Differences in Reproductive Behavior between Spawning and Non-Spawning Zebrafish Pairs and the Effects of 17α-Ethinylestradiol (EE2)
Abstract
:1. Introduction
2. Results
2.1. The Reproductive Behavior in Spawning and Non-Spawning Zebrafish Pairs
2.2. Effect of 17α-Ethinylestradiol (EE2)
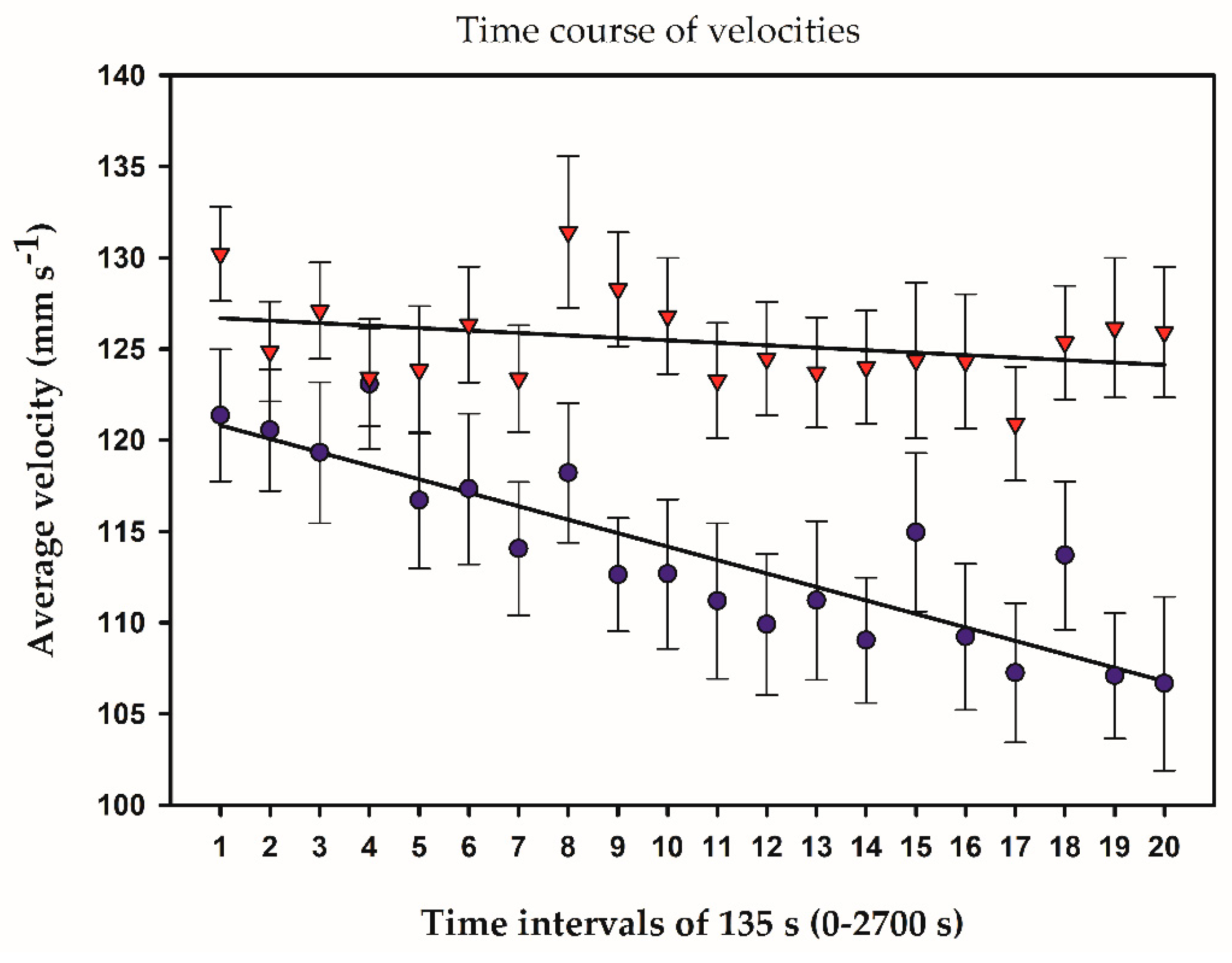
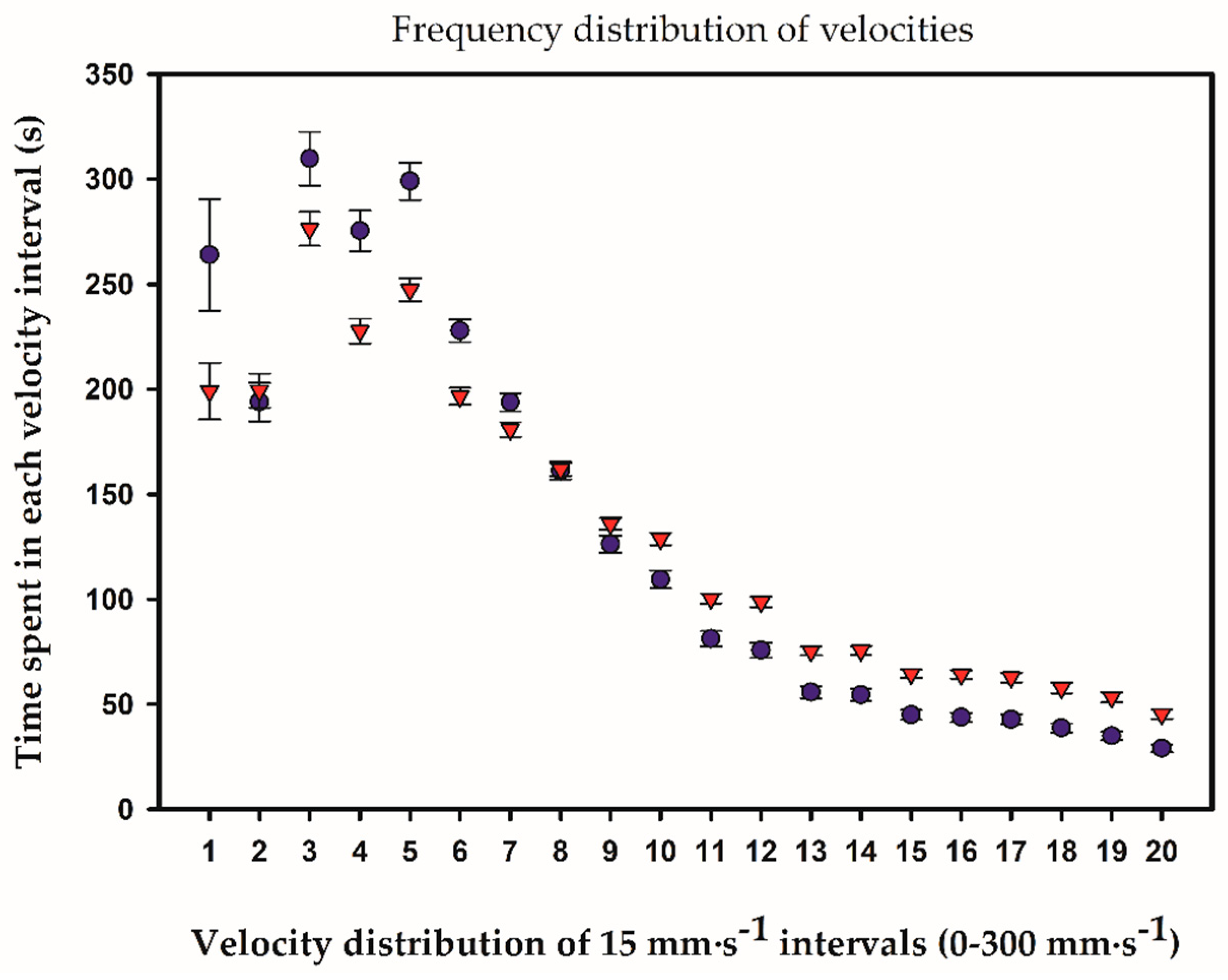
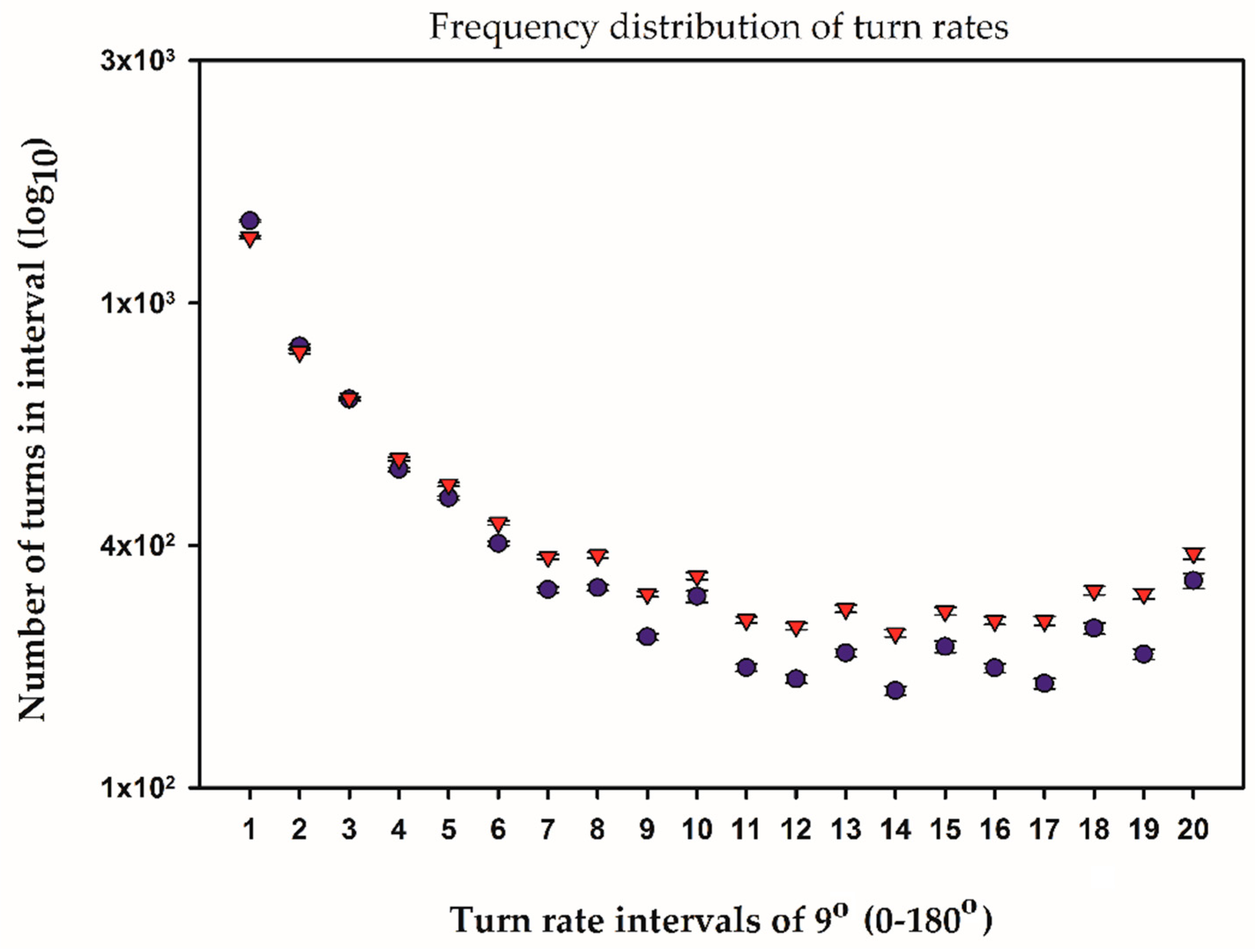
2.3. Temporal and Frequency Distributions
3. Discussion
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darrow, K.O.; Harris, W.A. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish 2004, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Penn, D.J.; Magee, S.; Zala, S.M. Reproductive behavior of wild zebrafish (Danio rerio) in large tanks. Behaviour 2010, 147, 641–660. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyza, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0, and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Hurk, R.; Lambert, J.G.D. Ovarian steroid glucuronides function as sex pheromones for male zebrafish, Brachydanio rerio. Can. J. Zool. 1983, 61, 2382–2387. [Google Scholar]
- Van den Hurk, R.; Schoonen, W.G.; Van Zoelen, G.A.; Lambert, J.G. The biosynthesis of steroid glucuronides in the testis of the zebrafish, Brachydanio rerio, and their pheromonal function as ovulation inducers. Gen. Comp. Endocrinol. 1987, 68, 179–188. [Google Scholar] [CrossRef]
- Gerlach, G. Pheromonal regulation of reproductive success in female zebrafish: Female suppression and male enhancement. Anim. Behav. 2006, 72, 1119–1124. [Google Scholar] [CrossRef]
- Larsen, M.G.; Hansen, K.B.; Henriksen, P.G.; Baatrup, E. Male zebrafish (Danio rerio) courtship behaviour resists the feminizing effects of 17α-ethinylestradiol—Morphological sexual characteristics do not. Aquat. Toxicol. 2008, 87, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, C.; Routledge, E.J.; Brighty, G.C.; Sumpter, J.P.; Waldock, M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 1998, 32, 1549–1558. [Google Scholar] [CrossRef]
