Evaluation of Different Dose-Response Models for High Hydrostatic Pressure Inactivation of Microorganisms
Abstract
:1. Introduction
2. The Theory
2.1. Discrete Model
If P ≥ Pmin S(P) = exp(– k(P − Pmin))
2.2. Fermi Equation
2.3. Shoulder Model
2.4. Weibull Model
2.5. Calculation of P5
3. The Methodology
3.1. Data Selection, Fit of the Models, and Statistical Analysis
4. Results and Discussion
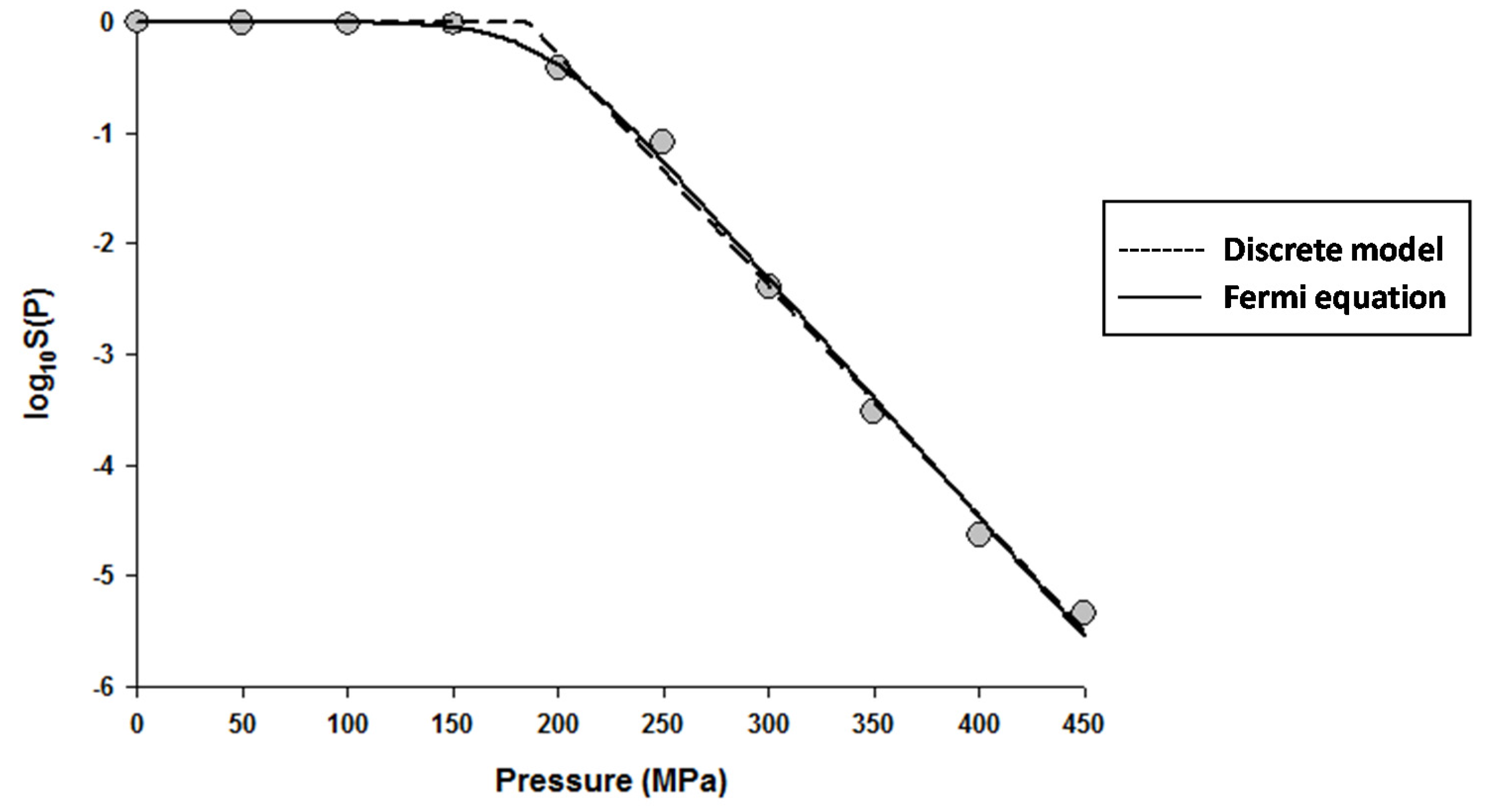
4.1. Goodness-of-Fit of the Models
4.2. Parameters of the Models
4.3. Effects of Substrate on Model Parameters and P5 Values
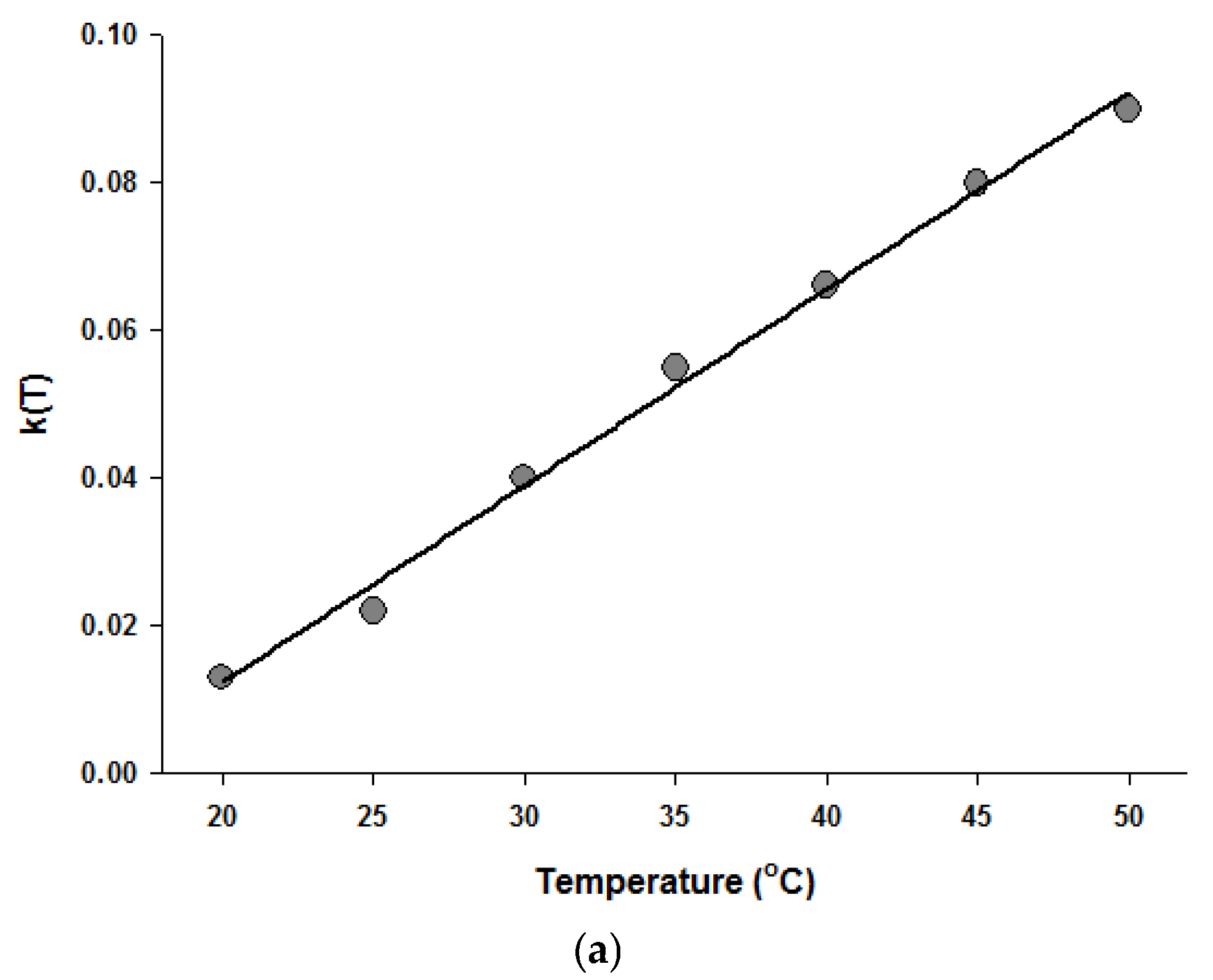
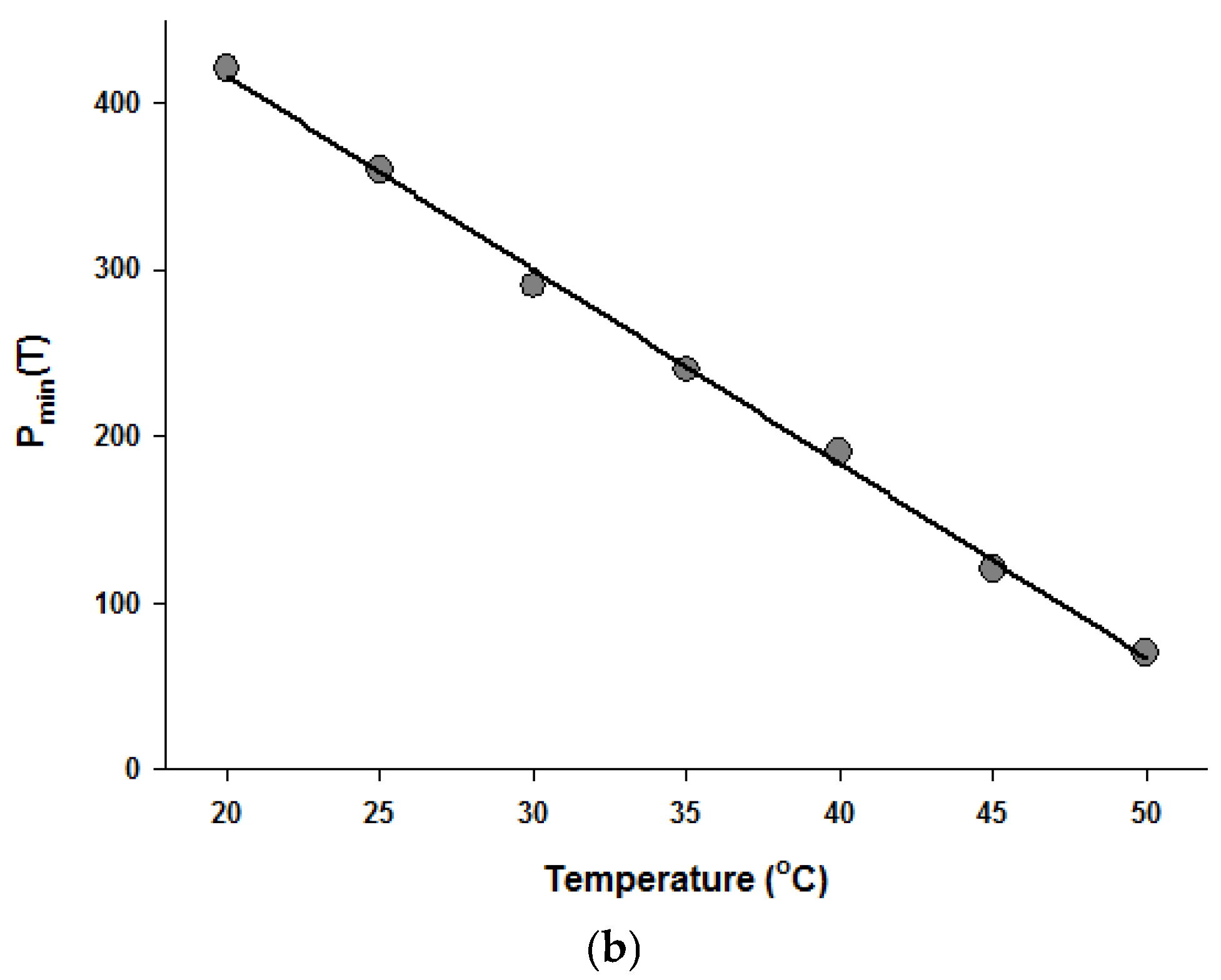
4.4. Effect of Holding Time, Temperature, and Compression and Decompression Rates on Model Parameters and P5 Values
4.5. Possible Applications of the Fermi Equation
5. Conclusions and Future Outlook
Conflicts of Interest
References
- Buzrul, S.; Alpas, H.; Bozoglu, F. Effects of high hydrostatic pressure on shelf life of lager beer. Eur. Food Res. Technol. 2005, 220, 615–618. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov. Food Sci. Emerg. Technol. 2011, 12, 104–110. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Eng. Rev. 2011, 3, 44–61. [Google Scholar] [CrossRef]
- Buzrul, S. High hydrostatic pressure treatment of beer and wine: A review. Innov. Food Sci. Emerg. Technol. 2012, 13, 1–12. [Google Scholar] [CrossRef]
- Buzrul, S. Multi-pulsed high hydrostatic pressure inactivation of microorganisms: A review. Innov. Food Sci. Emerg. Technol. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Huang, H.-W.; Lung, H.-M.; Yang, B.B.; Wang, C.-Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Guan, D.; Chen, H.; Hoover, D.G. Inactivation of Salmonella typhimurium DT 104 in UHT whole milk by high hydrostatic pressure. Int. J. Food Microbiol. 2005, 104, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.M.; Ting, E.Y.; Stewart, C.M.; Robbins, J.A. Recommended laboratory practices for conducting high pressure microbial inactivation experiments. Innov. Food Sci. Emerg. Technol. 2004, 5, 299–306. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Balasubramaniam, V.M. Fundamentals of Food Processing Using High Pressure. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Cánovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA; Institute of Food Technologists: Chicago, IL, USA, 2011; pp. 3–19. [Google Scholar]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Tonello Samson, C.; Welti-Chanes, J.; Torres, J.A. High-pressure processing technologies for the pasteurization and sterilization of foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Damrau, E. Mathematical interpretation of dose-response curves. Bull. Math. Biol. 1997, 59, 747–761. [Google Scholar] [CrossRef]
- Peleg, M. Advanced Quantitative Microbiology for Food and Biosystems: Models for Predicting Growth and Inactivation; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Norton, T.; Sun, D.-W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S.; Zwietering, M.H. Experimental design, data processing and model fitting in predictive microbiology. In Modelling Microorganisms in Food; Brul, S., van Gerwen, S., Zwietering, M., Eds.; CRC Press: Boca Raton, FL, USA; Woodhead Publishing: Cambridge, UK, 2007; pp. 22–43. [Google Scholar]
