β-Cyclodextrin Attenuates Perfluorooctanoic Acid Toxicity in the Zebrafish Embryo Model
Abstract
:1. Introduction
2. Materials and Methods
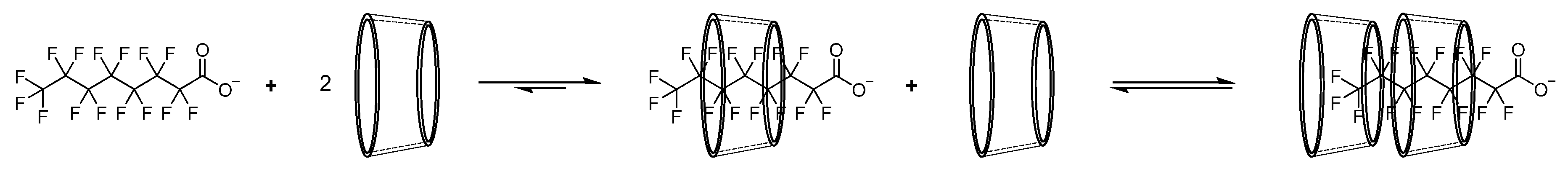
2.1. Test Chemicals
2.2. Zebrafish Rearing and Breeding
2.3. Zebrafish Embryo Toxicity Assay
2.4. Statistical Analyses
3. Results
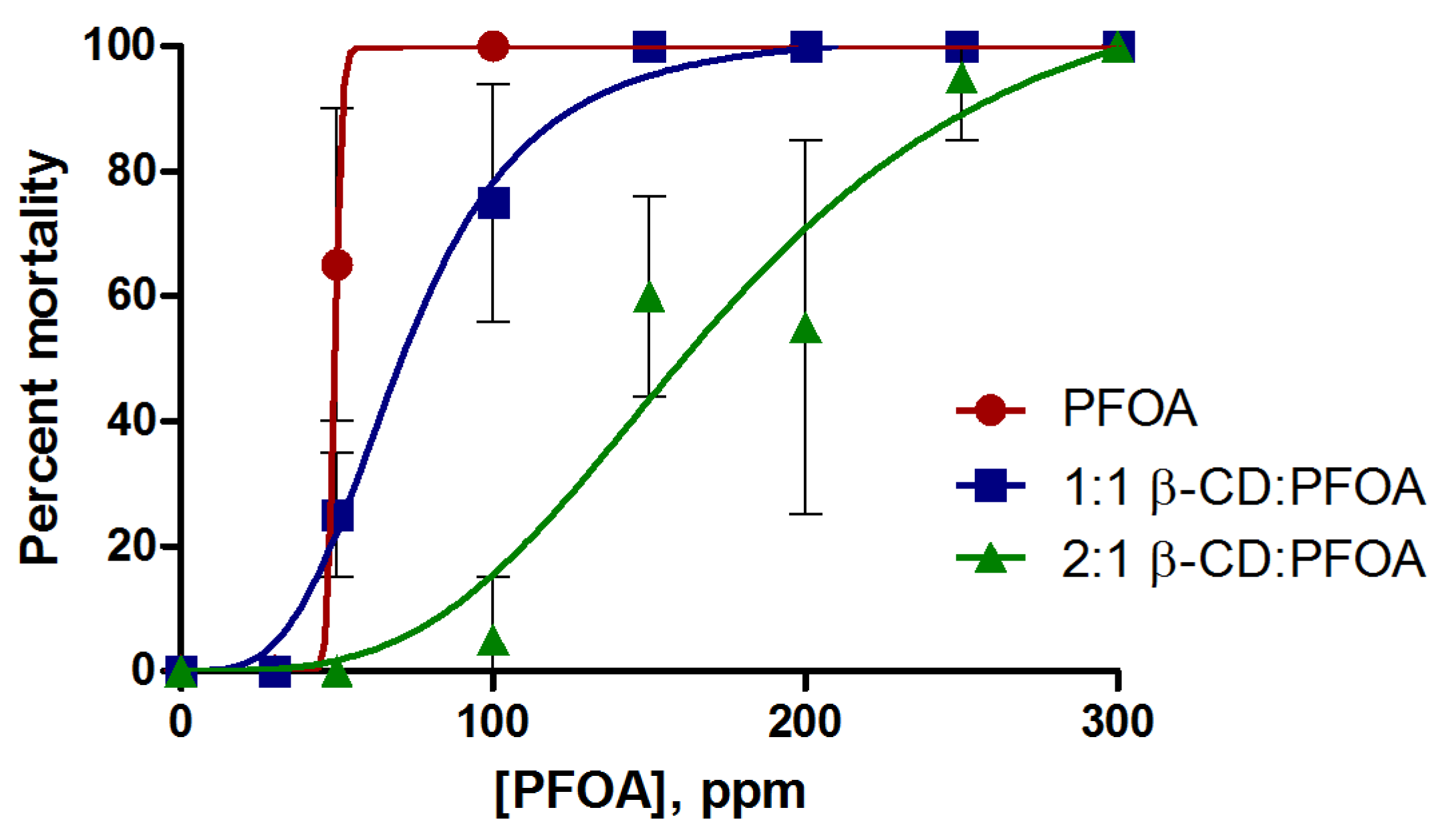
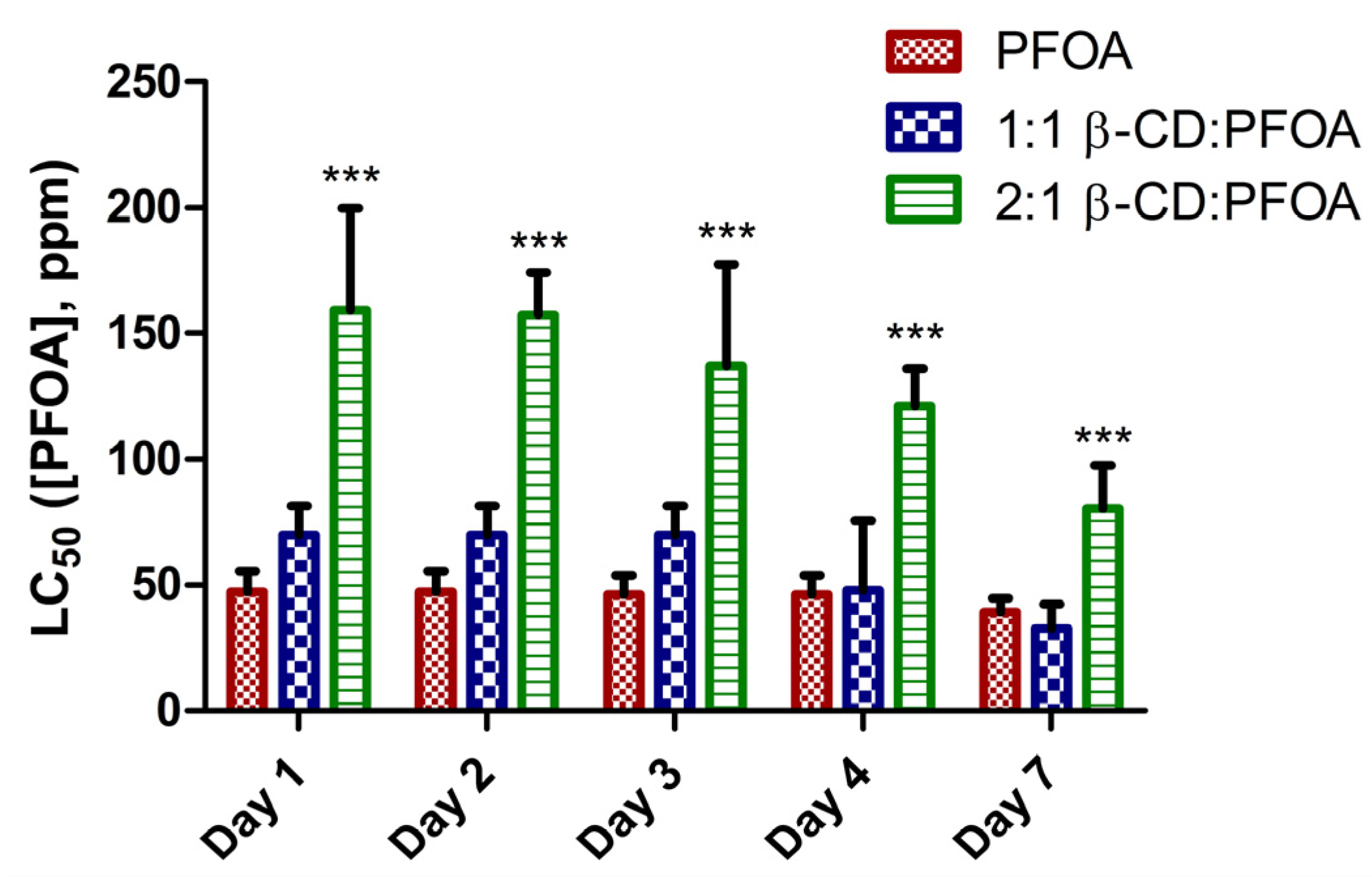
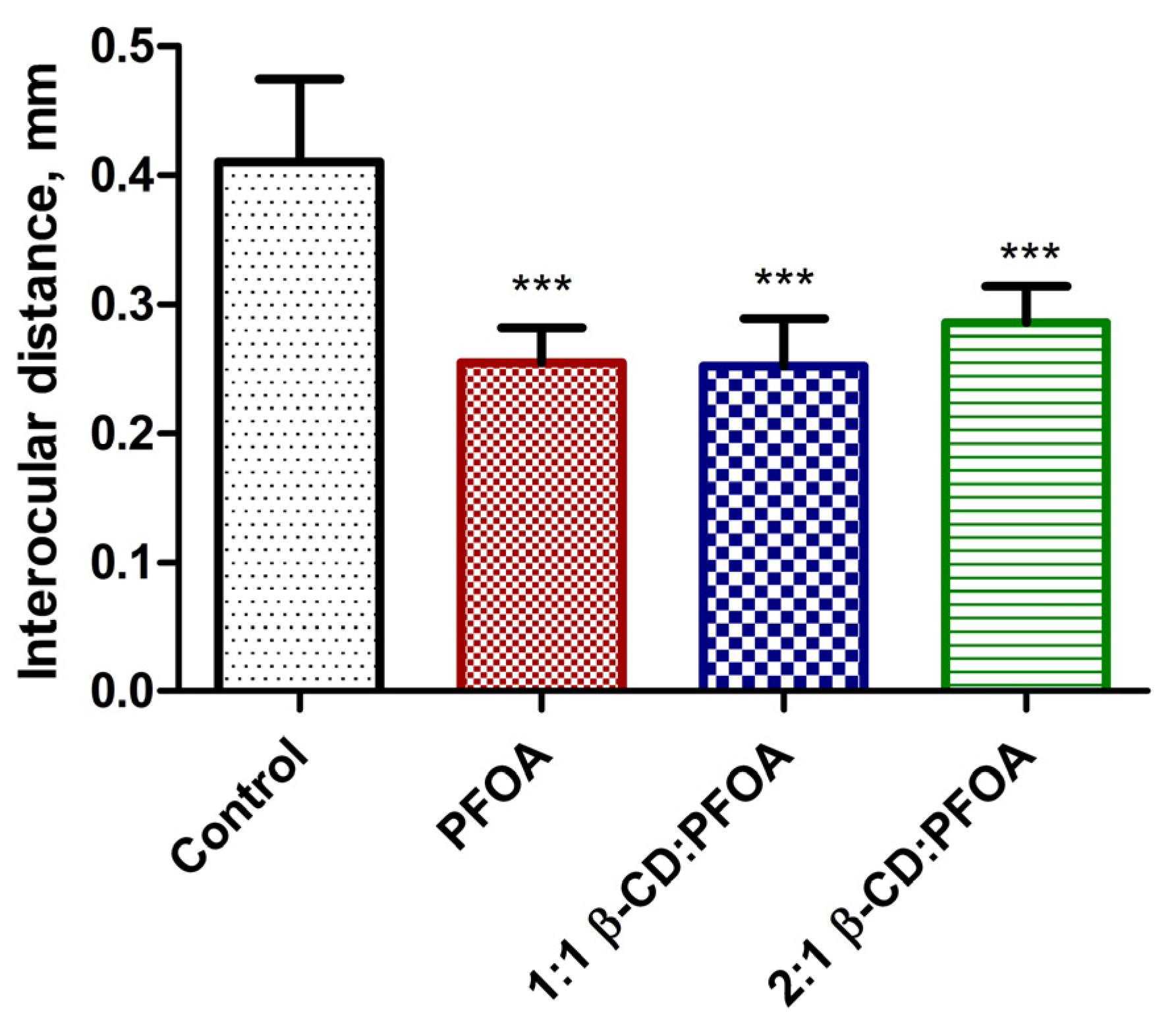
3.1. PFOA Toxicity in the Zebrafish Embryo Model
3.2. Attenuation of PFOA Toxicity by β-CD
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Source, fate, and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated compounds in U.S. fast food packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef]
- Barzen-Hanson, K.A.; Roberts, S.C.; Choyke, S.; Oetjen, K.; McAlees, A.; Riddell, N.; McCrindle, R.; Ferguson, P.L.; Higgins, C.P.; Field, J.A. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.A.; Leffers, H. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 2008, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K. Perfluoroalkyl and polyfluoroalkyl substances: Current and future perspectives. Environ. Chem. 2011, 8, 333–338. [Google Scholar] [CrossRef]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C.G. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, W.; de Voogt, P.; Coen, W.D.; Bervoets, L. Perfluorinated substances in human food and other sources of human exposure. Rev. Environ. Contam. Toxicol. 2010, 208, 179–215. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, S.J.; Brooks, A.P.; Maher, A.; Flensborg, P.; Arnold, S.; Fletcher, T.; Steenland, K.; Shankar, A.; Knox, S.S.; Pollard, C.; et al. The C8 health project: Design, methods, and participants. Environ. Health Perspect. 