Hydrophobic Sand Is a Non-Toxic Method of Urine Collection, Appropriate for Urinary Metal Analysis in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Urine Collection Methods
2.2.1. Metabolic Cages
2.2.2. Hydrophobic Sand
2.3. Experimental Design for Urine Collection
2.4. Creatinine Concentration in Urine
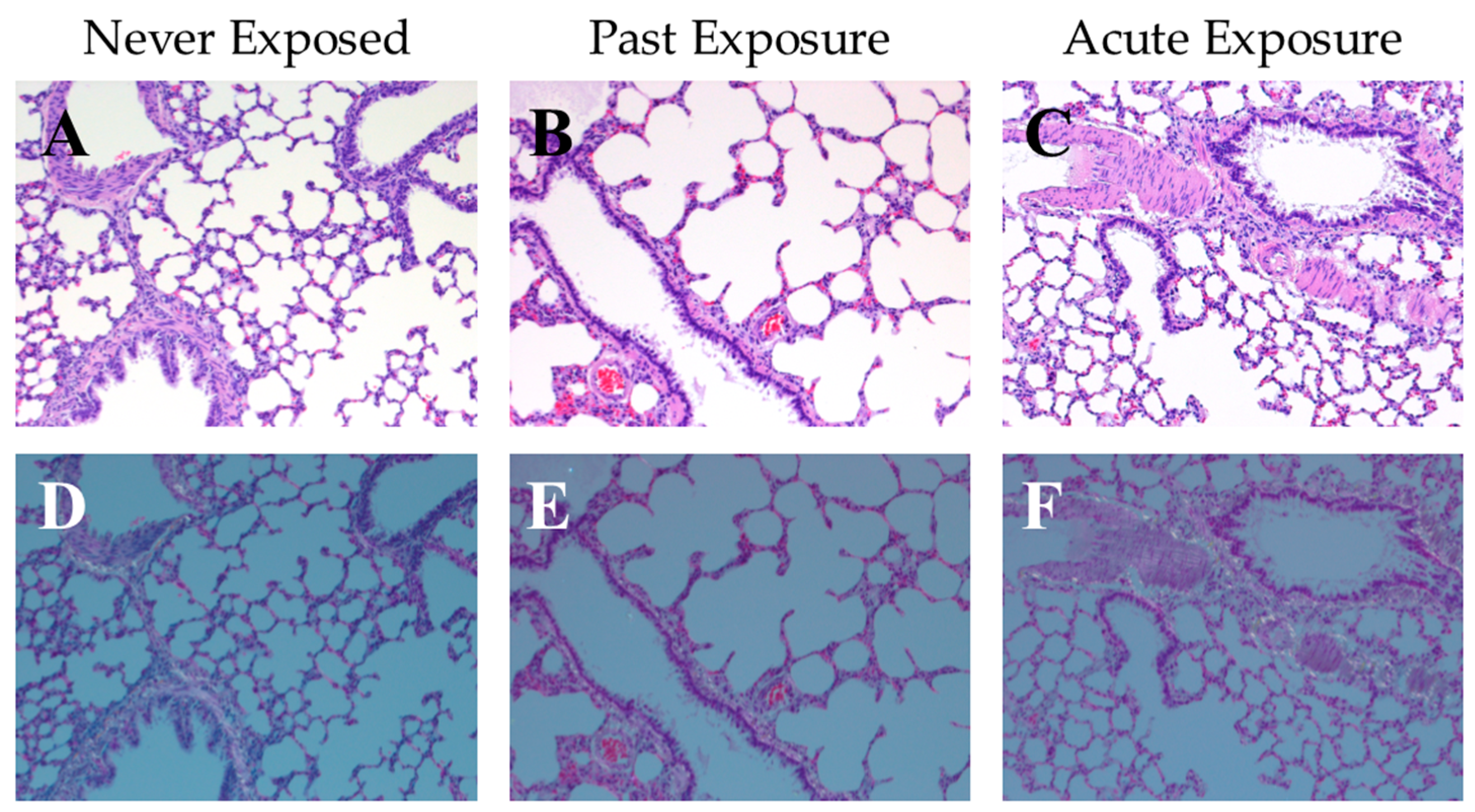
2.5. Examining Potential Internalization of Hydrophobic Sand by Rats
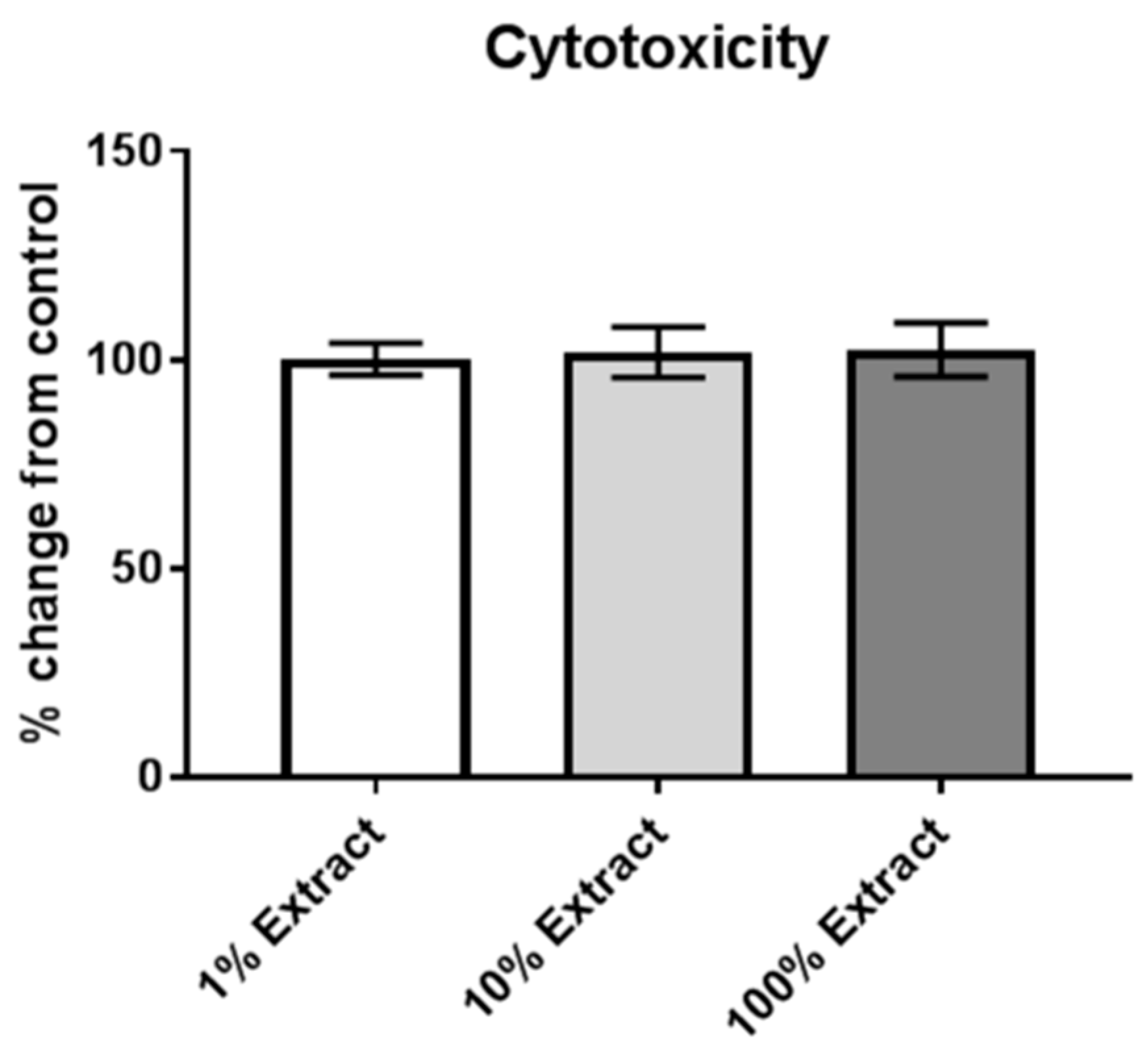
2.6. Assessment of Cytotoxicity
2.6.1. Cell Line and Media
2.6.2. Cell Treatment
2.6.3. Viability Assay
2.7. Analyses of Physical Properties of LabSand
2.7.1. Determination of Hydrophobic Sand Particle Size
2.7.2. Imaging of Hydrophobic Sand Particles
2.8. Metal Analysis by ICP-MS
2.8.1. Urine Metal Analysis
2.8.2. Analysis of Metal Recovery from Hydrophobic Sand
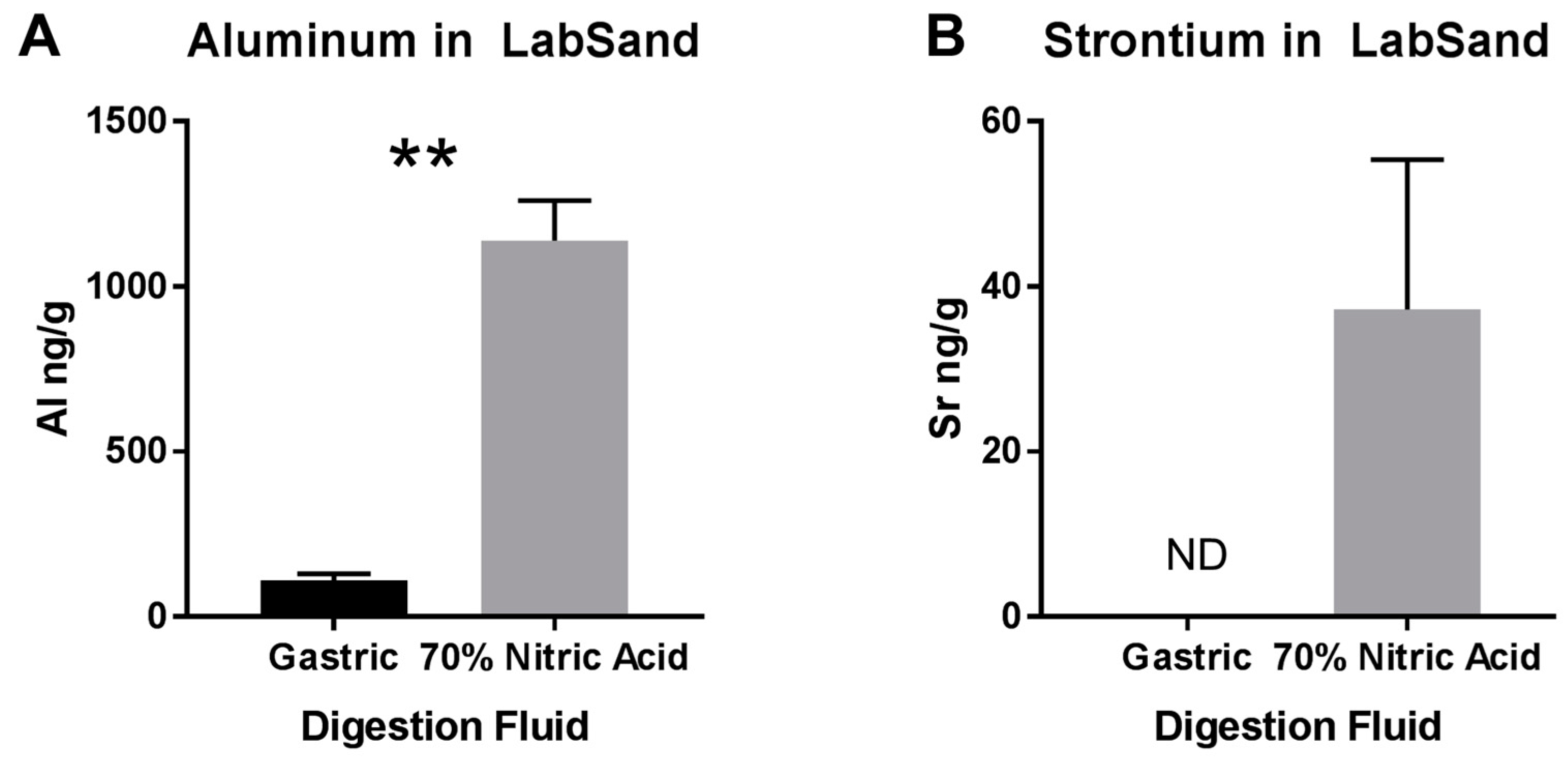
2.8.3. Digestion of Hydrophobic Sand by Synthetic Rat Gut Fluid and Nitric Acid
2.8.4. Metal Analysis of HydroGel®
2.8.5. Treatment of Metabolic Cage Pieces with Simulated Urine
2.9. Statistical Analysis
3. Results
3.1. Risk of Internalization and Cytotoxicity
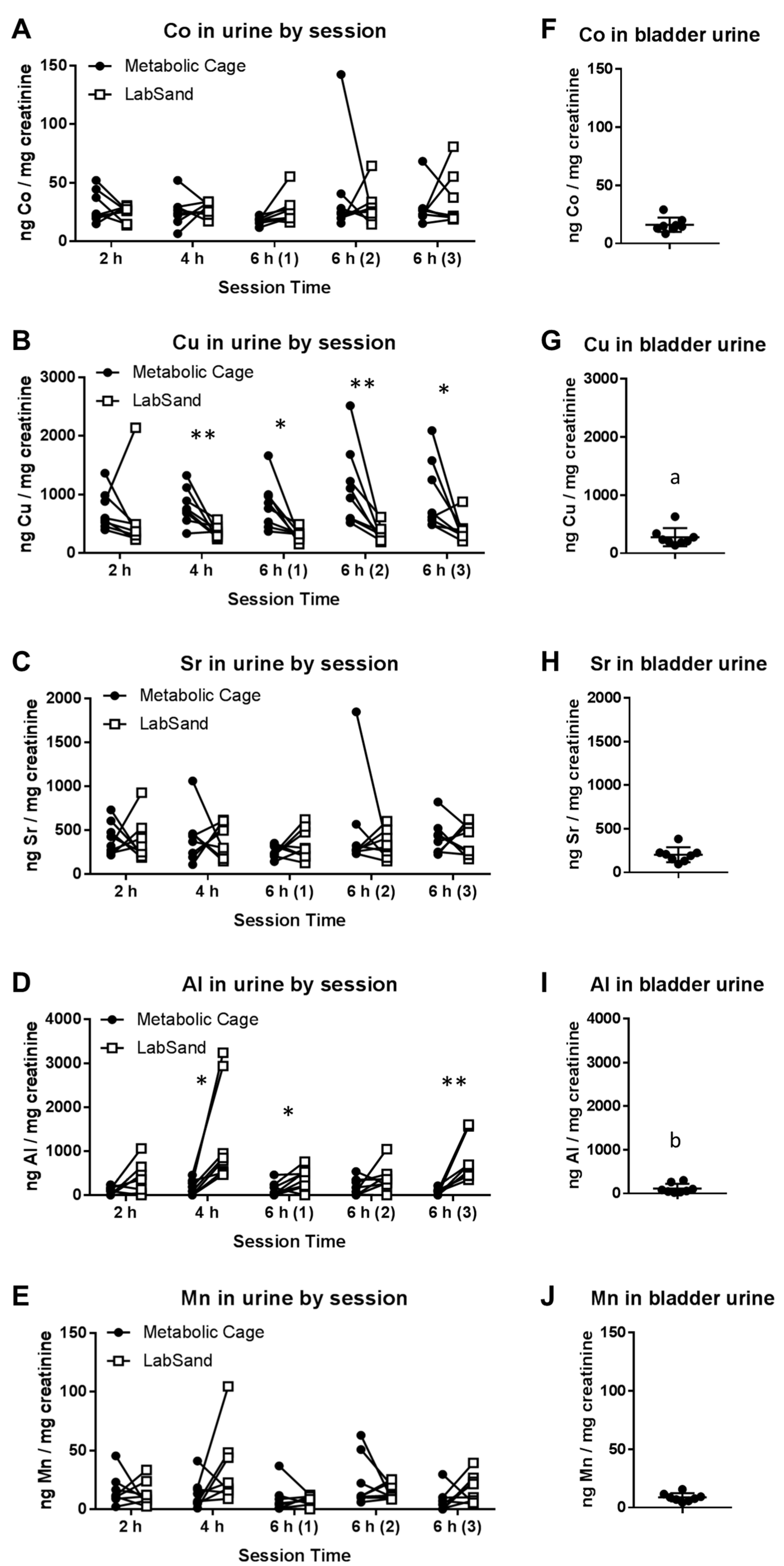
