Heavy Metal Pollution of Chari River Water during the Crossing of N’Djamena (Chad)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sampling
2.3. Analytical Procedures
2.4. Data Analysis
3. Results
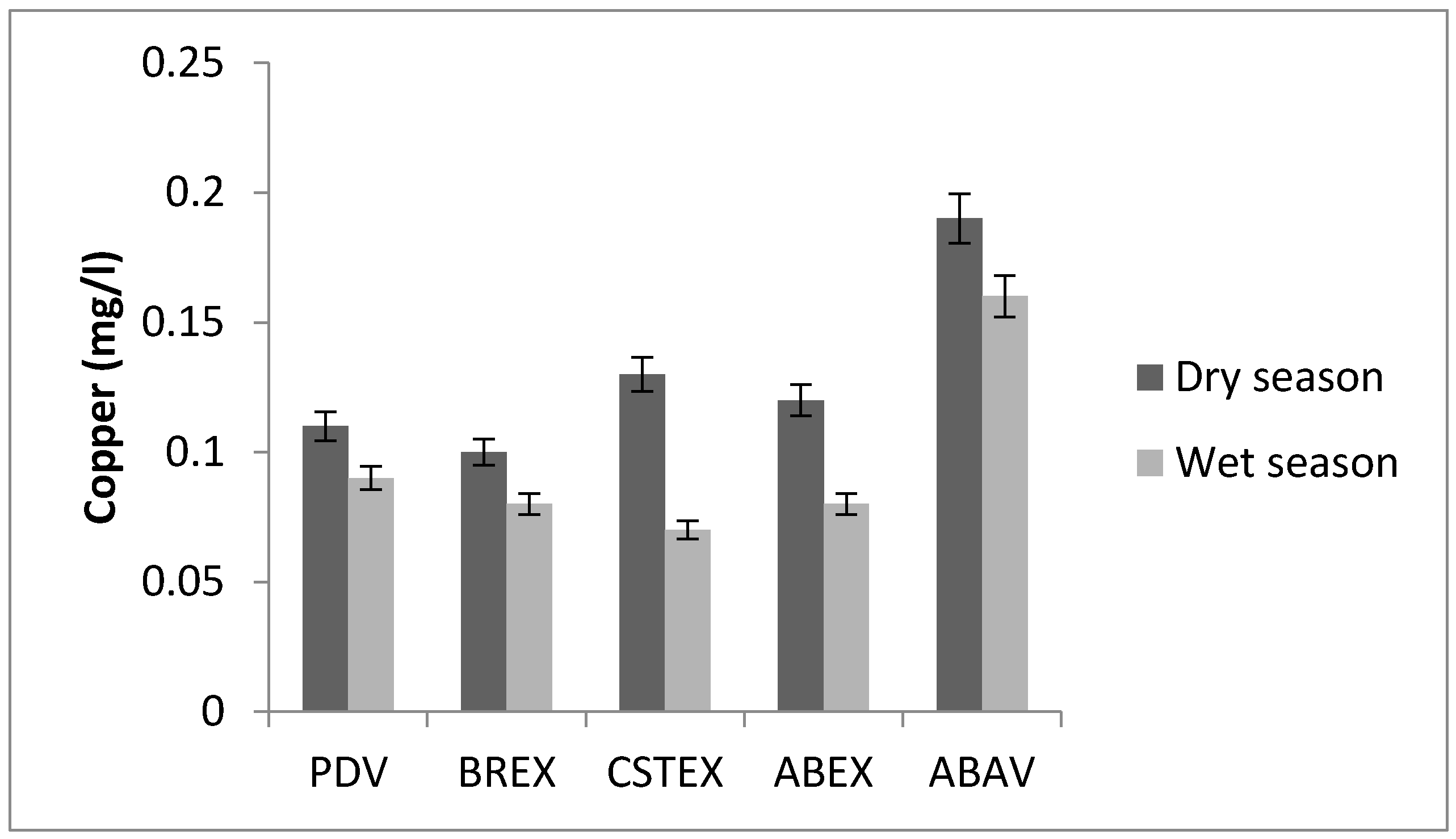
3.1. Copper
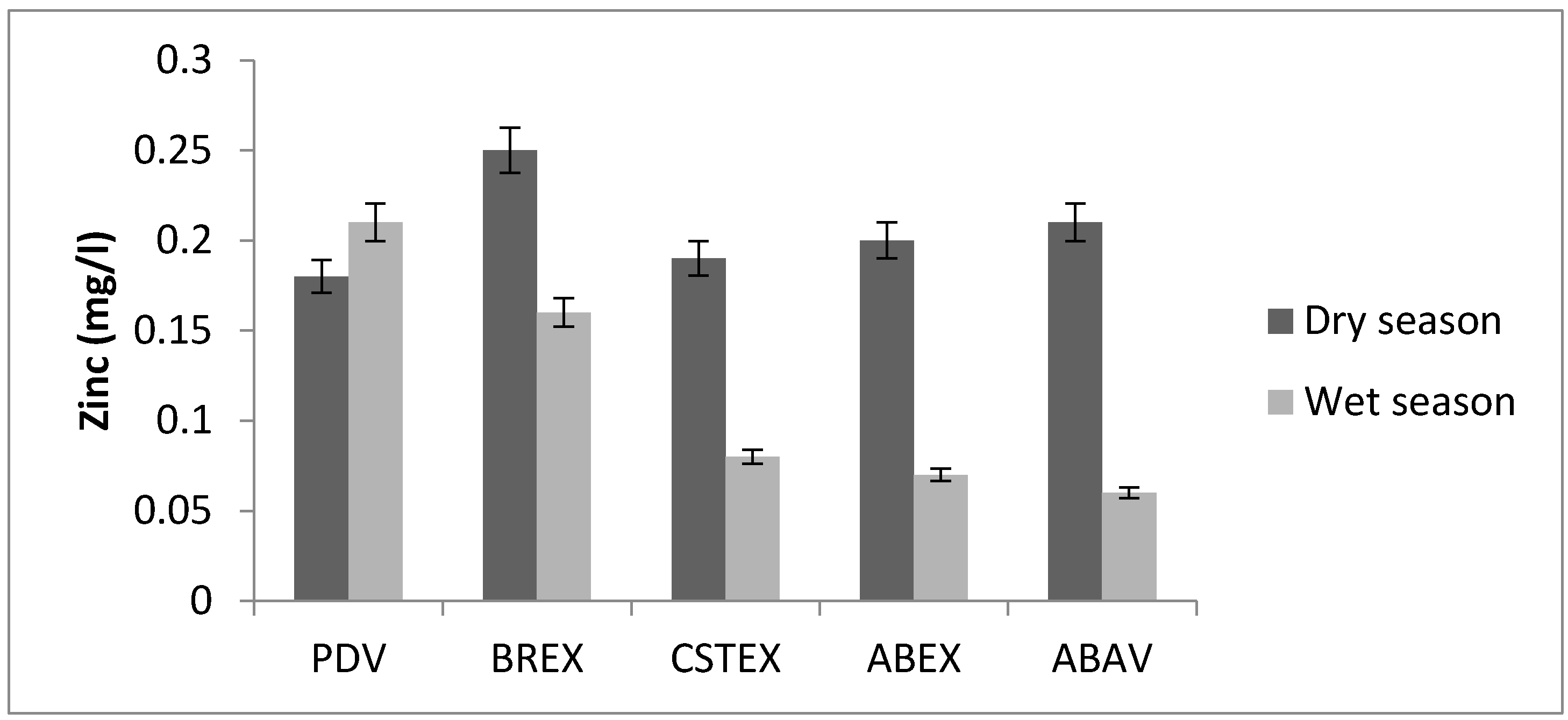
3.2. Zinc
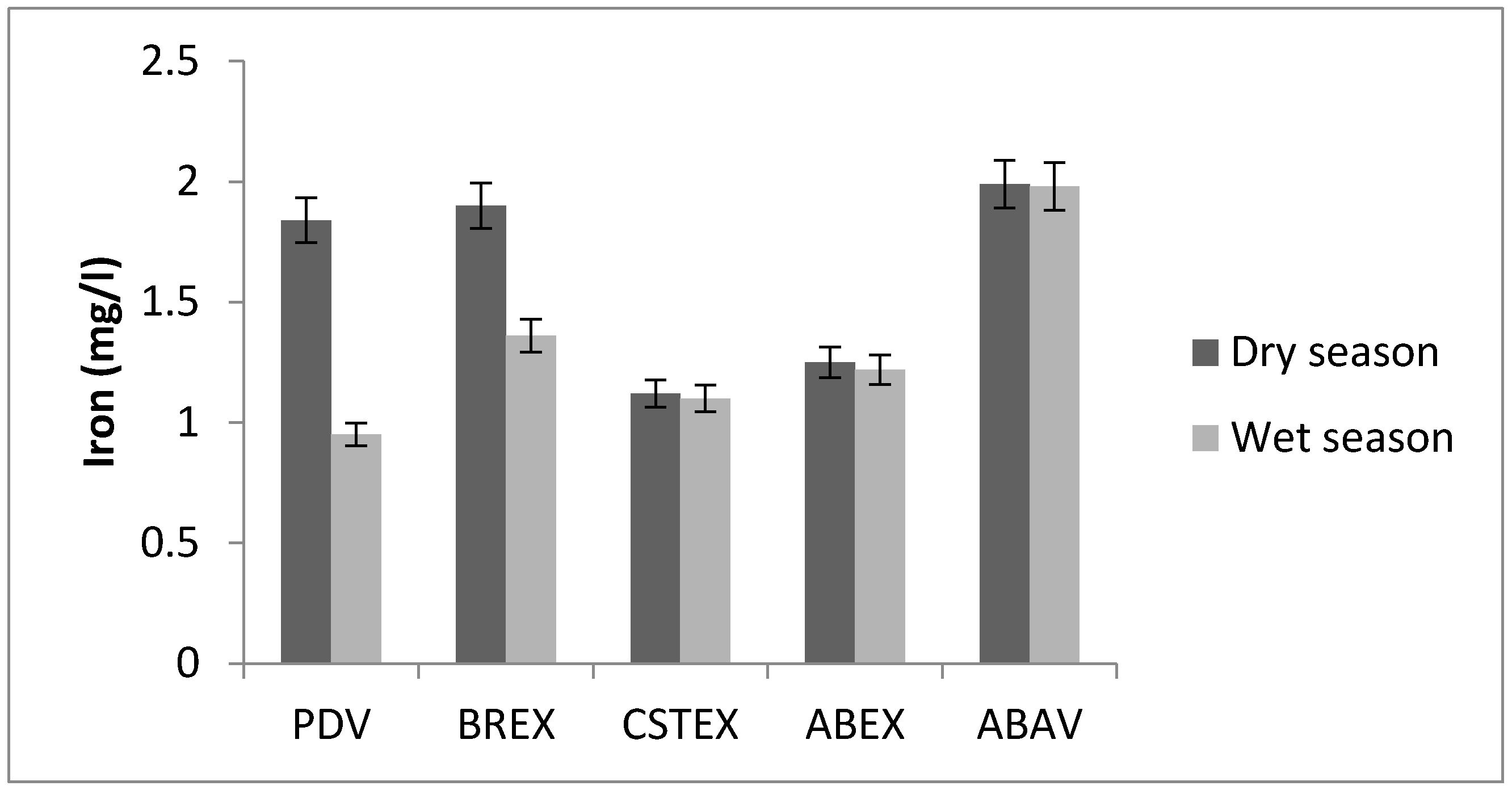
3.3. Iron
3.4. Nickel
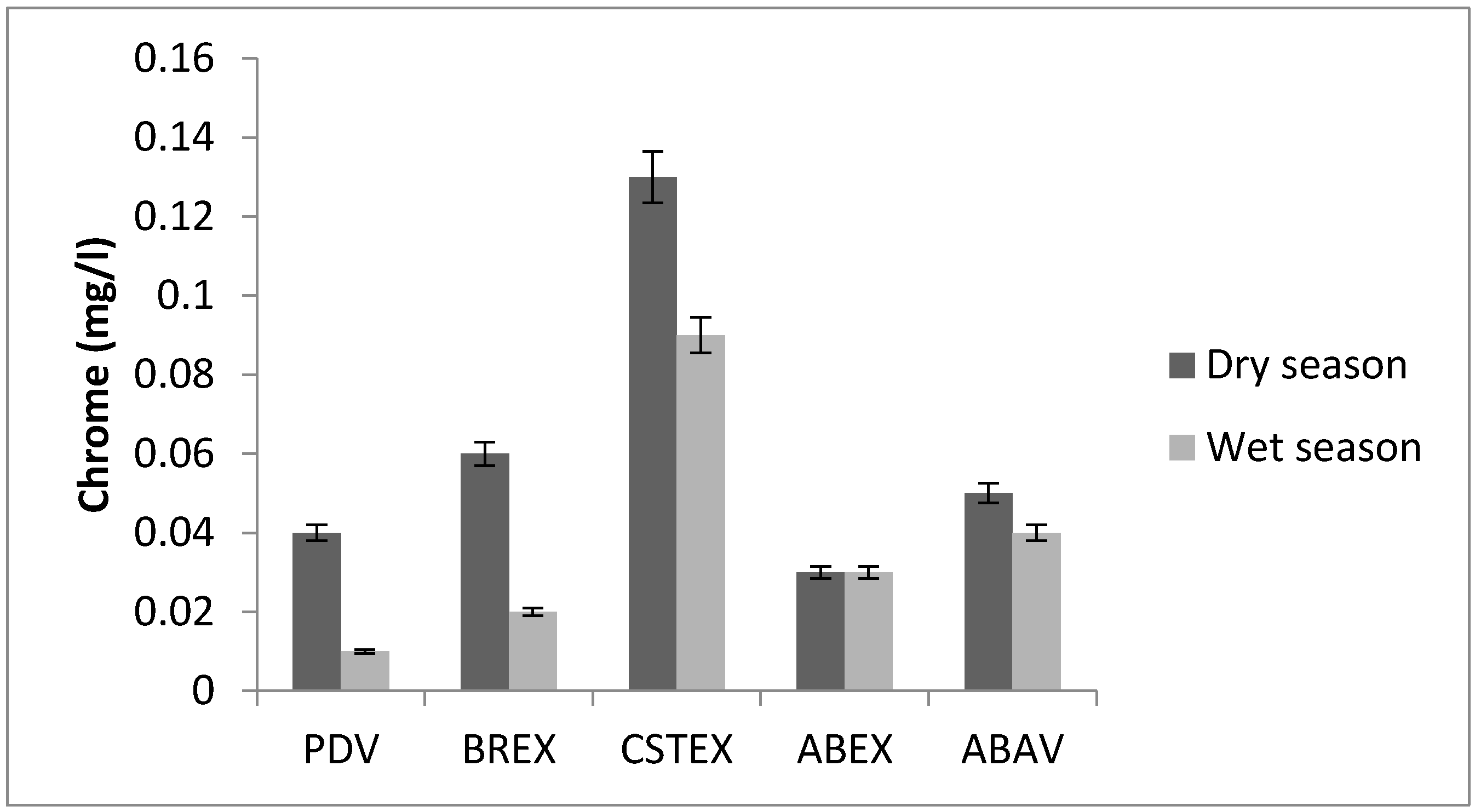
3.5. Chromium
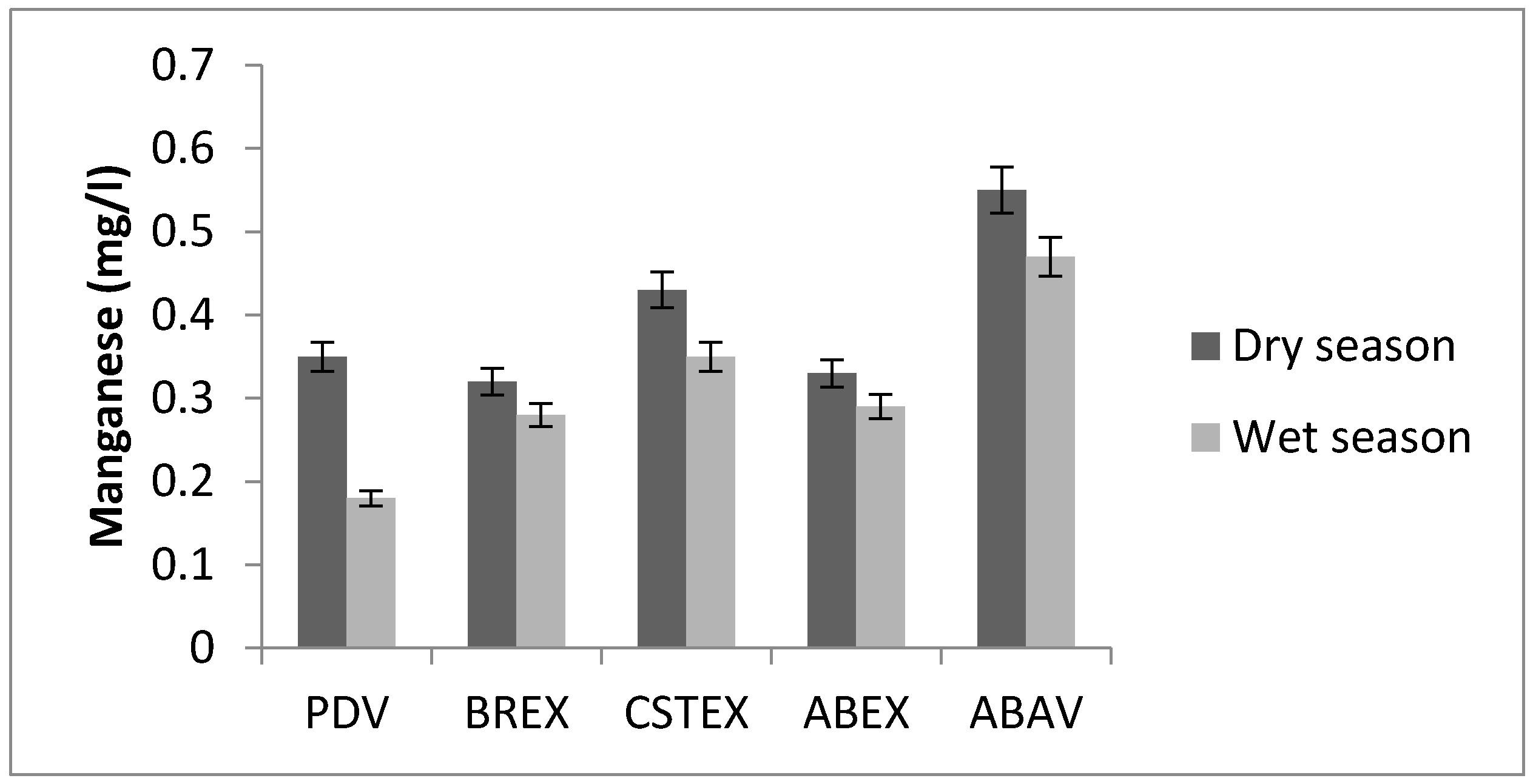
3.6. Manganese
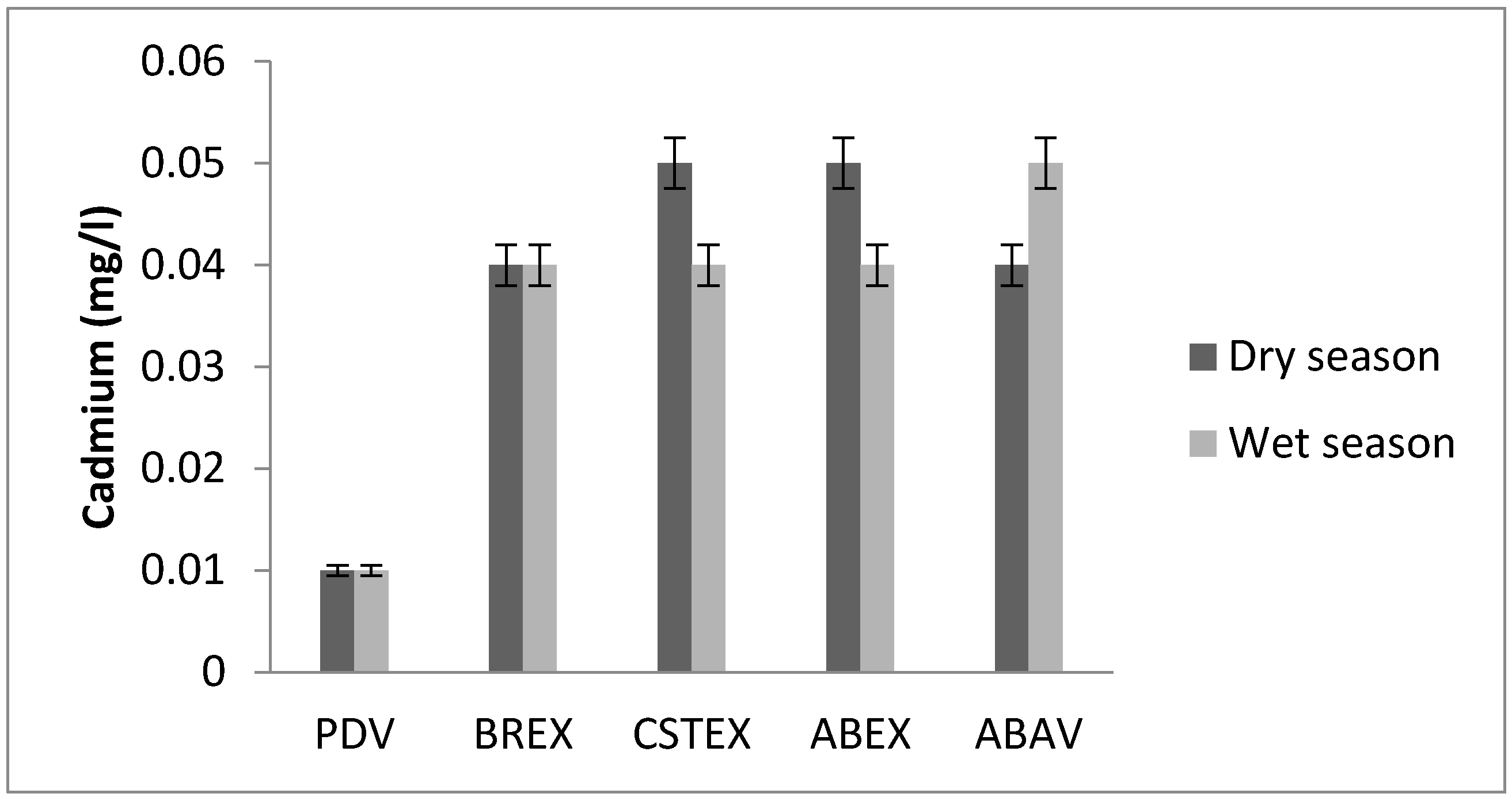
3.7. Cadmium
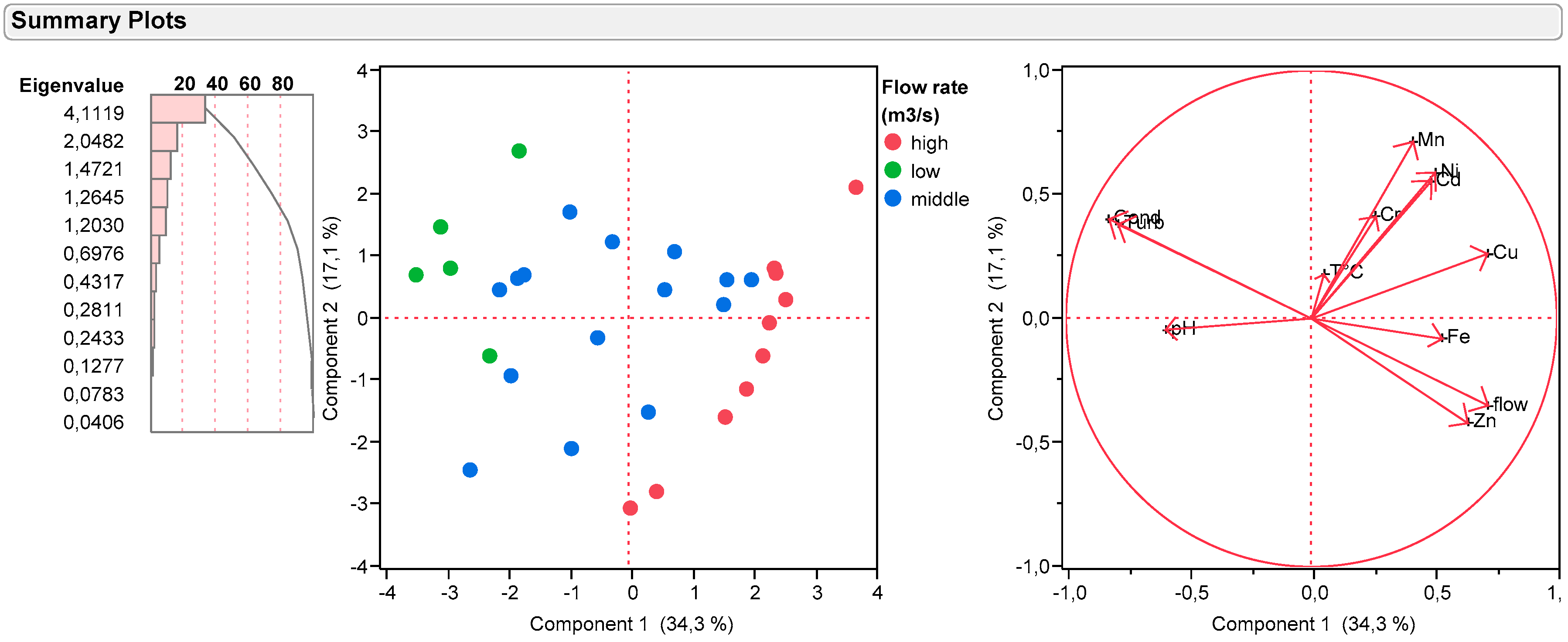
3.8. Principal Component Analysis (PCA)
- Positive correlation: x and y vary in the same direction
- Negative correlation: x and y vary in the opposite direction of the other
- No correlation: x and y vary independently