- Peleg, M. Evaluation of the Fermi equation as a model of dose-response curves. Appl. Microbiol. Biotechnol. 1996, 46, 303–306. [Google Scholar] [CrossRef]
- Geeraerd, A.; Valdramidis, V.; Van Impe, J. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Buzrul, S. A suitable model of microbial growth. Afr. J. Microbiol. Res. 2009, 3, 468–474. [Google Scholar]
- Buzrul, S. A predictive model for high-pressure carbon dioxide inactivation of microorganisms. J. Food Saf. 2009, 29, 208–223. [Google Scholar] [CrossRef]
- Buzrul, S. Modeling and predicting inactivation of Escherichia coli under isobaric and dynamic high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2009, 10, 391–395. [Google Scholar] [CrossRef]
- Moussa, M.; Perrier-Cornet, J.-M.; Gervais, P. Synergistic and antagonistic effects of combined subzero temperature and high pressure on inactivation of Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.B.; Hoover, D.G. Inactivation of Campylobacter jejuni by high hydrostatic pressure. Lett. Appl. Microbiol. 2004, 38, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Doğan, C.; Erkmen, O. Ultra high hydrostatic pressure inactivation of Escherichia coli in milk, and orange and peach juices. Food Sci. Technol. Int. 2003, 9, 403–405. [Google Scholar] [CrossRef]
- Yamamoto, K.; Matsubara, M.; Kawasaki, S.; Bari, M.L.; Kawamoto, S. Modeling the pressure inactivation dynamics of Escherichia coli. Braz. J. Med. Biol. Res. 2005, 38, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, J.; Xie, H.; Yang, Y.; Lin, C. Inactivation of Staphylococcus aureus and Escherichia coli by the synergistic action of high hydrostatic pressure and dissolved CO2. Int. J. Food Microbiol. 2010, 144, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.L.; Kish, A.; Steele, A.; Hemley, R.J. Differential high pressure survival in stationary-phase Escherichia coli MG1655. High Press. Res. 2011, 31, 325–333. [Google Scholar] [CrossRef]
- Perrier-Cornet, J.-M.; Tapin, S.; Gaeta, S.; Gervais, P. High-pressure inactivation of Saccharomyces cerevisiae and Lactobacillus plantarum at subzero temperatures. J. Biotechnol. 2005, 115, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Sohn, K.H.; Shin, J.H.; Lee, H.J. High hydrostatic pressure inactivation of Lactobacillus viridescens and its effects on ultrastructure of cells. Int. J. Food Sci. Technol. 2001, 36, 775–781. [Google Scholar] [CrossRef]
- Simpson, R.K.; Gilmour, A. The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J. Appl. Microbiol. 1997, 83, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tholozan, J.L.; Ritz, M.; Jugiau, F.; Federighi, M.; Tissier, J.P. Physiological effects of high hydrostatic pressure treatments on Listeria monocytogenes and Salmonella typhimurium. J. Appl. Microbiol. 2000, 88, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O.; Dogan, C. Effects of ultra high hydrostatic pressure on Listeria monocytogenes and natural flora in broth, milk and fruit juices. Int. J. Food Sci. Technol. 2004, 39, 91–97. [Google Scholar] [CrossRef]
- Koseki, Y.; Yamamoto, K. pH and solute concentration of suspension media affect the outcome of high hydrostatic pressure treatment of Listeria monocytogenes. Int. J. Food Microbiol. 2006, 111, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Donsì, G.; Ferrari, G.; Maresca, P. On the modelling of the inactivation kinetics of Saccharomyces cerevisiae by means of combined temperature and high pressure treatments. Innov. Food Sci. Emerg. Technol. 2003, 4, 35–44. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hsu, C.-P.; Huang, H.-W.; Yang, B.B. The relationship between inactivation and morphological damage of Salmonella enterica treated by high hydrostatic pressure. Food Res. Int. 2013, 54, 1482–1487. [Google Scholar] [CrossRef]
