Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Process
2.3. Encapsulation of the Extracts
2.4. Sample Characterization
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Ferric Ion Reducing Antioxidant Power (FRAP)
2.4.4. Encapsulation Productivity (EP)
2.4.5. Moisture Content
2.4.6. Water Activity (aw)
2.4.7. X-ray Diffraction (XRD) Analysis
2.4.8. Morphology of Particles by Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Content and Antioxidant Capacity of the Microparticles
3.2. Encapsulation Productivity (EP)
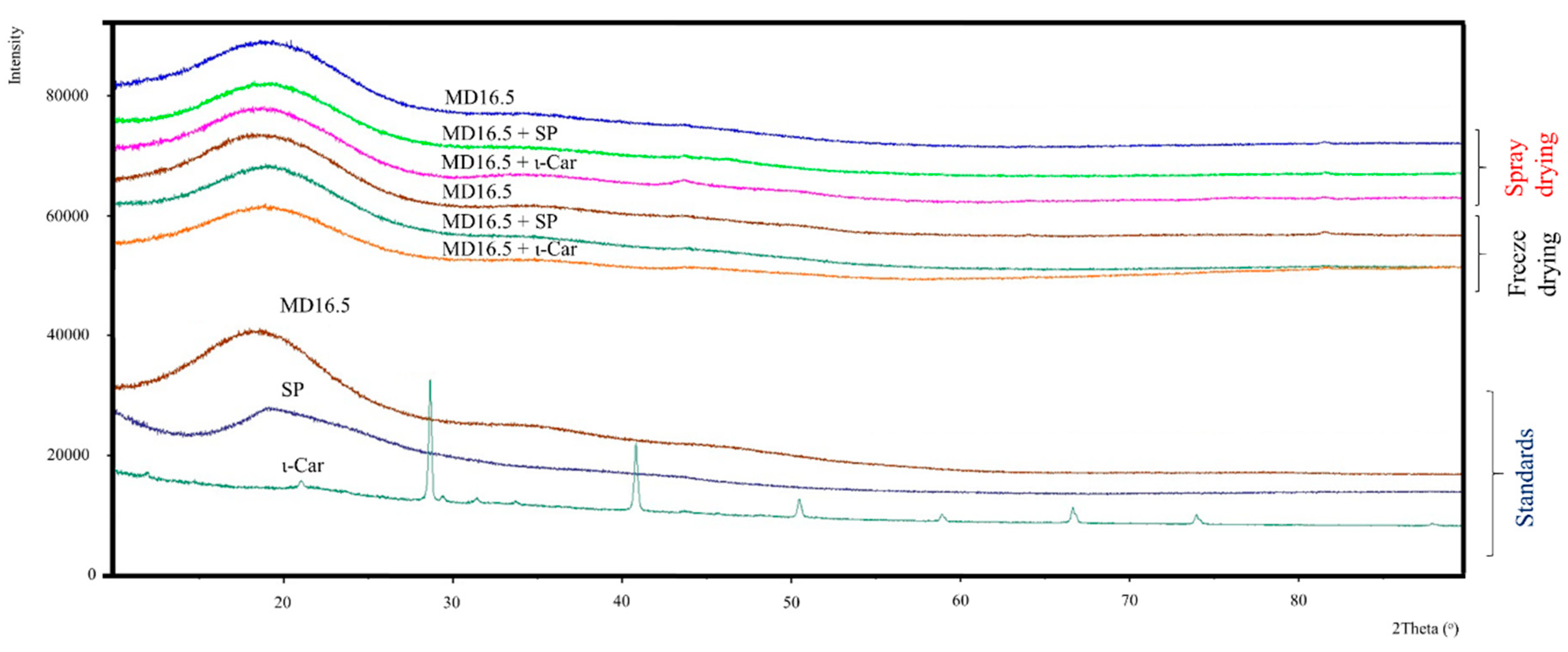
3.3. Morphology and X-ray Diffraction (XRD) Analysis of the Powders
3.4. Moisture Content and Water Activity of the Powders
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papoutsis, K.; Vuong, Q.V.; Golding, J.B.; Hasperué, J.H.; Pristijono, P.; Bowyer, M.C.; Scarlett, J.S.; Stathopoulos, E.C. Pretreatment of citrus by-products affects polyphenol recovery: A review. Food Rev. Int. 2018. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds: A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res. Int. 2012, 48, 478–483. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT Food Sci. Technol. 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of phenolic compounds from olive pomace using spray drying: A study of operative parameters. LWT Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Chronakis, I.S. On the Molecular Characteristics, Compositional Properties, and Structural-Functional Mechanisms of Maltodextrins: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 599–637. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Dumont, M.J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid. Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, A.; Fabra, M.J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Rasouli Ghahroudi, F.; Mizani, M.; Rezaei, K.; Bameni Moghadam, M. Mixed extracts of green tea and orange peel encapsulated and impregnated on black tea bag paper to be used as a functional drink. Int. J. Food. Sci. Technol. 2017, 52, 1534–1542. [Google Scholar] [CrossRef]
- Shofinita, D.; Langrish, T.A.G. Redox (pro-oxidant/antioxidant) balance in the spray drying of orange peel extracts. Drying Technol. 2016, 34, 1719–1725. [Google Scholar] [CrossRef]
- Edrisi Sormoli, M.; Langrish, T.A.G. The use of a plug-flow model for scaling-up of spray drying bioactive orange peel extracts. Innov. Food Sci. Emerg. Technol. 2016, 37, 27–36. [Google Scholar] [CrossRef]
- Edrisi Sormoli, M.; Langrish, T.A.G. Spray drying bioactive orange-peel extracts produced by Soxhlet extraction: Use of WPI, antioxidant activity and moisture sorption isotherms. LWT Food Sci. Technol. 2016, 72, 1–8. [Google Scholar] [CrossRef]
- Shofinita, D.; Feng, S.; Langrish, T.A.G. Comparing yields from the extraction of different citrus peels and spray drying of the extracts. Adv. Powder Technol. 2015, 26, 1633–1638. [Google Scholar] [CrossRef]