- Belfroid, A.C.; Van der Horst, A.; Vethaak, A.D.; Schäfer, A.J.; Rijs, G.B.J.; Wegener, J.; Cofino, W.P. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci. Total Environ. 1999, 225, 101–108. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants. I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- Johnson, A.C.; Belfroid, A.; Di Corcia, A. Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci. Total Environ. 2000, 256, 163–173. [Google Scholar] [CrossRef]
- Svenson, A.; Örn, S.; Allard, A.S.; Viktor, T.; Parkkonen, J.; Olsson, P.E.; Förlin, L.; Norrgren, L. Estrogenicity of domestic and industrial effluents in Sweden. Aquat. Ecosyst. Health Manag. 2002, 5, 423–434. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Response to comment on “Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams 1999–2000: A national reconnaissance”. Environ. Sci. Technol. 2002, 36, 4007–4008. [Google Scholar] [CrossRef]
- Länge, R.; Hutchinson, T.H.; Croudace, C.P.; Siegmund, F.; Schweinfurth, H.; Hampe, P.; Panter, G.H.; Sumpter, J.P. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.P.; Kime, D.E.; Van der Ven, L.T.M.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect. 2004, 112, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.; Maack, G.; Schäfers, C.; Segner, H. An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio. Environ. Toxicol. Chem. 2005, 24, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E.; Henriksen, P. Disrupted reproductive behavior in unexposed female zebrafish (Danio rerio) paired with males exposed to low concentrations of 17α-ethinylestradiol (EE2). Aquat. Toxicol. 2015, 160, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Colman, J.R.; Baldwin, D.; Johnson, L.L.; Scholz, N.L. Effects of the synthetic estrogen 17α-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio). Aquat. Toxicol. 