- Lee, J.; Kaletunç, G. Inactivation of Salmonella Enteritidis strains by combination of high hydrostatic pressure and nisin. Int. J. Food Microbiol. 2010, 140, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O. Effects of high hydrostatic pressure on Salmonella typhimurium and aerobic bacteria in milk and fruit juices. Roman. Biotechnol. Lett. 2011, 16, 6540–6547. [Google Scholar]
- Wang, C.-Y.; Huang, H.-W.; Hsu, C.-P.; Shyu, Y.-T.; Yang, B.B. Inactivation and morphological damage of Vibrio parahaemolyticus treated with high hydrostatic pressure. Food Control 2013, 32, 348–353. [Google Scholar] [CrossRef]
- Ponce, E.; Pla, R.; Capellas, M.; Guamis, B.; Mor-Mur, M. Inactivation of Escherichia coli inoculated in liquid whole egg by high hydrostatic pressure. Food Microbiol. 1998, 15, 265–272. [Google Scholar] [CrossRef]
- Doona, C.J.; Feeherry, F.E.; Ross, E.W. A quasi-chemical model for the growth and death of microorganisms in foods by non-thermal and high-pressure processing. Int. J. Food Microbiol. 2005, 100, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Buzrul, S.; Largeteau, A.; Alpas, H.; Demazeau, G. Pulsed pressure treatment for inactivation of Escherichia coli and Listeria innocua in whole milk. J. Phy. Conf. Ser. 2008, 121, 142001. [Google Scholar] [CrossRef]
- Buzrul, S.; Alpas, H.; Largeteau, A.; Demazeau, G. Modeling high pressure inactivation of Escherichia coli and Listeria innocua in whole milk. Eur. Food Res. Technol. 2008, 227, 443–448. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, M.; Chen, H. Inactivation of Escherichia coli O157:H7 and Salmonella spp. in strawberry puree by high hydrostatic pressure with/without subsequent frozen storage. Int. J. Food Microbiol. 2013, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Carreño, J.M.; Gurrea, M.C.; Sampedro, F.; Carbonell, J.V. Effect of high hydrostatic pressure and high-pressure homogenisation on Lactobacillus plantarum inactivation kinetics and quality parameters of mandarin juice. Eur. Food Res. Technol. 2011, 232, 265–274. [Google Scholar] [CrossRef]
- Huang, H.-W.; Lung, H.-M.; Chang, Y.-H.; Yang, B.B.; Wang, C.-Y. Inactivation of pathogenic Listeria monocytogenes in raw milk by high hydrostatic pressure. Foodborne Pathog. Dis. 2015, 12, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.; Pulido, R.P.; Abriouel, H.; Grande, M.J.; Gálvez, A. Inactivation of Salmonella enterica cells in Spanish potato omelette by high hydrostatic pressure treatments. Innov. Food Sci. Emerg. Technol. 2012, 14, 25–30. [Google Scholar] [CrossRef]
- Pulido, R.P.; del Árbol, J.T.; Burgos, J.G.; Gálvez, A. Bactericidal effects of high hydrostatic pressure treatment singly or in combination with natural antimicrobials on Staphylococcus aureus in rice pudding. Food Control 2012, 28, 19–24. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Bi, X.; Gou, X.; Fu, S.; Liao, X. Comparison of microbial inactivation and rheological characteristics of mango pulp after high hydrostatic pressure treatment and high temperature short time treatment. Food Bioprocess Technol. 2013, 6, 2675–2684. [Google Scholar] [CrossRef]