2009, 117, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.M.; Hoffman, K.; Shin, H.M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 2013, 121, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.J.; Mondal, D.; Armstrong, B.; Bloom, M.S.; Fletcher, T. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect. 2012, 120, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Del Martin Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Weiss-Errico, M.J.; O’Shea, K.E. Detailed NMR investigation of cyclodextrin-perfluorinated surfactant interactions in aqueous media. J. Hazard. Mater. 2017, 329, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.B.; Wilson, L.D. Tunable macromolecular-based materials for the adsorption of perfluorooctanoic and octanoic acid anions. J. Colloid Interface Sci. 2013, 402, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. Nano-sized cyclodextrin-based molecularly imprinted polymer adsorbents for perfluorinated compounds—A mini review. Nanomaterials 2015, 5, 981–1003. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. Investigation of the adsorption processes of fluorocarbon and hydrocarbon anions at the solid-solution interface of macromolecular imprinted polymer materials. J. Phys. Chem. C 2016, 120, 6553–6568. [Google Scholar] [CrossRef]
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin polymer network sequesters perfluorooctanoic acid at environmentally relevant concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Verrall, R.E. 19F and 1H-NMR investigation of cyclodextrin/fluorocarbon alkyl carboxylate surfactant inclusion complexes. Langmuir 1998, 14, 4710–4717. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Borisov, A.S.; Wilson, L.D.; Hazendonk, P. Formation of host-guest complexes of β-cyclodextrin and perfluorooctanoic acid. J. Phys. Chem. B 2011, 115, 9511–9527. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Sidhu, P.; Wilson, L.D.; Hazendonk, P. Characterization and Dynamic Properties for the Solid Inclusion Complexes of β-Cyclodextrin and Perfluorooctanoic Acid. J. Phys. Chem. B 2013, 117, 8269–8282. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Sidhu, P.S.; Wilson, L.D.; Hazendonk, P.; Borisov, A. Counterion anchoring effect on the structure of the solid-state inclusion complexes of β-cyclodextrin and sodium perfluorooctanoate. J. Phys. Chem. C 2015, 119, 22225–22243. [Google Scholar] [CrossRef]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish embryos as models for embryotoxicity and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Mattingly, C.J.; Allen, D.; Ceger, P.; Casey, W.; Hinton, D.; Kanungo, J.; Kullman, S.W.; Tal, T.; Bondesson, M.; et al. Advancing toxicology research using in vivo high throughput toxicology with small fish models. ALTEX 2016, 33, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.E.; Kullman, S.W.; Hardman, R.C.; Volz, D.C.; Chen, P.J.; Carney, M.; Bencic, D.C. Resolving mechanisms of toxicity while pursuing ecotoxicological relevance? Mar. Pollut. Bull. 2005, 51, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Fischer, S.; Gündel, U.; Küster, E.; Luckenbach, T.; Voelker, D. The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. Int. 2008, 15, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Marrs, J.A. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, A.; Vergauwen, L.; De Coen, W.; Knapen, D. Structure-activity relationship assessment of four perfluorinated chemicals using a prolonged zebrafish early life stage test. Chemosphere 2011, 82, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Liu, H.L.; Shi, W.; Wei, S.; Giesy, J.P.; Yu, H.X. Effects of perfluorinated compounds on development of zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2011, 19, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, A.; Vergauwen, L.; Benoot, D.; Laukens, K.; Knapen, D. Mechanistic toxicity study of perfluorooctanoic acid in zebrafish suggests mitochondrial dysfunction to play a key role in PFOA toxicity. Chemosphere 2013, 91, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhang, J.; Chen, Y.; Wang, L.; Wang, M.; Xiong, D.; Sun, Y. Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch. Environ. Contam. Toxicol. 2013, 64, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, M.; Sundström, M.; Larsson, P.; Gabrielsson, J.; Bergman, A.; Norrgren, L.; Örn, S. Tissue uptake, distribution and elimination of (14)C-PFOA in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 163, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Annunziato, K.A.; Bugel, S.M.; Cooper, K.R. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Annunziato, K.M.; Cooper, K.R. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS and PFNA. Aquat. Toxicol. 2016, 180, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Toor, F.; Annunziato, K.A.; Cooper, K.R. Effects of chronic perfluorooctanoic acid (PFOA) at low concentrations on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod. Toxicol. 2017, 69, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Conlledo, N.; Langerholc, T.; Madorran, E.; Sala, M.; Barranco, A. Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate. Food Chem. Toxicol. 2017, 104, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P.; Gantar, M.; Gibbs, P.D.