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Van der Bruggen, B.; Borghgraef, K.; Vinckier, C. Causes of Water Supply Problems in Urbanised Regions in Developing Countries. Water Resour. Manag. 2010, 24, 1885–1902. [Google Scholar] [CrossRef]
- Sorlini, S.; Palazzini, D.; Sieliechi, J.M.; Ngassoum, M.B. Assessment of Physical-Chemical Drinking Water Quality in the Logone Valley (Chad-Cameroon). Sustainability 2013, 5, 3060–3076. [Google Scholar] [CrossRef]
- Rodier, J. 2009- L’analyse de L’eau, 9th ed.; Dunod: Paris, France, 2009; p. 1526. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Volume Recommendations; Available online: http://www.who.int/water_sanitation_health/dwq/GDWQ2004web (accessed on 1 September 2012).
- Desjardins, R. Le Traitement Des Eaux, 2nd ed.; Ecole Polytechnique de Montreal: Montreal, QC, Canada, 1997; p. 304. [Google Scholar]
- Rose, J.B.; Gerba, C.P.; Jakubowski, W. Survey of Potable water supplies for cryptosporium and giardia. Environ. Sci. Technol. 1991, 25, 1393–1400. [Google Scholar] [CrossRef]
- Philippe, M.; Marina, C. Approches Analytiques Pour L’évaluation de la Disponibilité des Métaux Dans les Milieux Aquatiques; Rapport INERIS DRC-03-46820-PMo/JL-03.0672; Chimie Analytique et Environnementale. Direction des Risques Chroniques: Paris, France, 2003; p. 87. [Google Scholar]
- Zakaria, H.; Abderaman, M.A. Profil National du Tchad sur la Gestion des Produits Chimiques; MEE-UNITAR: Manhattan, NY, USA, 2002; p. 103. [Google Scholar]
- Chouret, A.; Mathieu, P. La nappe phréatique à la périphérie du Lac Tchad: Résultats préliminaires des travaux récents de l’ORSTOM. In Proceedings of the 3è Conférence de Géologie Africaine, Khartoum, Soudan, 3–17 January 1976; p. 10. [Google Scholar]