- Black, E.P.; Huppertz, T.; Fitzgerald, G.F.; Kelly, A.L. Baroprotection of vegetative bacteria by milk constituents: A study on Listeria innocua. Int. Dairy J. 2007, 17, 104–110. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. Generating microbial survival curves during thermal processing in real time. J. Appl. Microbiol. 2005, 98, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, N.; Ritz, M.; Delépine, S.; Jugiau, F.; Federighi, M.; de Lamballerie, M. Influence of kinetic parameters of high pressure processing on bacterial inactivation in a buffer system. Int. J. Food Microbiol. 2006, 106, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. A model of temperature effects on microbial populations from growth to lethality. J. Sci. Food Agric. 1995, 68, 83–89. [Google Scholar] [CrossRef]
- Peleg, M. A model of microbial survival after exposure to pulsed electric fields. J. Sci. Food Agric. 1995, 67, 93–99. [Google Scholar] [CrossRef]
- Tassou, C.C.; Panagou, E.Z.; Samaras, F.J.; Galiatsatou, P.; Mallidis, C.G. Temperature-assisted high hydrostatic pressure inactivation of Staphylococcus aureus in a ham model system: Evaluation in selective and nonselective medium. J. Appl. Microbiol. 2008, 104, 1764–1773. [Google Scholar] [CrossRef] [PubMed]


| R2adj | MSE | |||||
|---|---|---|---|---|---|---|
| Discrete | Fermi | Weibull | Discrete | Fermi | Weibull | |
| Mean | 0.970 | 0.973 | 0.967 | 0.224 | 0.201 | 0.295 |
| Standard deviation | 0.025 | 0.022 | 0.031 | 0.145 | 0.137 | 0.317 |
| Min | 0.900 | 0.917 | 0.872 | 0.009 | 0.007 | 0.005 |
| Max | 0.999 | 0.999 | 0.999 | 0.640 | 0.638 | 1.36 |
| Microorganism | Strain | Substrate | CR/CT a | DR/DT b | Process Conditions c | Discrete | Fermi | Weibull | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Campylobacter jejuni | ATCC 35921 | Bolton broth | ND d | ND | 25 °C, 10 min | k = 0.307 ± 0.021 MPa−1 | k = 0.309 ± 0.021 MPa−1 | n = 8.6 ± 0.4 | Solomon & Hoover [21] |
| Pmin = 241.0 ± 2.7 MPa | Pmin = 241.3 ± 2.8 MPa | P1 = 235.0 ± 2.7 MPa | |||||||
| P5 = 278.5 MPa | P5 = 278.6 MPa | P5 = 283.4 MPa | |||||||
| Escherichia coli | KUEN 1504 | Brain heart infusion broth | 100 MPa·s−1 | 200 MPa·s−1 | 25 °C, 3 min | k = 0.022 ± 0.001 MPa−1 | k = 0.023 ± 0.001 MPa−1 | n = 1.7 ± 0.2 | Doğan & Erkmen [22] |
| Pmin = 175.9 ± 10.9 MPa | Pmin = 190.8 ± 12.5 MPa | P1 = 262.1 ± 21.4 MPa | |||||||
| P5 = 699.2 MPa | P5 = 691.4 MPa | P5 = 675.5 MPa | |||||||
| 25 °C, 5 min | k = 0.031 ± 0.001 MPa−1 | k = 0.032 ± 0.002 MPa−1 | n = 1.6 ± 0.2 | ||||||
| Pmin = 165.8 ± 15.1 MPa | Pmin = 172.8 ± 16.6 MPa | P1 = 202.2 ± 22.0 MPa | |||||||
| P5 = 537.2 MPa | P5 = 532.6 MPa | P5 = 552.9 MPa | |||||||
| 25 °C, 10 min | k = 0.040 ± 0.003 MPa−1 | k = 0.040 ± 0.003 MPa−1 | n = 1.5 ± 0.2 | ||||||
| Pmin = 138.6 ± 20.5 MPa | Pmin = 139.1 ± 21.8 MPa | P1 = 147.2 ± 27.1 MPa | |||||||
| P5 = 426.4 MPa | P5 = 426.9 MPa | P5 = 430.4 MPa | |||||||
| E. coli | ATCC 25922 | Phosphate-buffered saline | ND | ND | 25 °C, 5 min | k = 0.113 ± 0.010 MPa−1 | k = 0.113 ± 0.010 MPa−1 | n = 2.5 ± 0.4 | Yamamoto et al. [23] |
| Pmin = 134.1 ± 9.8 MPa | Pmin = 134.4 ± 9.9 MPa | P1 = 127.8 ± 14.7 MPa | |||||||
| P5 = 236.0 MPa | P5 = 236.3 MPa | P5 = 243.3 MPa | |||||||