3.2. LabSand vs. Metabolic Cage: Metal in Urine
3.3. Potential Sources of Contamination
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DU | Depleted uranium |
| AFRRI | Armed Forces Radiobiology Research Institute |
| LS | LabSand |
| MC | Metabolic cage |
| ICP-MS | Inductively coupled plasma mass spectroscopy |
References
- Dougherty, P.J.; Eidt, H.C. Wound ballistics: Minie ball vs. full metal jacketed bullets—A comparison of Civil War and Spanish-American War firearms. Mil. Med. 2009, 174, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Schenck, N.L.; Kronman, B.S. Hoarseness and mass in the neck 30 years after penetrating shrapnel injury. Ann. Otol. Rhinol. Laryngol. 1977, 86, 259. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Wilkinson, A. Shrapnel presenting with symptoms 62 years after wounding. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 193. [Google Scholar] [CrossRef] [PubMed]
- Symonds, R.P.; Mackay, C.; Morley, P. The late effect of grenade fragments. J. R. Army Med. Corps 1985, 131, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, G.; McKay, M.J.; Taubman, K.L.; Bilous, A.M. Malignant fibrous histiocytoma developing in bone 44 years after shrapnel trauma. Cancer 1990, 66, 2229–2232. [Google Scholar] [CrossRef] [PubMed]