- Djoret, D. Etude de la Recharge de la Nappe du Chari Baguirmi (Tchad) Par les Méthodes Chimiques et Isotopiques. Ph.D. Thesis, Université d’Avignon et des Pays de Vaucluse, Avignon, France, 2000. [Google Scholar]
- Rodier, J.A.; Roche, M. La sécheresse actuelle en Afrique tropicale, Quelques données hydrologiques. Hydrol. Sci. Bull. 1973, 18, 411–418. [Google Scholar] [CrossRef]
- Kadjangaba, E. Synthèse des Données Isotopiques et Hydrochimiques du Bassin du Lac Tchad (Niger, Nigeria, Cameroun, Tchad); DEA Science de l’Eau dans l’Environnement Continental. Université d’Avignon et des Pays du Vaucluse: Avignon, France, 2000; p. 85. [Google Scholar]
- Massuel, S. Modélisation Hydrodynamique de la Nappe Phréatique Quaternaire du Bassin du Lac Tchad; DEA Science de l’eau dans l’environnement continental, Université de Montpellier II: Montpellier, France, 2001; p. 85. [Google Scholar]
- Schneider, J.-L. Les principaux événements hydroclimatiques survenus en Afrique sahélosaharienne depuis 1200 ans. A.D.C.R. Acad. Sci. Paris t.312 1991, Série II, 93–96. [Google Scholar]
- Schéma Directeur de l’Eau et de l’Assainissement (SDEA). Schéma Directeur de L’eau et de L’assainissement au Tchad 2003–2020; HCNE-MEE-PNUD-DAES; SDEA: N’Djamena, Tchad, 2003; 260p. [Google Scholar]
- Bruno, L.; Hach Modèle, D.R. 2400 Spectrophotomètre de Laboratoire Procédure; Doc 022.77.00567; Hach Company: Loveland, CO, USA, 2002; 405p. [Google Scholar]
- Richard, G.B. Chemometrics, Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Zacarias, I.; Garmen, G.; Yanez, M.A.; Chinwe, O.; Manuel, O.; Ricardo, U. Determination of the taste threshold of copper in water. Chem. Senses 2001, 26, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Janus, J.A.; Krajnc, E.L. Integrated criteria document chromium: Effects. Health Environ. Res. Online 2000, 47, 112–125. [Google Scholar]
- Järup, L. Cadmium overload and toxicity. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 2), 35–39. [Google Scholar] [CrossRef] [PubMed]
- Savary, P. Guides des Analyses de la Qualité de L’eau; Techni. Cités: Paris, France, 2003; 283p. [Google Scholar]
- Coe, M.T.; Foley, A. Human and natural impacts on the water resources of the Lake basin and the observed level of Lake Chad for the 288 months of available of Africa showing a continuous network. J. Geophys. Res. 2001, 106, 3349–3356. [Google Scholar] [CrossRef]
- Cadihlac, L.; Henry, C.; Fournier, I.; Marchet, P.; Vallée, K.; Bonnefille, M.; Chery, L. Système D’évaluation de la Qualité des Eaux Souterraine SEQ-Eau Souterraines; Rapport de présentation Version 0.1-Août 2003; Bureau de Recherches Géologiques et Minières: Paris, France, 2003; 75p.