| 25 °C, 10 min | k = 0.116 ± 0.011 MPa−1 | k = 0.116 ± 0.012 MPa−1 | n = 2.4 ± 0.5 | ||||||
| Pmin = 131.6 ± 9.5 MPa | Pmin = 131.3 ± 10.2 MPa | P1 = 122.2 ± 19.1 MPa | |||||||
| P5 = 230.9 MPa | P5 = 230.6 MPa | P5 = 239.0 MPa | |||||||
| E. coli | K12TG1 | Luria-Bertani broth | 3 min | 3 min | 25 °C, 10 min | k = 0.048 ± 0.002 MPa−1 | k = 0.050 ± 0.002 MPa−1 | n = 2.4 ± 0.2 | Moussa et al. [20] |
| Pmin = 185.7 ± 5.2 MPa | Pmin = 192.3 ± 5.6 MPa | P1 = 218.9 ± 13.4 MPa | |||||||
| P5 = 425.6 MPa | P5 = 422.6 MPa | P5 = 428.0 MPa | |||||||
| E. coli | ATCC 11775 | Luria-Bertani broth | 10 s | 10 s | 20 °C, 10 min | k = 0.062 ± 0.007 MPa−1 | k = 0.078 ± 0.008 MPa−1 | n = 4.3 ± 0.1 | Wang et al. [24] |
| Pmin = 249.9 ± 14.2 MPa | Pmin = 278.6 ± 11.5 MPa | P1 = 293.4 ± 2.6 MPa | |||||||
| P5 = 435.6 MPa | P5 = 426.2 MPa | P5 = 426.6 MPa | |||||||
| 30 °C, 10 min | k = 0.050 ± 0.006 MPa−1 | k = 0.051 ± 0.006MPa−1 | n = 2.6 ± 0.2 | ||||||
| Pmin = 168.6 ± 22.3 MPa | Pmin = 172.8 ± 22.3 MPa | P1 = 214.2 ± 11.5 MPa | |||||||
| P5 = 398.9 MPa | P5 = 398.5 MPa | P5 = 397.8 MPa | |||||||
| E. coli | MG1655 | Luria-Bertani broth | 50 MPa·s−1 | <1 s | 25 °C, 10 min | k = 0.054 ± 0.002 MPa−1 | k = 0.054 ± 0.002 MPa−1 | n = 2.6 ± 0.2 | Griffin et al. [25] |
| Pmin = 182.1 ± 5.3 MPa | Pmin = 184.1 ± 5.0 MPa | P1 = 211.4 ± 9.0 MPa | |||||||
| P5 = 395.3 MPa | P5 = 397.3 MPa | P5 = 392.6 MPa | |||||||
| Lactobacillus plantarum | 103151T | MRS broth | 1.5 MPa·s−1 | 1.5 MPa·s−1 | 25 °C, 10 min | k = 0.182 ± 0.010 MPa−1 | k = 0.214 ± 0.009 MPa−1 | n = 5.5 ± 0.6 | Perrier-Cornet et al. [26] |
| Pmin = 199.6 ± 3.6 MPa | Pmin = 212.2 ± 2.7 MPa | P1 = 203.9 ± 8.3 MPa | |||||||
| P5 = 262.9 MPa | P5 = 266.0 MPa | P5 = 273.2 MPa | |||||||
| L. viridescens | IFO 3949 | MRS broth | 1 min | ≈2 s | 25 °C, 5 min | k = 0.065 ± 0.013 MPa−1 | k = 0.065 ± 0.013 MPa−1 | n =3.0 ± 0.5 | Park et al. [27] |
| Pmin = 319.6 ± 39.6 MPa | Pmin = 319.7 ± 37.7 MPa | P1 = 298.2 ± 55.4 MPa | |||||||
| P5 = 496.7 MPa | P5 = 496.8 MPa | P5 = 509.9 MPa | |||||||
| Listeria monocytogenes | Poultry isolate | Buffered saline | ≈ 2 min | 1 min | AT e, 5 min | k = 0.101 ± 0.004 MPa−1 | k = 0.104 ± 0.006 MPa−1 | n = 4.5 ± 0.7 | Simpson & Gilmour [28] |
| Pmin = 296.3 ± 3.7 MPa | Pmin = 299.0 ± 5.8 MPa | P1 = 291.2 ± 18.0 MPa | |||||||
| P5 = 410.3 MPa | P5 = 409.7 MPa | P5 = 416.4 MPa | |||||||
| AT, 10 min | k = 0.112 ± 0.012 MPa−1 | k = 0.112 ± 0.013MPa−1 | n =3.5 ± 0.8 | ||||||
| Pmin = 279.8 ± 11.5 MPa | Pmin = 279.4 ± 12.6 MPa | P1 = 246.4 ± 31.2 MPa | |||||||
| P5 = 382.6 MPa | P5 = 382.2 MPa | P5 = 390.3 MPa | |||||||
| Scott A | k = 0.080 ± 0.011 MPa−1 | k = 0.081 ± 0.011 MPa−1 | n = 5.3 ± 0.9 | ||||||
| Pmin = 313.7 ± 12.8 MPa | Pmin = 315.5 ± 12.3 MPa | P1 = 333.2 ± 14.6 MPa | |||||||
| P5 = 457.6 MPa | P5 = 457.6 MPa | P5 = 451.4 MPa | |||||||
| L. monocytogenes | Scott A | Citrate buffer | ND | ND | 20 °C, 10 min | k = 0.105 ± 0.028 MPa−1 | k = 0.105 ± 0.028 MPa−1 | n = 3.9 ± 0.9 | Tholozan et al. [29] |
| Pmin = 227.2 ± 31.8 MPa | Pmin = 227.4 ± 31.7 MPa | P1 = 231.8 ± 25.7 MPa | |||||||
| P5 = 336.9 MPa | P5 = 337.1 MPa | P5 = 350.2 MPa | |||||||
| Phosphate buffer | k = 0.061 ± 0.008 MPa−1 | k = 0.061 ± 0.008 MPa−1 | n = 2.4 ± 0.5 | ||||||
| Pmin = 271.6 ± 28.2 MPa | Pmin = 271.6 ± 28.4 MPa | P1 = 245.9 ± 45.0 MPa | |||||||
| P5 = 460.3 MPa | P5 = 460.3 MPa | P5 = 480.8 MPa | |||||||