- Ligtenstein, D.A.; Krijnen, J.L.M.; Jansen, B.R.H.; Eulderink, F. Forgotten injury: A late benign complication of an unremoved shrapnel fragment—Case report. J. Trauma 1994, 36, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Eylon, S.; Mosheiff, R.; Liebergall, M.; Wolf, E.; Brocke, L.; Peyser, A. Delayed reaction to shrapnel retained in soft tissue. Inj. Int. J. Care Inj. 2005, 36, 275–281. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans. Surgical Implants and Other Foreign Bodies; IARC: Lyon, France, 1999; Volume 74, pp. 113–229. [Google Scholar]
- Kane, M.A.; Kasper, C.E.; Kalinich, J.F. Protocol for the assessment of potential health effects from embedded metal fragments. Mil. Med. 2009, 174, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.A.; Benson, K.A.; Bogo, V.; Daxon, E.G.; Hogan, J.B.; Jacocks, H.M.; Landauer, M.R.; McBride, S.A.; Shehata, C.W. Establishment of an Animal Model to Evaluate the Biological Effects of Intramuscularly Embedded Depleted Uranium Fragments; Technical Report 96-3; Armed Forces Radiobiology Research Institute: Bethesda, MD, USA, 1996. [Google Scholar]
- OTSG/MEDCOM. Medical Management of Army Personnel Exposed to Depleted Uranium (DU). OTSG/MEDCOM Policy Memo 11-047. 2011. Available online: www.pdhealth.mil/downloads/OTSG_MEDCOM_Policy_11–047_Med_M.pdf (accessed on 2 September 2015).
- Kalinich, J.F.; Emond, C.A.; Dalton, T.K.; Mog, S.R.; Colman, G.D.; Kordell, J.E.; Miller, A.C.; McClain, D.E. Embedded weapons-grade tungsten alloy shrapnel rapidly induces metastatic high-grade rhabdomyosarcomas in F344 rats. Environ. Health Perspect. 2005, 113, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.E.; Roszell, L.E.; Murr, L.E.; Ramirez, D.A.; Demaree, J.D.; Klotz, B.R.; Rosencrance, A.B.; Dennis, W.E.; Bao, W.; Perkins, E.J.; et al. In vivo corrosion, tumor outcome, and microarray gene expression for two types of muscle-implanted tungsten alloys. Toxicol. Appl. Pharmacol. 2012, 265, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Emond, C.A.; Vergara, V.B.; Lombardini, E.D.; Mog, S.R.; Kalinich, J.F. Induction of rhabdomyosarcoma by embedded military-grade tungsten/nickel/cobalt not by tungsten/nickel/iron in the B6C3F1 mouse. Int. J. Toxicol. 2015, 34, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Health Affairs Policy Letter 07-029 (2007). Analysis of Metal Fragments Removed from Department of Defense Personnel. Available online: www.health.mil/~/media/MHS/Policy%20Files/Import/07-029.ashx (accessed on 2 September 2015).
- Kalinich, J.F.; Vergara, V.B.; Emond, C.A. Urinary and serum metal levels as indicators of embedded tungsten alloy fragments. Mil. Med. 2008, 173, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.C.; Aguirre, J.A.; Lemoine, A.P.; Segura, E.T.; Barontini, M.; Armando, I. Influence of age on stress responses to metabolic cage housing in rats. Cell. Mol. Neurobiol. 1999, 19, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Royo, F.; Lyberg, K.; Carlsson, H.-E.; Hau, J. Effect of metabolic cage housing on immunoglobin A and corticosterone excretion in faeces and urine of young rats. Exp. Physiol. 2004, 89, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Everds, N.E.; Scofield, R.H. Experimental animal urine collection: A review. Lab. Anim. 2004, 38, 333–361. [Google Scholar] [CrossRef] [PubMed]
- LabSand MSDS. Available online: www.labsand.com/wp-content/uploads/2016/09/MSDS-LabSand.pdf (accessed on 1 March 2017).