- Shofinita, D.; Langrish, T.A.G. Spray drying of orange peel extracts: Yield, total phenolic content, and economic evaluation. J. Food Eng. 2014, 139, 31–42. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Quan, V.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Quan, V.V. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Quan, V.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food Sci. Technol. 2016, 51, 2009–2018. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins, B.D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.H.; Roach, P.D. Production of caffeinated and decaffeinated green tea catechin powders from underutilised old tea leaves. J. Food Eng. 2012, 110, 1–8. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Andrade, L.R.; Farina, M.; Rocha-Leão, M.H.M. Characterization of short chain fatty acid microcapsules produced by spray drying. Mater. Sci. Eng. C. 2004, 24, 653–658. [Google Scholar]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J. Food Eng. 2013, 117, 513–520. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, CE. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Nizori, A.; Bui, L.T.T.; Jie, F.; Small, D.M. Impact of varying hydrocolloid proportions on encapsulation of ascorbic acid by spray drying. Int. J. Food Sci. Technol. 2018. [Google Scholar] [CrossRef]




| Citrus Species | Coating Agents | Encapsulation Technique | Refs |
|---|---|---|---|
| Orange peels | Gelatin and Gum arabic | Coacervation | [16] |
| Orange peels | Whey protein isolate | Spray-drying | [17] |
| Orange peels | Whey protein isolate | Spray-drying | [18,19] |
| Citrus peels | Whey protein isolate | Spray-drying | [20] |
| Orange peels | N.M. | Spray-drying | [21] |
| MD (16.5–19.0 DE) | Soybean Protein | ι-Carrageenan |
|---|---|---|
| 1 | 0 | 0 |
| 5 | 1 | 0 |
| 9 | 0 | 1 |
| Method | Coating Agents | TPC | TFC | FRAP | EPTPC | EPTFC | EPFRAP |
|---|---|---|---|---|---|---|---|
| Spray-drying | MD | 1.26 ± 0.03 d | 0.34 ± 0.01 c | 2.99 ± 0.02 e | 56.52 ± 1.57 d | 58.14 ± 1.51 b | 54.84 ± 0.35 d |
| MD + SP | 1.49 ± 0.03 c | 0.34 ± 0.01 c | 3.17 ± 0.07 d | 66.97 ± 1.16 b | 58.14 ± 0.75 b | 58.23 ± 1.33 c | |
| MD + ι-Car | 1.26 ± 0.02 d | 0.34 ± 0.01 c | 3.10 ± 0.04 d,e | 56.46 ± 0.84 d | 58.67 ± 1.51 b | 56.90 ± 0.73 c | |
| Freeze-drying | MD | 1.41 ± 0.10 c,d | 0.33 ± 0.00 c | 3.36 ± 0.02 c | 63.45 ± 4.78 b,c | 56.53 ± 0.01 b | 61.74 ± 0.41 b |
| MD + SP | 1.66 ± 0.02 b | 0.43 ± 0.02 b | 3.70 ± 0.05 b | 74.84 ± 1.05 a | 74.40 ± 2.64 a | 68.02 ± 0.86 a | |
| MD + ι-Car | 1.30 ± 0.01 d | 0.33 ± 0.02 c | 3.43 ± 0.04 c | 58.46 ± 0.32 c,d | 57.07 ± 3.02 b | 63.07 ± 0.76 b | |
| Extract | 2.22 ± 0.14 a,* | 0.58 ± 0.02 a | 5.44 ± 0.10 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. https://doi.org/10.3390/foods7070115
Papoutsis K, Golding JB, Vuong Q, Pristijono P, Stathopoulos CE, Scarlett CJ, Bowyer M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods. 2018; 7(7):115. https://doi.org/10.3390/foods7070115
Chicago/Turabian StylePapoutsis, Konstantinos, John B. Golding, Quan Vuong, Penta Pristijono, Costas E. Stathopoulos, Christopher J. Scarlett, and Michael Bowyer. 2018. "Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan" Foods 7, no. 7: 115. https://doi.org/10.3390/foods7070115