| L. monocytogenes | 4a KUEN 136 | Brain heart infusion broth | 100 MPa·s−1 | 200 MPa·s−1 | 25 °C, 5 min | k = 0.024 ± 0.002 MPa−1 | k = 0.024 ± 0.003 MPa−1 | n = 1.3 ± 0.2 | Erkmen & Dogan [30] |
| Pmin = 119.0 ± 37.7 MPa | Pmin = 122.5 ± 40.0 MPa | P1 = 178.5 ± 33.7 MPa | |||||||
| P5 = 598.7 MPa | P5 = 602.2 MPa | P5 = 615.6 MPa | |||||||
| 25 °C, 10 min | k = 0.031 ± 0.003 MPa−1 | k = 0.031 ± 0.003 MPa−1 | n = 1.2 ± 0.2 | ||||||
| Pmin = 91.2 ± 31.7 MPa | Pmin = 91.6 ± 32.2 MPa | P1 = 124.1 ± 23.7 MPa | |||||||
| P5 = 462.6 MPa | P5 = 463.0 MPa | P5 = 474.5 MPa | |||||||
| L. monocytogenes | ATCC 19117 | 1% buffered peptone water | 5 MPa·s−1 | <10 s | 25 °C, 10 min | k = 0.069 ± 0.010 MPa−1 | k = 0.071 ± 0.009MPa−1 | n = 2.9 ± 0.8 | Koseki & Yamamoto [31] |
| Pmin = 304.9 ± 30.2 MPa | Pmin = 311.3 ± 26.8 MPa | P1 = 284.9 ± 53.3 MPa | |||||||
| P5 = 471.8 MPa | P5 = 473.5 MPa | P5 = 496.3 MPa | |||||||
| 5% buffered peptone water | k = 0.084 ± 0.010 MPa−1 | k = 0.112 ± 0.009MPa−1 | n = 5.9 ± 0.4 | ||||||
| Pmin = 398.5 ± 15.3 MPa | Pmin = 441.9 ± 9.5 MPa | P1 = 423.5 ± 8.8 MPa | |||||||
| P5 = 535.6 MPa | P5 = 544.7 MPa | P5 = 556.3 MPa | |||||||
| Saccharomyces cerevisiae | ND | MRS broth | ND | ≈2 s | 25 °C, 10 min | k = 0.145 ± 0.012 MPa−1 | k = 0.152 ± 0.014 MPa−1 | n = 3.9 ± 0.2 | Donsì et al. [32] |
| Pmin = 141.3 ± 6.1 MPa | Pmin = 145.6 ± 7.0 MPa | P1 = 151.4 ± 1.9 MPa | |||||||
| P5 = 220.7 MPa | P5 = 221.3 MPa | P5 = 228.7 MPa | |||||||
| 45 °C, 3 min | k = 0.140 ± 0.012 MPa−1 | k = 0.144 ± 0.023 MPa−1 | n =3.8 ± 0.2 | ||||||
| Pmin = 109.6 ± 9.3 MPa | Pmin = 111.7 ± 9.5 MPa | P1 = 126.4 ± 3.9 MPa | |||||||
| P5 = 191.8 MPa | P5 = 191.7 MPa | P5 = 193.1 MPa | |||||||
| 45 °C, 6 min | k = 0.140 ± 0.01 MPa−1 | k = 0.146 ± 0.014 MPa−1 | n =3.0 ± 0.1 | ||||||
| Pmin = 93.1 ± 5.9 MPa | Pmin = 96.2 ± 6.2 MPa | P1 = 105.1 ± 2.3 MPa | |||||||
| P5 = 175.3 MPa | P5 = 175.1 MPa | P5 = 179.7 MPa | |||||||
| 45 °C, 10 min | k = 0.141 ± 0.011 MPa−1 | k = 0.144 ± 0.012 MPa−1 | n = 2.8 ± 0.09 | ||||||
| Pmin = 89.9 ± 5.2 MPa | Pmin = 91.7 ± 5.3 MPa | P1 = 98.9 ± 2.1 MPa | |||||||
| P5 = 171.6 MPa | P5 = 171.7 MPa | P5 = 175.7 MPa | |||||||
| S. cerevisiae | CBS 1171 | Malt Wickerham medium | 1.5 MPa·s−1 | 1.5 MPa·s−1 | 25 °C, 10 min | k = 0.165 ± 0.013 MPa−1 | k = 0.166 ± 0.014 MPa−1 | n = 4.1 ± 0.2 | Perrier-Cornet et al. [26] |
| Pmin = 184.1 ± 6.3 MPa | Pmin = 184.8 ± 6.5 MPa | P1 = 177.7 ± 3.4 MPa | |||||||
| P5 = 253.9 MPa | P5 = 254.2 MPa | P5 = 263.1 MPa | |||||||
| Salmonella enterica | BCRC 12947 | Nutrient broth | 45 MPa·s−1 | <10 s | 25 °C, 5 min | k = 0.122 ± 0.028 MPa−1 | k = 0.122 ± 0.027MPa−1 | n = 2.9 ± 0.3 | Wang et al. [33] |
| Pmin = 175.5 ± 2.6 MPa | Pmin = 175.6 ± 2.5 MPa | P1 = 162.0 ± 11.4 MPa | |||||||
| P5 = 269.9 MPa | P5 = 270.0 MPa | P5 = 282.2 MPa | |||||||
| 25 °C, 10 min | k = 0.086 ± 0.006 MPa−1 | k = 0.090 ± 0.006MPa−1 | n = 1.8 ± 0.2 | ||||||
| Pmin = 94.1 ± 10.0 MPa | Pmin = 102.5 ± 10.8 MPa | P1 = 97.8 ± 12.1 MPa | |||||||
| P5 = 228.0 MPa | P5 = 230.4 MPa | P5 = 239.1 MPa | |||||||
| S. enteritidis | FDA | Trypticase soy broth + yeast extract | 6.7 MPa·s−1 | ND | 25 °C, 10 min | k = 0.073 ± 0.006 MPa−1 | k = 0.074 ± 0.007 MPa−1 | n = 2.9 ± 0.4 | Lee & Kaletunç [34] |
| Pmin = 235.7 ± 14.2 MPa | Pmin = 237.3 ± 16.2 MPa | P1 = 235.0 ± 20.7 MPa | |||||||
| P5 = 393.4 MPa | P5 = 392.9 MPa | P5 = 409.4 MPa | |||||||