- Kit4Cat MSDS. Available online: www.kit4cat.com/wp-content/uploads/2011/04/MSDS-Kkit4Ccat.pdf (accessed on 1 March 2017).
- Hoffman, J.F.; Fan, A.X.; Neuendorf, E.H.; Vergara, V.B.; Kalinich, J.F. Hydrophobic Sand versus metabolic cages: A comparison of urine collection methods for the rat (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 2017, in press. [Google Scholar]
- Ansoborlo, E.; Henge-Napoli, M.H.; Chazel, V.; Gibert, R.; Guilmette, R.A. Review and critical analysis of available in vitro dissolution tests. Health Phys. 1999, 77, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Issacson, L.C. Urinary ionic strength, osmolality, and specific conductivity. Investig. Urol. 1968, 5, 406–413. [Google Scholar]
- Enck, P.; Merlin, V.; Erckenbright, J.F.; Wienbeck, M. Stress effects on gastrointestinal transit in the rat. Gut 1989, 30, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Lancaster, H.; Ridley, D.; McClure, F.; Whelan, G. 3Rs Refinement—Use of Hydrophobic Sand in Collection of Analytical Urine Samples. Available online: http://labsand.com/wp-content/uploads/2015/05/GSK-poster.pdf (accessed on 26 May 2017).

| Metal | Time Spent Mixing with LabSand | ||
|---|---|---|---|
| 5 min | 15 min | 60 min | |
| Aluminum | 1.254 (0.644) | 0.654 (0.064) ** | 2.35 (1.484) |
| Strontium | 1.143 (0.192) ** | 0.810 (0.220) * | 1.090 (0.162) ** |
| Copper | −0.013 (0.14) | 0.074 (0.117) | 0.057 (0.001) ** |
| Cobalt | 0.204 (0.136) | 0.100 (0.180) | −0.006 (0.240) |
| Zinc | −2.917 (0.340) ** | −2.717 (0.251) ** | −2.26 (0.265) ** |
| Lead | 0.205 (0.015) ** | 0.270 (0.022) ** | 0.189 (0.036) * |
| Source | Metal Concentration, PPB (ng/mL) | ||||
|---|---|---|---|---|---|
| Co | Cu | Sr | Al | Mn | |
| 18 Ω | −0.020 | 0.065 | 0.190 | 0.560 | −0.010 |
| Sink 1 | 0.095 | 413.150 | 270.950 | 5.610 | 3.380 |
| Sink 2 | 0.180 | 1411.000 | 238.700 | 5.950 | 3.170 |
| Sink 3 | 0.100 | 303.950 | 258.400 | 3.635 | 7.780 |
| Sink 4 | 0.315 | 105.550 | 179.200 | 0.995 | 4.225 |
| Treatment | Copper Concentration, PPB (ng/mL) | ||
|---|---|---|---|
| Ring | Funnel | Container | |
| Millipore Rinse | 0.616 (0.459) | 0.153 (0.050) | 0.934 (0.538) |
| Tap Water alone | 0.520 (0.211) | 0.100 (0.061) | 0.694 (0.218) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, J.F.; Vergara, V.B.; Mog, S.R.; Kalinich, J.F. Hydrophobic Sand Is a Non-Toxic Method of Urine Collection, Appropriate for Urinary Metal Analysis in the Rat. Toxics 2017, 5, 25. https://doi.org/10.3390/toxics5040025
Hoffman JF, Vergara VB, Mog SR, Kalinich JF. Hydrophobic Sand Is a Non-Toxic Method of Urine Collection, Appropriate for Urinary Metal Analysis in the Rat. Toxics. 2017; 5(4):25. https://doi.org/10.3390/toxics5040025
Chicago/Turabian StyleHoffman, Jessica F., Vernieda B. Vergara, Steven R. Mog, and John F. Kalinich. 2017. "Hydrophobic Sand Is a Non-Toxic Method of Urine Collection, Appropriate for Urinary Metal Analysis in the Rat" Toxics 5, no. 4: 25. https://doi.org/10.3390/toxics5040025