- Tchoroun, M.; Noumi, G.B.; Tchadanaye, N.M.; Dangwang, D.J.M. Heavy Metals Pollution Level in Water, Fish and Sediments from the Logone River Within Moundou City (Chad). Int. J. Environ. Monit. Anal. 2015, 3, 275–281. [Google Scholar] [CrossRef]
- Abderahmane, M.A. Profil National du Tchad sur la Gestion des Produits Chimiques; Ministère de l’Environnement et de l’eau: N’Djamena, Tchad, 2001; 101p.
- Siéliéchi, J.M.; Noumi, G.B.; Fadimatou, M.; Ali, A.; Kapseu, C. Speciation of heavy metals in sediments sampled from different pollution sources of lake Dang, Ngaoundere–Cameroon. Int. J. Environ. Prot. 2013, 2, 10–19. [Google Scholar]
- Ngaram, N.; Tchadanaye, N.M.; Merle, A.; Lanteri, P.; Baskali-Bouregaa, N. Caractérisation Physicochimique des Eaux du Fleuve Chari au Niveau de la Ville de N’djaména (Tchad); Annales de l’Université de Ndjamena-Série C –N°05; Annales de l’Université de Ndjamena: N’Djamena, Chad, 2011; 102p. [Google Scholar]


| Mean Values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Weather 2008 | Rainy Weather 2009 | |||||||||
| Parameters | PDV | BREX | CSTEX | ABEX | ABAV | PDV | BREX | CSTEX | ABEX | ABAV |
| Zn (mg/L) | 0.18 | 0.25 | 0.19 | 0.20 | 0.21 | 0.21 | 0.16 | 0.08 | 0.07 | 0.06 |
| Cu (mg/L) | 0.11 | 0.10 | 0.13 | 0.12 | 0.19 | 0.09 | 0.08 | 0.07 | 0.08 | 0.16 |
| Cr (mg/L) | 0.04 | 0.06 | 0.13 | 0.03 | 0.05 | 0.01 | 0.02 | 0.09 | 0.03 | 0.04 |
| Mn (mg/L) | 0.35 | 0.32 | 0.43 | 0.33 | 0.55 | 0.18 | 0.28 | 0.35 | 0.29 | 0.47 |
| Ni (mg/L) | 0.10 | 0.21 | 0.28 | 0.60 | 0.65 | 0.08 | 0.09 | 0.16 | 0.52 | 0.57 |
| Fe (mg/L) | 1.84 | 1.90 | 1.12 | 1. 25 | 1.99 | 0.95 | 1.36 | 1.10 | 1.22 | 1.98 |
| Cd (mg/L) | 0.01 | 0.04 | 0.05 | 0.05 | 0.04 | 0.01 | 0.04 | 0.04 | 0.04 | 0.05 |
| Row | Flow | T, °C | pH | Cond | Turb | Cd | Zn | Cu | Cr | Mn | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| flow | 1.00 | 0.35 | −0.50 | −0.69 | −0.59 | 0.24 | 0.44 | 0.30 | 0.09 | −0.04 | 0.08 | 0.43 |
| T °C | 0.35 | 1.00 | −0.16 | 0.12 | 0.21 | 0.13 | −0.18 | 0.12 | −0.06 | −0.04 | 0.09 | 0.07 |
| pH | −0.50 | −0.16 | 1.00 | 0.44 | 0.24 | −0.25 | −0.15 | −0.51 | −0.19 | −0.11 | −0.20 | −0.29 |
| Cond | −0.69 | 0.12 | 0.44 | 1.00 | 0.89 | −0.09 | −0.59 | −0.55 | −0.12 | −0.13 | −0.17 | −0.24 |
| Turb | −0.59 | 0.21 | 0.24 | 0.89 | 1.00 | −0.17 | −0.64 | −0.52 | −0.16 | −0.12 | −0.16 | −0.22 |
| Cd | 0.24 | 0.13 | −0.25 | −0.09 | −0.17 | 1.00 | 0.20 | 0.17 | 0.54 | 0.43 | 0.46 | 0.36 |
| Zn | 0.44 | −0.18 | −0.15 | −0.59 | −0.64 | 0.20 | 1.00 | 0.23 | 0.00 | 0.08 | 0.05 | 0.52 |
| Cu | 0.30 | 0.12 | −0.51 | −0.55 | −0.52 | 0.17 | 0.23 | 1.00 | 0.12 | 0.55 | 0.56 | 0.15 |
| Cr | 0.09 | −0.06 | −0.19 | −0.12 | −0.16 | 0.54 | 0.00 | 0.12 | 1.00 | 0.35 | −0.03 | −0.12 |