| S. typhimurium | ATCC 13311 | Citrate buffer | ND | ND | 20 °C, 10 min | k = 0.110 ± 0.005MPa−1 | k = 0.111 ± 0.005MPa−1 | n =3.2 ± 0.3 | Tholozan et al. [29] |
| Pmin = 177.5 ± 5.6 MPa | Pmin = 178.6 ± 5.7 MPa | P1 = 180.3 ± 11.7 MPa | |||||||
| P5 = 282.2 MPa | P5 = 282.3 MPa | P5 = 298.1 MPa | |||||||
| Phosphate buffer | k = 0.157 ± 0.013MPa−1 | k = 0.156 ± 0.012MPa−1 | n = 4.9 ± 0.4 | ||||||
| Pmin = 276.4 ± 7.5 MPa | Pmin = 275.9 ± 7.5 MPa | P1 = 258.0 ± 7.7 MPa | |||||||
| P5 = 349.7 MPa | P5 = 349.7 MPa | P5 = 358.3 MPa | |||||||
| S. typhimurium | KUEN 1357 | Tryptone soy broth | 100 MPa·s−1 | 200 MPa·s−1 | 25 °C, 10 min | k = 0.057 ± 0.005 MPa−1 | k = 0.057 ± 0.005 MPa−1 | n = 2.1 ± 0.4 | Erkmen [35] |
| Pmin = 163.3 ± 12.7 MPa | Pmin = 163.8 ± 14.1 MPa | P1 = 171.8 ± 22.9 MPa | |||||||
| P5 = 365.3 MPa | P5 = 365.8 MPa | P5 = 369.7 MPa | |||||||
| Vibrio parahaemolyticus | BCRC 10806 | Tryptic soy broth | 45 MPa·s−1 | <10 s | 25 °C, 5 min | k = 0.185 ± 0.013 MPa−1 | k = 0.190 ± 0.014MPa−1 | n = 5.7 ± 0.3 | Wang et al. [36] |
| Pmin = 241.8 ± 5.0 MPa | Pmin = 244.2 ± 5.7 MPa | P1 = 237.2 ± 4.6 MPa | |||||||
| P5 = 304.0 MPa | P5 = 304.8 MPa | P5 = 314.6 MPa | |||||||
| 25 °C, 10 min | k = 0.132 ± 0.012 MPa−1 | k = 0.162 ± 0.014MPa−1 | n = 3.9 ± 0.4 | ||||||
| Pmin = 196.1 ± 9.1 MPa | Pmin = 218.8 ± 8.1 MPa | P1 = 196.6 ± 11.2 MPa | |||||||
| P5 = 283.3 MPa | P5 = 289.9 MPa | P5 = 297.0 MPa |
| Microorganism | Strain | Substrate | CR/CT a | DR/DT b | Process Conditions c | Discrete | Fermi | Weibull | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Campylobacter jejuni | ATCC 35921 | Whole milk | ND d | ND | 25 °C, 10 min | k = 0.203 ± 0.025 MPa−1 | k = 0.217 ± 0.030 MPa−1 | n = 9.0 ± 0.3 | Solomon & Hoover [21] |
| pmin = 293.6 ± 6.5 MPa | pmin = 298.0 ± 7.3 MPa | p1 = 298.3 ± 2.1 MPa | |||||||
| p5 = 350.3 MPa | p5 = 351.1 MPa | p5 = 356.7 MPa | |||||||
| E. coli | 405 CECT | Liquid whole egg | 3–4 min | 90–120 s | 50 °C, 5 min | k = 0.061 ± 0.012 MPa−1 | k = 0.074 ± 0.016MPa−1 | n = 5.3 ± 0.4 | Ponce et al. [37] |
| pmin = 286.9 ± 21.0 MPa | pmin = 309.7 ± 20.9 MPa | p1 = 334.3 ± 7.3 MPa | |||||||
| p5 = 475.6 MPa | p5 = 465.3 MPa | p5 = 452.9 MPa | |||||||
| E. coli | ATCC 11229 | Whey protein solution | ND | ND | 50 °C, 5 min | k = 0.095 ± 0.003 MPa−1 | k = 0.098 ± 0.003 MPa−1 | n = 5.0 ± 0.8 | Doona et al. [38] |
| pmin = 229.1 ± 2.7 MPa | pmin = 231.8 ± 2.8 MPa | p1 = 250.3 ± 12.4 MPa | |||||||
| p5 = 350.3 MPa | p5 = 349.3 MPa | p5 = 345.4 MPa | |||||||
| 50 °C, 10 min | k = 0.137 ± 0.006 MPa−1 | k = 0.138 ± 0.006 MPa−1 | n = 5.6 ± 1.0 | ||||||
| pmin = 222.0 ± 2.6 MPa | pmin = 222.4 ± 2.7 MPa | p1 = 229.4 ± 11.9 MPa | |||||||
| p5 = 306.0 MPa | p5 = 305.8 MPa | p5 = 305.8 MPa | |||||||
| E. coli | ATCC 11775 | Whole milk | 5 MPa·s−1 | 5 MPa·s−1 | 22 °C, 10 min | k = 0.057 ± 0.006 MPa−1 | k = 0.057 ± 0.006 MPa−1 | n = 4.4 ± 0.2 | Buzrul et al. [39,40] |
| pmin = 378.8 ± 14.9 MPa | pmin = 380.1 ± 15.7 MPa | p1 = 402.1 ± 4.8 MPa | |||||||
| p5 = 580.8 MPa | p5 = 582.1 MPa | p5 = 579.7 MPa | |||||||
| E. coli, O157:H7 | Cocktail of 5 strains | puree | 22 MPa·s−1 | <10 s | 21 °C, 2 min | k = 0.050 ± 0.009 MPa−1 | k = 0.056 ± 0.010 MPa−1 | n = 3.3 ± 0.3 | Huang et al. [41] |
| pmin = 183.3 ± 24.2 MPa | pmin = 201.8 ± 24.6 MPa | p1 = 240.4 ± 10.6 MPa | |||||||
