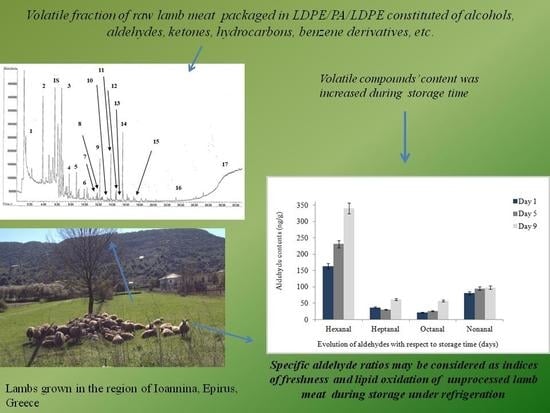
Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lamb Meat Samples, Packaging and Analysis Conditions
2.2. Determination of Lipid Oxidation
2.3. Determination of Volatile Compounds
2.4. GC/MS Instrumentation and Method Conditions
2.5. Mass Spectral Data Processing
2.6. Formatting of Mathematical Components
2.7. Statistical Analysis
3. Results
3.1. Alcohols
3.1.1. Aldehydes
3.1.2. Ketones
3.1.3. Hydrocarbons: Aliphatic and Benzene Derivatives
3.1.4. Sulfur Compounds
3.1.5. Ethers
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
Funding
References
- Soncin, S.; Chiesa, L.M.; Cantoni, C.; Biondi, P.A. Preliminary study of the volatile fraction in the raw meat of pork, duck and goose. J. Food Comp. Anal. 2007, 20, 436–439. [Google Scholar] [CrossRef]
- Gravador, R.S.; Serra, A.; Luciano, G.; Pennisi, P.; Vasta, V.; Mele, M.; Pauselli, M.; Priolo, A. Volatiles in raw and cooked meat from lambs fed olive cake and linseed. Animal 2015, 9, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.; Morcuende, D.; Ventanas, S.; Cava, R. Analysis of volatiles in meat from Iberian pigs and lean pigs after refrigeration and cooking by using SPME-GC-MS. J. Agric. Food Chem. 2003, 51, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Creixell, W.; Pavez-Barra, C.; Sánchez, E.; Albornoz, F.; Young, M.E. Modeling volatile organic compounds released by bovine fresh meat using an integration of solid phase microextraction and databases. Food Bioprocess Technol. 2012, 5, 2557–2567. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, S111–S123. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J. The effects of temperature and pressure on the performance of Carboxen/PDMS fibres during solid phase microextraction (SPME) of headsapace volatiles from cooked and raw turkey breast. Flavour Fragr. J. 2001, 16, 294–302. [Google Scholar] [CrossRef]
- Moon, S.Y.; Cliff, M.A.; Li-Chan, E.C.Y. Odour-active components of simulated beef flavour analysed by solid phase microextraction and gas chromatography-mass spectrometry and-olfactometry. Food Res. Int. 2006, 39, 294–308. [Google Scholar] [CrossRef]
- Pérez, R.A.; Rojo, M.D.; González, G.; De Lorenzo, C. Solid-phase microextraction for the determination of volatile compounds in the spoilage of raw ground beef. J. AOAC Int. 2008, 91, 1409–1415. [Google Scholar] [PubMed]
- Verzera, A.; Dima, G.; Tripodi, G.; Ziino, M.; Lanza, C.M.; Mazzaglia, A. Fast quantitative determination of aroma volatile constituents in melon fruits by headspace-solid-phase microextraction and gas chromatography-mass spectrometry. Food Anal. Methods 2011, 4, 141–149. [Google Scholar] [CrossRef]
- Condurso, C.; Tripodi, G.; Cincotta, F.; Lanza, C.M.; Mazzaglia, A.; Verzera, A. Quality assessment of Mediterranean shrimps during frozen storage. Ital. J. Food Sci. 2016, 28, 497–509. [Google Scholar]
- Ahn, D.U.; Jo, C.; Olson, D.G. Volatile profiles of raw and cooked turkey thigh as affected by purge temperature and holding time before purge. J. Food Sci. 1999, 64, 230–233. [Google Scholar] [CrossRef]