| Mn | −0.04 | −0.04 | −0.11 | −0.13 | −0.12 | 0.43 | 0.08 | 0.55 | 0.35 | 1.00 | 0.58 | 0.03 |
| Ni | 0.08 | 0.09 | −0.20 | −0.17 | −0.16 | 0.46 | 0.05 | 0.56 | −0.03 | 0.58 | 1.00 | 0.40 |
| Fe | 0.43 | 0.07 | −0.29 | −0.24 | −0.22 | 0.36 | 0.52 | 0.15 | −0.12 | 0.03 | 0.40 | 1.00 |
| Location | Flow (m3/s) | Cd | Pb | Zn | Cu | Cr | Mn | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|
| PDV | High | 0.02 | 0.02 | 0.16 | 0.13 | 0.02 | 0.10 | 0.01 | 1.84 |
| Middle | 0.01 | 0.02 | 0.20 | 0.10 | 0.01 | 0.20 | 0.13 | 0.80 | |
| Low | 0.01 | 0.01 | 0.20 | 0.08 | 0.03 | 0.40 | 0.12 | 1.49 | |
| BRAM | High | 0.04 | 0.11 | 0.11 | 0.03 | 0.11 | 0.01 | 1.47 | |
| Middle | 0.04 | 0.08 | 0.06 | 0.02 | 0.25 | 0.20 | 1.16 | ||
| Low | 0.03 | 0.03 | 0.03 | 0.06 | 0.07 | 0.03 | 0.50 | 0.15 | |
| BREX | High | 0.04 | 0.07 | 0.27 | 0.13 | 0.06 | 0.30 | 0.20 | 2.02 |
| Middle | 0.04 | 0.05 | 0.21 | 0.09 | 0.03 | 0.30 | 0.15 | 1.56 | |
| Low | 0.04 | 0.03 | 0.16 | 0.07 | 0.05 | 0.30 | 0.11 | 1.42 | |
| BRAV | High | 0.04 | 0.16 | 0.23 | 0.01 | 0.25 | 0.02 | 1.48 | |
| Middle | 0.03 | 0.03 | 0.01 | 0.07 | 0.07 | 0.01 | 0.25 | 0.08 | |
| Low | 0.03 | 0.03 | 0.08 | 0.14 | 0.04 | 0.37 | 0.17 | 1.66 | |
| CSTAM | High | 0.03 | 0.09 | 0.13 | 0.03 | 0.23 | 0.04 | 1.46 | |
| Middle | 0.02 | 0.04 | 0.12 | 0.04 | 0.11 | 0.05 | 0.35 | ||
| Low | 0.03 | 0.28 | 0.08 | 0.07 | 0.22 | 0.52 | 0.33 | ||
| ABAM | High | 0.04 | 0.20 | 0.10 | 0.03 | 0.40 | 0.11 | 1.87 | |
| Middle | 0.06 | 0.06 | 0.08 | 0.11 | 0.03 | 0.35 | 0.94 | 1.21 | |
| Low | 0.05 | 0.03 | 0.10 | 0.11 | 0.03 | 0.43 | 0.71 | 1.69 | |
| ABEX | High | 0.06 | 0.06 | 0.22 | 0.13 | 0.02 | 0.34 | 0.59 | 2.58 |
| Middle | 0.05 | 0.03 | 0.16 | 0.10 | 0.04 | 0.31 | 0.58 | 1.87 | |
| Low | 0.04 | 0.02 | 0.09 | 0.10 | 0.03 | 0.29 | 0.53 | 1.67 | |
| ABAV | High | 0.05 | 0.07 | 0.21 | 0.20 | 0.06 | 0.60 | 0.68 | 2.23 |
| Middle | 0.04 | 0.04 | 0.15 | 0.17 | 0.04 | 0.55 | 0.58 | 1.62 | |
| Low | 0.04 | 0.03 | 0.11 | 0.17 | 0.05 | 0.43 | 0.59 | 1.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nambatingar, N.; Clement, Y.; Merle, A.; New Mahamat, T.; Lanteri, P. Heavy Metal Pollution of Chari River Water during the Crossing of N’Djamena (Chad). Toxics 2017, 5, 26. https://doi.org/10.3390/toxics5040026
Nambatingar N, Clement Y, Merle A, New Mahamat T, Lanteri P. Heavy Metal Pollution of Chari River Water during the Crossing of N’Djamena (Chad). Toxics. 2017; 5(4):26. https://doi.org/10.3390/toxics5040026
Chicago/Turabian StyleNambatingar, N’garam, Yohann Clement, Alain Merle, Tchadanaye New Mahamat, and Pierre Lanteri. 2017. "Heavy Metal Pollution of Chari River Water during the Crossing of N’Djamena (Chad)" Toxics 5, no. 4: 26. https://doi.org/10.3390/toxics5040026