| p5 = 413.6 MPa | p5 = 407.4 MPa | p5 = 391.5 MPa | |||||||
| Lactobacillus plantarum | CECT 220 | Mandarin juice | 90 s | 15 s | 30 °C, 1 min | k = 0.106 ± 0.018 MPa−1 | k = 0.099 ± 0.015 MPa−1 | n = 6.8 ± 0.5 | Carreño et al. [42] |
| pmin = 307.6 ± 10.6 MPa | pmin = 302.8 ± 9.8 MPa | p1 = 321.1 ± 4.7 MPa | |||||||
| p5 = 416.2 MPa | p5 = 419.1 MPa | p5 = 406.9 MPa | |||||||
| 45 °C, 1 min | k = 0.081 ± 0.007 MPa−1 | k = 0.081 ± 0.007 MPa−1 | n = 3.4 ± 0.3 | ||||||
| pmin = 225.5 ± 10.2 MPa | pmin = 226.7 ± 10.3 MPa | p1 = 231.5 ± 9.7 MPa | |||||||
| p5 = 367.6 MPa | p5 = 368.8 MPa | p5 = 371.7 MPa | |||||||
| Listeria innocua | ATCC 33090 | Whole milk | 5 MPa·s−1 | 5 MPa·s−1 | 22 °C, 10 min | k = 0.086 ± 0.013 MPa−1 | k = 0.086 ± 0.013 MPa−1 | n = 5.6 ± 0.4 | Buzrul et al. [39,40] |
| pmin = 426.5 ± 17.1 MPa | pmin = 426.9 ± 17.9 MPa | p1 = 424.4 ± 9.9 MPa | |||||||
| p5 = 560.4 MPa | p5 = 560.7 MPa | p5 = 565.7 MPa | |||||||
| L.monocytogenes | BCRC 15354 | Whole milk | ND | ND | 25 °C, 5 min | k = 0.114 ± 0.007 MPa−1 | k = 0.114 ± 0.007 MPa−1 | n = 3.9 ± 0.8 | Huang et al. [43] |
| pmin = 275.1 ± 6.9 MPa | pmin = 275.2 ± 7.3 MPa | p1 = 257.8 ± 24.5 MPa | |||||||
| p5 = 376.1 MPa | p5 = 376.2 MPa | p5 = 389.5 MPa | |||||||
| 25 °C, 10 min | k = 0.099 ± 0.009 MPa−1 | k = 0.100 ± 0.010 MPa−1 | n = 2.9 ± 0.8 | ||||||
| pmin = 237.5 ± 11.3 MPa | pmin = 237.7 ± 13.4 MPa | p1 = 208.4 ± 33.0 MPa | |||||||
| p5 = 353.8 MPa | p5 = 352.8 MPa | p5 = 363.0 MPa | |||||||
| Salmonella enteritidis | Cocktail of 4 strains | Potato omelet | 1.25 MPa·s−1 | <1 s | 21 °C, 5 min | k = 0.023 ± 0.002 MPa−1 | k = 0.024 ± 0.002 MPa−1 | n = 2.1 ± 0.2 | Toledo et al. [44] |
| pmin = 245.3 ± 12.7 MPa | pmin = 256.6 ± 19.0 MPa | p1 = 325.7 ± 23.4 MPa | |||||||
| p5 = 745.8 MPa | p5 = 736.3 MPa | p5 = 700.9 MPa | |||||||
| S. typhimurium | DT 104 | Whole milk | 6.7 MPa·s−1 | <10 s | 21 °C, 10 min | k = 0.050 ± 0.006 MPa−1 | k = 0.050 ± 0.007 MPa−1 | n = 3.1 ± 0.8 | Guan et al. [7] |
| pmin = 334.6 ± 19.4 MPa | pmin = 337.4 ± 23.5 MPa | p1 = 339.9 ± 40.8 MPa | |||||||
| p5 = 564.9 MPa | p5 = 567.7 MPa | p5 = 571.3 MPa | |||||||
| Staphylococcus aureus | Cocktail of 3 strains | Rice pudding | 1.25 MPa·s−1 | <1 s | 23–27 °C, 10 min | k = 0.053 ± 0.007 MPa−1 | k = 0.058 ± 0.008MPa−1 | n = 3.2 ± 0.1 | Pulido et al. [45] |
| pmin = 284.5 ± 24.6 MPa | pmin = 305.8 ± 28.7 MPa | p1 = 314.0 ± 7.5 MPa | |||||||
| p5 = 501.7 MPa | p5 = 504.3 MPa | p5 = 519.2 MPa | |||||||
| Total aerobic bacteria | Mango pulp | 2 MPa·s−1 | 200 MPa·s−1 | ND, 1 min | k = 0.032 ± 0.012 MPa−1 | k = 0.033 ± 0.012 MPa−1 | n = 2.6 ± 0.3 | Lui et al. [46] | |
| pmin = 269.0 ± 8.2 MPa | pmin = 279.7 ± 7.8 MPa | p1 = 327.8 ± 21.5 MPa | |||||||
| p5 = 628.8 MPa | p5 = 628.6 MPa | p5 = 608.8 MPa |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzrul, S. Evaluation of Different Dose-Response Models for High Hydrostatic Pressure Inactivation of Microorganisms. Foods 2017, 6, 79. https://doi.org/10.3390/foods6090079
Buzrul S. Evaluation of Different Dose-Response Models for High Hydrostatic Pressure Inactivation of Microorganisms. Foods. 2017; 6(9):79. https://doi.org/10.3390/foods6090079
Chicago/Turabian StyleBuzrul, Sencer. 2017. "Evaluation of Different Dose-Response Models for High Hydrostatic Pressure Inactivation of Microorganisms" Foods 6, no. 9: 79. https://doi.org/10.3390/foods6090079