- Nam, K.C.; Ahn, D.U. Combination of aerobic and vacuum packaging to control lipid oxidation and off-odor volatiles of irradiated raw turkey breast. Meat Sci. 2003, 63, 389–395. [Google Scholar] [CrossRef]
- Vasta, V.; Ratel, J.; Engel, E. Mass spectrometry analysis of volatile compounds in raw meat for the authentication of the feeding background of farm animals. J. Agric. Food Chem. 2007, 55, 4630–4639. [Google Scholar] [CrossRef] [PubMed]
- Insausti, K.; Beriain, M.J.; Gorraiz, C.; Purroy, A. Volatile compounds of raw beef from 5 local Spanish cattle breeds stored under modified atmosphere. J. Food Sci. 2002, 67, 1580–1589. [Google Scholar] [CrossRef]
- Vasta, V.; D’Alessandro, A.G.; Priolo, A.; Petrotos, K.; Martemucci, G. Volatile compound profile of ewe’s milk and meat of their suckling lambs in relation to pasture vs. indoor feeding system. Small Rumin. Res. 2012, 105, 16–21. [Google Scholar] [CrossRef]
- Gąsior, R.; Wojtycza, K. Sense of smell and volatile aroma compounds and their role in the evaluation of the quality of products of animal origin—A review. Ann. Anim. Sci. 2016, 16, 3–31. [Google Scholar] [CrossRef]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Berdague, J.L.; Antequera, T.; López-Bote, C. Volatile components of dry-cured Iberian ham. Food Chem. 1991, 41, 23–32. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Parolari, G.; Virgili, R.; Buttini, R.; Careri, M.; Mangia, A. Flavour compounds of dry-cured ham. J. Agric. Food Chem. 1992, 40, 2389–2394. [Google Scholar] [CrossRef]
- Leffingwell and Associates. 2017. Available online: http://www.leffingwell.com/ (accessed on 10 October 2017).
- Wilson, R.A.; Katz, I. Review of literature on chicken flavour and report of isolation of several new chicken flavour components from aqueous cooked chicken broth. J. Agric. Food Chem. 1972, 20, 741–747. [Google Scholar] [CrossRef]
- Larick, D.K.; Turner, B.E. Headspace volatiles and sensory characteristics of ground beef from forage- and grain-fed heifers. J. Food Sci. 1990, 54, 649–654. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Raes, K.; Balcaen, A.; Dirinck, P.; De Winne, A.; Claeys, E.; Demeyer, D.; De Smet, S. Meat quality, fatty acid composition and flavour analysis in Belgian retail beef. Meat Sci. 2003, 65, 1237–1246. [Google Scholar] [CrossRef]
- Young, O.A.; Lane, G.A.; Priolo, A.; Fraser, K. Pastoral and species flavour in lambs raised on pasture, lucerne or maize. J. Sci. Food Agric. 2003, 83, 93–104. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Cheraghi, T.; Roozen, J.P. Lipid-derived off flavours in meat by-products: Effect of antioxidants and Maillard reactants. In Flavour of Meat, Meat Products and Seafood; Shahidi, F., Ed.; Blackie Academic & Professional: London, UK, 1998; p. 260. ISBN 978-0-7514-0484-5. [Google Scholar]
- Ajuyah, A.O.; Fenton, T.W.; Hardin, R.T.; Sim, J.S. Measuring lipid oxidation volatiles in meats. J. Food Sci. 1993, 58, 270–273. [Google Scholar] [CrossRef]
- Brewer, M.S.; Vega, J.D. Detectable odor thresholds of selected lipid oxidation compounds in a meat model system. J. Food Sci. 1995, 60, 592–595. [Google Scholar] [CrossRef]
- Acree, T.; Aru, H. Flavornet. 1997. Available online: http://www.nysaes.cornell.edu/flavornet/chem.html (accessed on 30 October 2001).