2009, 91, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.G.; Bilberg, K.; Baatrup, E. Reversibility of oestrogenic sex-changes in zebrafish (Danio rerio). Environ. Toxicol. Chem. 2009, 28, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.G.; Baatrup, E. Functional behavior and reproduction in androgenic sex reversed zebrafish (Danio rerio). Environ. Toxicol. Chem. 2010, 29, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Smith, C. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav. Ecol. 2006, 17, 779–783. [Google Scholar] [CrossRef]
- Hutter, S.; Zala, S.M.; Penn, D. Sex recognition in zebrafish (Danio rerio). J. Ethol. 2011, 29, 55–61. [Google Scholar] [CrossRef]
- Coe, T.S.; Söffker, M.K.; Filby, A.L.; Hodgson, D.; Tyler, C.R. Impact of early life exposure to estrogen on subsequent breeding behaviour and reproductive success in zebrafish. Environ. Sci. Technol. 2010, 44, 6481–6487. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Lin, C.Y.; Chen, T.H. Environmentally relevant exposure of 17α-ethinylestradiol impairs spawning and reproductive behavior in the brackish medaka Oryzias memastigma. Mar. Pollut. Bull. 2014, 85, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Majewski, A.R.; Blanchfield, P.J.; Palace, V.P.; Wautier, K. Waterborne 17α-ethinylestradiol affects aggressive behaviour of male fathead minnows (Pimephales promelas) under artificial spawning conditions. Water Qual. Res. J. Can. 2002, 37, 697–710. [Google Scholar]
- Salierno, J.D.; Kane, A.S. 17α-ethinylestradiol alters reproductive behaviors, circulating hormones, and sexual morphology in male fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.; Blunt, B.R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 2005, 20, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, M.; Craft, J.A.; Lehtonen, K.K.; Lindström, K. Exposure