 0.11 |
| Health Habit Screeners | |||||||||
| Calories Expended | 2574 | ± | 538 | 2818 | ± | 486 | 2440 | ± | 525 |
| F/V Intake (serving/week) | 6.87 | ± | 2.28 | 6.44 | ± | 2.30 | 7.12 | ± | 2.27 |
| Calc/Vit D Intake (serving/week) | 18.95 | ± | 6.39 | 19.88 | ± | 8.30 | 18.42 | ± | 5.09 |
| Age (years) | 22.52 | ± | 3.71 | 21.71 | ± | 4.09 | 23.03 | ± | 3.41 |
| Body Mass Index (kg/m2) | 22.87 | ± | 2.59 | 23.47 | ± | 2.33 | 22.51 | ± | 2.70 |
| Bone Mineral Density (g/cm2) | |||||||
|---|---|---|---|---|---|---|---|
| Lumbar Spine | Proximal Femur | ||||||
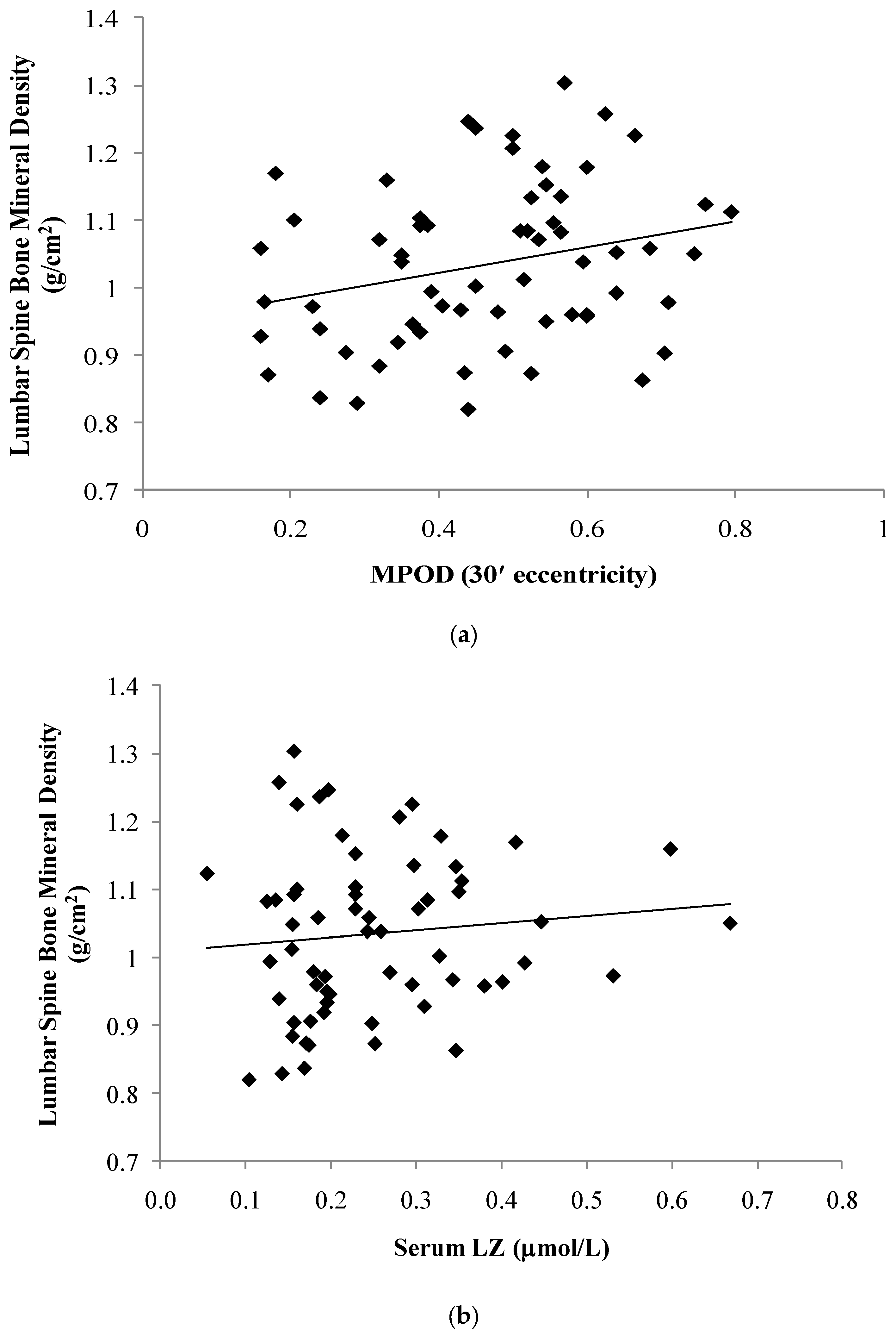
| Macular Pigment | MPOD 7.5′ | 0.29 | | | 0.02 | 0.32 | | | 0.02 |
| MPOD 30′ | 0.26 | | | 0.04 | 0.30 | | | 0.03 | |
| MPOD 60′ | 0.22 | | | 0.09 | 0.24 | | | 0.08 | |
| MPOD 105′ | 0.28 | | | 0.03 | 0.43 | | | 0.00 | |
| Serum (mol/L) | LZ | 0.10 | | | 0.42 | 0.06 | | | 0.65 |
| Lutein (L) | 0.09 | | | 0.50 | 0.02 | | | 0.88 | |
| Zeaxanthin (Z) | 0.14 | | | 0.27 | 0.18 | | | 0.19 | |
| Total Body Fat Percentage | 0.13 | | | 0.30 | -0.06 | | | 0.68 | |
| Calories Expended | 0.16 | | | 0.28 | 0.53 | | | 0.00 | |
| F/V Intake (serving/week) | 0.12 | | | 0.41 | 0.13 | | | 0.41 | |
| Calc/Vit D Intake (serving/week) | 0.34 | | | 0.02 | 0.38 | | | 0.02 | |
| Calories Expended | Calcium/Vitamin D Intake | ||||||
|---|---|---|---|---|---|---|---|
| Macular Pigment | MPOD 7.5′ | 0.31 | | | 0.03 | 0.30 | | | 0.04 |
| MPOD 30′ | 0.31 | | | 0.03 | 0.30 | | | 0.04 | |
| MPOD 60′ | 0.22 | | | 0.13 | 0.29 | | | 0.05 | |
| MPOD 105′ | 0.32 | | | 0.03 | 0.22 | | | 0.15 | |
| Serum (mol/L) | LZ | 0.18 | | | 0.23 | 0.12 | | | 0.44 |
| Lutein (L) | 0.12 | | | 0.41 | 0.10 | | | 0.50 | |
| Zeaxanthin (Z) | 0.33 | | | 0.02 | 0.15 | | | 0.33 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovier, E.R.; Hammond, B.R. The Macular Carotenoids Lutein and Zeaxanthin Are Related to Increased Bone Density in Young Healthy Adults. Foods 2017, 6, 78. https://doi.org/10.3390/foods6090078
Bovier ER, Hammond BR. The Macular Carotenoids Lutein and Zeaxanthin Are Related to Increased Bone Density in Young Healthy Adults. Foods. 2017; 6(9):78. https://doi.org/10.3390/foods6090078
Chicago/Turabian StyleBovier, Emily R., and Billy R. Hammond. 2017. "The Macular Carotenoids Lutein and Zeaxanthin Are Related to Increased Bone Density in Young Healthy Adults" Foods 6, no. 9: 78. https://doi.org/10.3390/foods6090078