- Wasowicz, E.; Kaminski, E.; Kollmannsberger, H.; Nitz, S.; Berger, G.; Drawert, F. Volatile components of sound and musty wheat grains. Chem. Mikrobiol. Technol. Lebensm. 1988, 11, 161–168. [Google Scholar]
- Meynerier, A.; Novelli, E.; Chizzolini, R.; Zanardi, E.; Gandemer, G. Volatile compounds of commercial Milano salami. Meat Sci. 1999, 51, 175–183. [Google Scholar] [CrossRef]
- Gorraiz, C.; Beriain, M.J.; Insausti, K. Effect of aging time on volatile compounds, odor, and flavor of cooked beef from Pirenaica and Friesian Bulls and Heifers. J. Food Sci. 2002, 67, 916–922. [Google Scholar] [CrossRef]
- Cha, Y.J.; Back, H.H.; Hsieh, T.C.Y. Volatile components in flavor concentrates from crayfish processing waste. J. Sci. Food Agric. 1992, 59, 239–248. [Google Scholar] [CrossRef]
- King, M.F.; Hamilton, B.L.; Mattews, M.A.; Rule, D.C.; Field, R.A. Isolation and identification of volatiles and condensable material in raw beef with supercritical carbon dioxide extraction. J. Agric. Food Chem. 1993, 41, 1974–1981. [Google Scholar] [CrossRef]
- Molo, L.; Dekimpe, J.; Etlevant, P.; Addeo, F. Neutral volatile compounds in the raw milks from different species. J. Dairy Res. 1993, 60, 199–213. [Google Scholar]
- Min, D.B.S.; Ina, K.; Peterson, R.J.; Chang, S.S. Preliminary identification of volatile flavor compounds in the neutral fraction of roasted beef. J. Food Sci. 1979, 44, 639–642. [Google Scholar] [CrossRef]
- The National Institute for Occupational Safety and Health. 2007. Available online: http://www.cdc.gov/niosh (accessed on 15 September 2007).
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2001; ISBN 100123526515. [Google Scholar]
- Le Bozec, L.; Moody, C.J. Naturally occurring nitrogen–sulfur compounds. The benzothiazole alkaloids. Aust. J. Chem. 2009, 62, 639–647. [Google Scholar] [CrossRef]
- Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 12th ed.; Van Nostrand Reinhold, Co.: New York, NY, USA, 1993; p. 132. [Google Scholar]
- Lorenz, G.; Stern, D.J.; Flath, R.A.; Haddon, W.F.; Tillin, S.J.; Teranishi, R. Identification of sheep liver volatiles. J. Agric. Food Chem. 1983, 31, 1052–1057. [Google Scholar] [CrossRef]
- Hoffmann, T.; Schieberle, P.; Grosch, W. Model studies on the oxidative stability of odor-active thiols occurring in food flavors. J. Agric. Food Chem. 1996, 44, 251–255. [Google Scholar] [CrossRef]
- Wade, L.G. Ether; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2017. [Google Scholar]
- Shahidi, F.; Pegg, R.C. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging and Shelf Life: A Practical Guide; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2009; p. 328. ISBN 9781420078442. [Google Scholar]
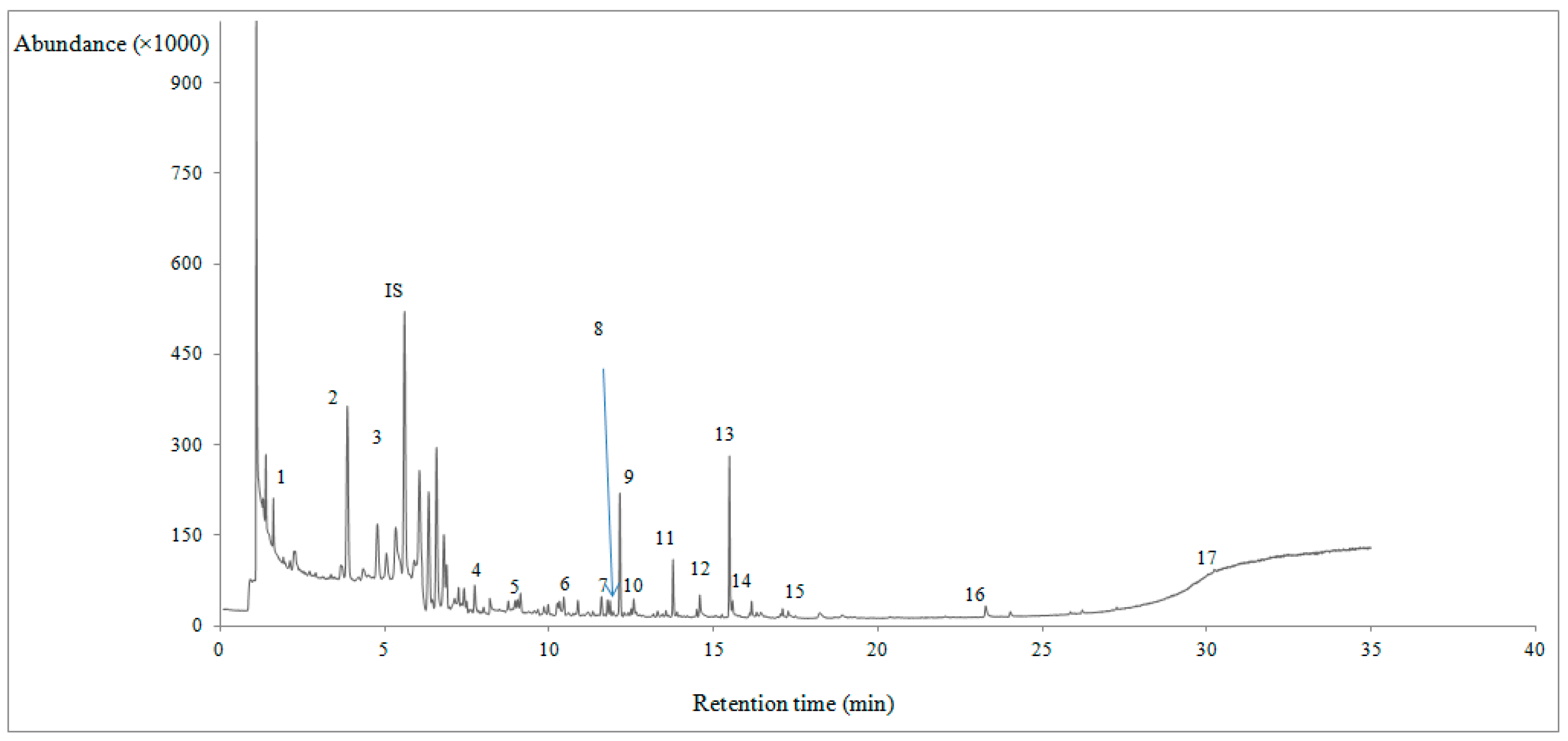
| VOCs | RT | KI | Day 1 | Day 5 | Day 9 | MSLM (%) | MI | p |
|---|---|---|---|---|---|---|---|---|
| Content (ng/g) | Content (ng/g) | Content (ng/g) | ||||||
| Alcohols | ||||||||
| 1-Pentanol | 11.77 | 1233 | 11.77 ± 0.01 | 24.73 ± 0.01 | 56.77 ± 7.83 | 83 | MS/KI | p = 0.144 |
| 1-Hexanol | 13.77 | 1342 | 27.70 ± 0.01 | 71.29 ± 47.72 | 236.92 ± 19.62 | 83 | MS/KI | p = 0.224 |
| 1-Octen-3-ol | 15.49 | 1459 | 96.45 ± 0.01 | 145.40 ± 156.77 | 397.11 ± 25.70 | 90 | MS/KI | p = 0.151 |
| 1-Heptanol | 15.59 | 1461 | 11.98 ± 0.01 | 37.40 ± 4.91 | 63.20 ± 8.69 | 86 | MS/KI | p = 0.121 |
| 1-Octanol | 17.29 | 1557 | 23.55 ± 0.02 | 30.90 ± 0.01 | 57.27 ± 16.65 | 91 | MS/KI | p = 0.057 |
| Aldehydes | ||||||||
| Hexanal | 7.75 | 1105 | 163.20 ± 0.01 | 231.02 ± 92.34 | 340.37 ± 165.89 | 96 | MS/KI | p = 0.040 |
| Heptanal | 10.34 | 1185 | 36.64 ± 0.02 | 29.38 ± 0.76 | 60.94 ± 6.75 | 98 | MS/KI | p = 0.043 |
| Octanal | 12.60 | 1299 | 21.02 ± 0.01 | 25.83 ± 26.67 | 56.59 ± 23.91 | 91 | MS/KI | p = 0.083 |