to 17α-ethinyl estradiol impairs courtship and aggressive behavior of male sand gobies (Pomatoschistus minutus). Chemosphere 2010, 79, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, M.; Craft, J.A.; Lehtonen, K.K.; Björk, H.; Lindström, K. Disruption of sexual selection in sand gobies (Pomatoschistus minutus) by 17α-ethinyl estradiol, an endocrine disruptor. Horm. Behav. 2009, 55, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, M.; Craft, J.A.; Lehtonen, K.K.; Lindström, K. An endocrine disrupting chemical changes courtship and parental care in the sand goby. Aquat. Toxicol. 2010, 97, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka, K.K.; Firlit, C.F. Ovarian cycle and egg production in the zebrafish, Brachydanio rerio. Copeia 1962, 4, 788–792. [Google Scholar] [CrossRef]
| Behavior Components | −Spawning (n = 59) | +Spawning (n = 30) | p-Value |
|---|---|---|---|
| Total path (m) Males Total path (m) Females | 304 ± 7.46 209 ± 8.03 | 339 ± 8.12 259 ± 8.52 | 0.005 <0.001 |
| Max velocity (mm·s−1) Males Max velocity (mm·s−1) Females | 738 ± 21.5 537 ± 23.9 | 709 ± 23.7 466 ± 29.2 | 0.403 0.077 |
| Average velocity (mm·s−1) Males Average velocity (mm·s−1) Females | 117 ± 2.72 87 ± 2.59 | 127 ± 3.30 100 ± 2.97 | 0.017 0.009 |
| Turn rate·s−1 (degrees) Males Turn rate·s−1 (degrees) Females | 407 ± 10.0 444 ± 13.6 | 542 ± 18.6 507 ± 21.7 | <0.001 0.012 |
| Turn bias·s−1 (degrees) Males Turn bias·s−1 (degrees) Females | −1.23 ± 1.01 −1.59 ± 1.37 | −3.39 ± 1.59 −1.18 ± 1.96 | 0.237 0.863 |
| Time in spawning area (s) Males Time in spawning area (s) Females | 544 ± 28.6 430 ± 34.7 | 762 ± 75.7 669 ± 74.3 | 0.002 0.001 |
| Path in spawning area M (m) Males Path in spawning area F (m) Females | 59.5 ± 2.76 42.0 ± 2.44 | 88.2 ± 7.89 68.4 ± 6.90 | <0.001 <0.001 |