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Sanchez, K.; Gantar, M.; Gibbs, P.; Schmale, M.C.; Berry, J.P. Carotenoid glycosides from cyanobacteria are teratogenic in the zebrafish (Danio rerio) embryo model. Chemosphere 2017, 174, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Granato, M.; Nüsslein-Volhard, C. Keeping and raising zebrafish. In Zebrafish; Nüsslein-Volhard, C., Dahm, R., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 7–37. [Google Scholar]
- International Business Machines Corporation (IBM Corp.). SPSS Statistics, version 22.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- GraphPad Software, Inc. Prism 5, version 5.03; GraphPad Software, Inc.: La Jolla, CA, USA, 2010. [Google Scholar]
- De Koning, C.; Beekhuijzen, M.; Tobor-Kaplon, M.; de Vries-Buitenweg, S.; Schoutsen, D.; Leeigen, N.; van de Waart, B.; Emmen, H. Visualizing compound distribution during zebrafish embryo development: The effects of lipophilicity and DMSO. Birth Defects Res. B Dev. Reprod. Toxicol. 2015, 104, 253–272. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss-Errico, M.J.; Berry, J.P.; O’Shea, K.E. β-Cyclodextrin Attenuates Perfluorooctanoic Acid Toxicity in the Zebrafish Embryo Model. Toxics 2017, 5, 31. https://doi.org/10.3390/toxics5040031
Weiss-Errico MJ, Berry JP, O’Shea KE. β-Cyclodextrin Attenuates Perfluorooctanoic Acid Toxicity in the Zebrafish Embryo Model. Toxics. 2017; 5(4):31. https://doi.org/10.3390/toxics5040031
Chicago/Turabian StyleWeiss-Errico, Mary Jo, John P. Berry, and Kevin E. O’Shea. 2017. "β-Cyclodextrin Attenuates Perfluorooctanoic Acid Toxicity in the Zebrafish Embryo Model" Toxics 5, no. 4: 31. https://doi.org/10.3390/toxics5040031