| Nonanal | 14.61 | 1393 | 80.98 ± 0.04 | 94.84 ± 1.20 | 97.52 ± 56.52 | 91 | MS/KI | p = 0.001 |
| Ketones | ||||||||
| 3-Octanone | 11.87 | 1266 | 23.44 ± 0.02 | 36.39 ± 46.46 | 66.98 ± 31.91 | 95 | MS/KI | p = 0.073 |
| Heterocyclic | ||||||||
| Benzothiazole | 23.27 | 1954 | 17.24 ± 0.02 | 24.53 ± 0.66 | 45.52 ± 0.30 | 91 | MS/KI | p = 0.062 |
| Benzene derivatives | ||||||||
| Toluene | 6.57 | 1027 | 381.95 ± 0.06 | 221.99 ± 51.56 | 735.19 ± 84.40 | 91 | MS/KI | p = 0.099 |
| o-Xylene | 9.13 | 1164 | 25.02 ± 0.10 | 27.24 ± 30.36 | 72.86 ± 9.48 | 95 | MS/KI | p = 0.114 |
| p-Cymene | 12.18 | 1280 | 29.35 ± 0.07 | 103.31 ± 15.33 | 181.68 ± 62.54 | 95 | MS/KI | p = 0.139 |
| Hydrocarbons | ||||||||
| 2,2,4,6,6-pentamethyl-heptane | 3.86 | <800 | 171.27 ± 26.42 | 226.12 ± 360.83 | 435.97 ± 196.77 | 83 | MS | p = 0.074 |
| Sulfur compounds | ||||||||
| Carbon disulfide | 1.62 | <800 | 825.58 ± 359.41 | 125.31 ± 44.37 | 376.24 ± 44.34 | 90 | MS | p = 0.168 |
| Ethers | ||||||||
| 15-Crown ether | 30.23 | 2432 | 5.11 ± 0.01 | 5.77 ± 2.82 | 20.41 ± 14.99 | 90 | MS/KI | p = 0.158 |
| Aldehyde Ratio-MDA | Day 1 | Day 5 | Day 9 | Pearson’s (r) |
|---|---|---|---|---|
| Hexanal–Nonanal | 2.02 | 2.44 | 3.49 | 1.00 |
| Heptanal–Nonanal | 0.45 | 0.31 | 0.62 | 0.87 |
| Octanal–Nonanal | 0.26 | 0.27 | 0.58 | 0.83 |
| Sum of (Hexanal, Heptanal, Octanal)–Nonanal | 2.73 | 3.02 | 4.70 | 0.89 |
| MDA (mg/kg) | 1.4 | 2.8 | 3.8 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, I.K. Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality. Foods 2018, 7, 40. https://doi.org/10.3390/foods7030040
Karabagias IK. Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality. Foods. 2018; 7(3):40. https://doi.org/10.3390/foods7030040
Chicago/Turabian StyleKarabagias, Ioannis Konstantinos. 2018. "Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality" Foods 7, no. 3: 40. https://doi.org/10.3390/foods7030040