| Visits to spawning area Males Visits to spawning area Females | 340 ± 14.3 241 ± 13.2 | 390 ± 27.2 286 ± 24.4 | 0.074 0.082 |
| Number of contacts between male and female | 1026 ± 55.3 | 1711 ± 85.5 | <0.001 |
| Total time (s) of contact between male and female | 454 ± 36.6 | 746 ± 69.2 | <0.001 |
| Average distance (mm) between male and female | 73.4 ± 2.52 | 47.7 ± 3.28 | <0.001 |
| Behavior Components | −Spawning (n = 47) | +Spawning (n = 14) | p-Value |
|---|---|---|---|
| Total path (m) Males Total path (m) Females | 293 ± 8.26 181 ± 8.92 | 335 ± 7.41 252 ± 7.53 | 0.009 <0.001 |
| Max velocity (mm·s−1) Males Max velocity (mm·s−1) Females | 742 ± 19.8 397 ± 19.0 | 632 ± 38.7 415 ± 51.4 | 0.011 0.700 |
| Average velocity (mm·s−1) Males Average velocity (mm·s−1) Females | 115 ± 3.19 79.3 ± 3.10 | 125 ± 2.80 96.4 ± 1.67 | 0.098 0.005 |
| Turn rate·s−1 (degrees) Males Turn rate·s−1 (degrees) Females | 423 ± 11.1 437 ± 16.6 | 555 ± 30.1 487 ± 28.0 | <0.001 0.145 |
| Turn bias·s−1 (degrees) Males Turn bias·s−1 (degrees) Females | −3.23 ± 1.35 −1.98 ± 1.30 | −0.44 ± 3.64 1.61 ± 2.67 | 0.380 0.203 |
| Time in spawning area (s) Males Time in spawning area (s) Females | 544 ± 46.1 436 ± 56.1 | 791 ± 88.7 639 ± 82.4 | 0.014 0.076 |
| Path in spawning area M (m) Males Path in spawning area F (m) Females | 58.7 ± 4.72 35.3 ± 4.04 | 92.7 ± 8.96 65.2 ± 9.08 | 0.001 0.006 |
| Visits to spawning area Males Visits to spawning area Females | 304 ± 13.6 189 ± 11.9 | 387 ± 19.3 276 ± 23.1 | 0.003 0.001 |
| Number of contacts between male and female | 959 ± 72.8 | 1816 ± 128 | <0.001 |
| Total time (s) of contact between male and female | 466 ± 44.8 | 785 ± 85.9 | 0.001 |
| Average distance (mm) between male and female | 74.6 ± 3.04 | 44.5 ± 4.39 | <0.001 |
| Behavior Components | 0 ng·L−1 (n = 59) | 1.26 ng·L−1 (n = 47) | p-Value |
|---|---|---|---|
| Total path (m) Males Total path (m) Females | 304 ± 7 256 ± 13 | 293 ± 8 194 ± 10 | 0.329 <0.001 |
| Max velocity (mm·s−1) Males Max velocity (mm·s−1) Females | 738 ± 22 1179 ± 72 | 742 ± 20 779 ± 67 | 0.901 <0.001 |
| Average velocity (mm·s−1) Males Average velocity (mm·s−1) Females | 113 ± 3 105 ± 4 | 110 ± 3 83 ± 3 | 0.504 <0.001 |
| Turn rate·s−1 (degrees) Males Turn rate·s−1 (degrees) Females | 394 ± 9 349 ± 12 | 407 ± 11 317 ± 12 | 0.346 0.070 |
| Turn bias·s−1 (degrees) Males Turn bias·s−1 (degrees) Females | −1.22 ± 0.98 −1.80 ± 1.16 | −3.19 ± 1.30 −2.07 ± 1.17 | 0.231 0.873 |
| Time in spawning area (s) Males Time in spawning area (s) Females | 621 ± 30 426 ± 20 | 609 ± 44 382 ± 29 | 0.828 0.221 |
| Path in spawning area M (m) Males Path in spawning area F (m) Females | 67 ± 3 35 ± 2 | 64 ± 5 25 ± 2 | 0.596 <0.001 |
| Visits to spawning area Males Visits to spawning area Females | 642 ± 23 313 ± 17 | 626 ± 28 241 ± 16 | 0.653 0.002 |
| Number of contacts between male and female | 1309 ± 55 | 1081 ± 45 | 0.002 |
| Total time (s) of contact between male and female | 743 ± 42 | 787 ± 55 | 0.531 |
| Average distance (mm) between male and female | 73 ± 3 | 75 ± 3 | 0.717 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksen, P.G.; Beedholm, K.; Baatrup, E. Differences in Reproductive Behavior between Spawning and Non-Spawning Zebrafish Pairs and the Effects of 17α-Ethinylestradiol (EE2). Toxics 2016, 4, 22. https://doi.org/10.3390/toxics4030022
Henriksen PG, Beedholm K, Baatrup E. Differences in Reproductive Behavior between Spawning and Non-Spawning Zebrafish Pairs and the Effects of 17α-Ethinylestradiol (EE2). Toxics. 2016; 4(3):22. https://doi.org/10.3390/toxics4030022
Chicago/Turabian StyleHenriksen, Per G., Kristian Beedholm, and Erik Baatrup. 2016. "Differences in Reproductive Behavior between Spawning and Non-Spawning Zebrafish Pairs and the Effects of 17α-Ethinylestradiol (EE2)" Toxics 4, no. 3: 22. https://doi.org/10.3390/toxics4030022





