County-Level Radon and Incidence of Female Thyroid Cancer in Iowa, New Jersey, and Wisconsin, USA
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Appleton, J.D. Radon in air and water. In Essentials of Medical Geology, Revised ed.; Sellenius, O., Alloway, B., Centeno, J.A., Finklelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 239–277. ISBN 978-94-007-4374-8. [Google Scholar]
- Ishikawa, T.; Narazaki, Y.; Yasuoka, Y.; Tokonami, S.; Yamada, Y. Bio-kinetics of radon ingested from drinking water. Radiat. Prot. Dosim. 2003, 105, 65–70. [Google Scholar] [CrossRef]
- Copes, R.; Scott, J. Radon exposure: Can we make a difference? Can. Med. Assoc. J. 2007, 177, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- George, A.C. The history, development and the present status of the radon measurement programme in the United States of America. Radiat. Prot. Dosim. 2015, 167, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lantz, P.M.; Mendes, D.; Philbert, M.A. Radon, smoking, and lung cancer: The need to refocus radon control policy. Am. J. Public Health 2013, 103, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wakeford, R. The cancer epidemiology of radiation. Oncogene 2004, 23, 6404–6428. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Preston, D.; Funamoto, S.; Yonehara, S.; Ito, M.; Tokuoka, S.; Sugiyama, H.; Soda, M.; Ozasa, K.; Mabuchi, K. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int. J. Cancer 2013, 132, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Riches, A.C.; Herceg, Z.; Bryant, P.E.; Wynford-Thomas, D. Radiation induced transformation of SV40-immortalized human thyroid epithelial cells by single 10 exposure to plutonium alpha particles in vitro. Int. J. Radiat. Biol. 1994, 66, 757–765. [Google Scholar] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Williams, D. Cancer after nuclear fallout: Lessons from the Chernobyl accident. Nat. Rev. Cancer 2002, 2, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zablotska, L.B.; Nadyrov, E.A.; Rozhko, A.V.; Gong, Z.; Polyanskaya, O.N.; McConnell, R.J.; O’Kane, P.; Brenner, A.V.; Little, M.P.; Ostroumova, E.; et al. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997–2008) of a cohort of children and adolescents from belarus exposed to radioiodines after the Chernobyl accident. Cancer 2015, 121, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kesminiene, A.; Evrard, A.S.; Ivanov, V.K.; Malakhova, I.V.; Kurtinaitise, J.; Stengrevics, A.; Tekkel, M.; Chekin, S.; Drozdovitch, V.; Gavrilin, Y.; Golovanov, I. Risk of thyroid cancer among Chernobyl liquidators. Radiat. Res. 2012, 178, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Gorski, A.I.; Maksioutov, M.A.; Vlasov, O.K.; Godko, A.M.; Tsyb, A.F.; Tirmarche, M.; Valenty, M.; Verger, P. Thyroid cancer incidence among adolescents and adults in the Bryansk region of Russia following the Chernobyl accident. Health Phys. 2003, 84, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, S.J.; Lee, C.; de Gonzalez, A.B. Medical exposure to radiation and thyroid cancer. Clin. Oncol. 2011, 23, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, R.; Malandrino, P.; Vigneri, P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr. Opin. Oncol. 2015, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kristbjornsdottir, A.; Rafnsson, V. Incidence of cancer among residents of high temperature geothermal areas in Iceland: A census based study 1981 to 2010. Environ. Health 2012, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Camacho, F.; Mangano, J.; Goldenberg, D. Evaluating for a geospatial relationship between radon levels and thyroid cancer in Pennsylvania. Laryngoscope 2015, 125. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.M.; Smith, T.J. Doses to organs and tissues from radon and its decay products. J. Radiol. Prot. 2002, 22, 389. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice, J.D., Jr. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat. Res. 1995, 141, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Ron, E.; Tokuoka, S.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007, 168, 1–64. [Google Scholar] [CrossRef] [PubMed]
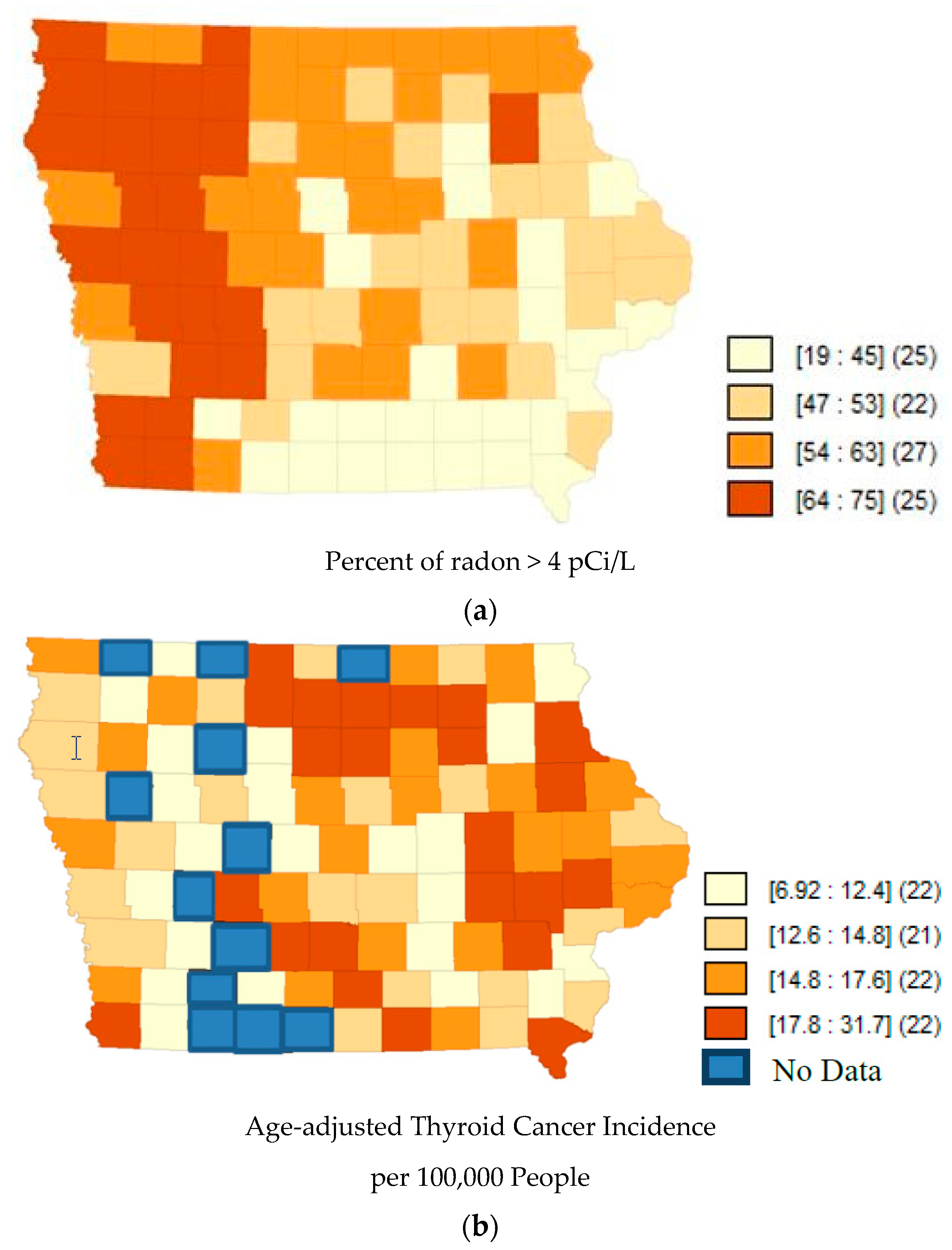
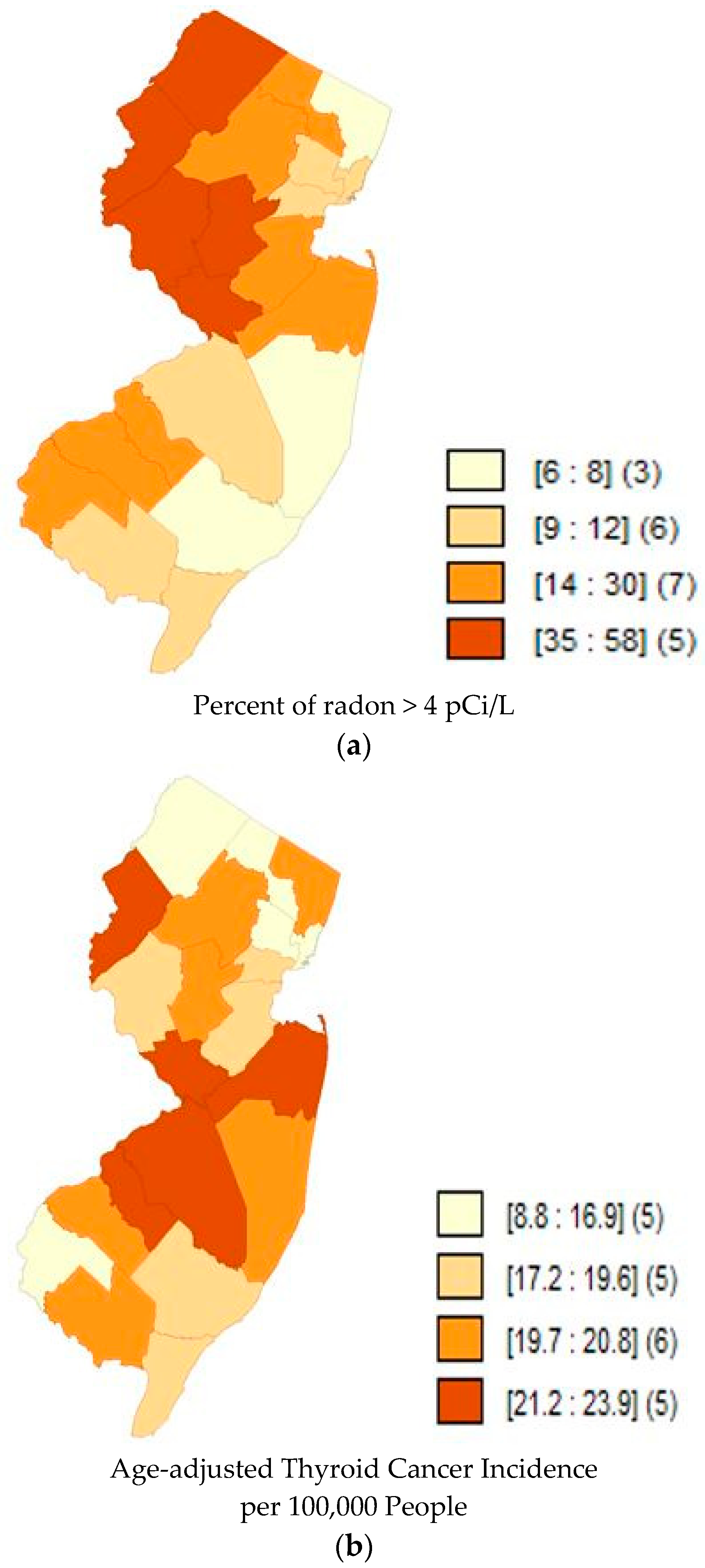
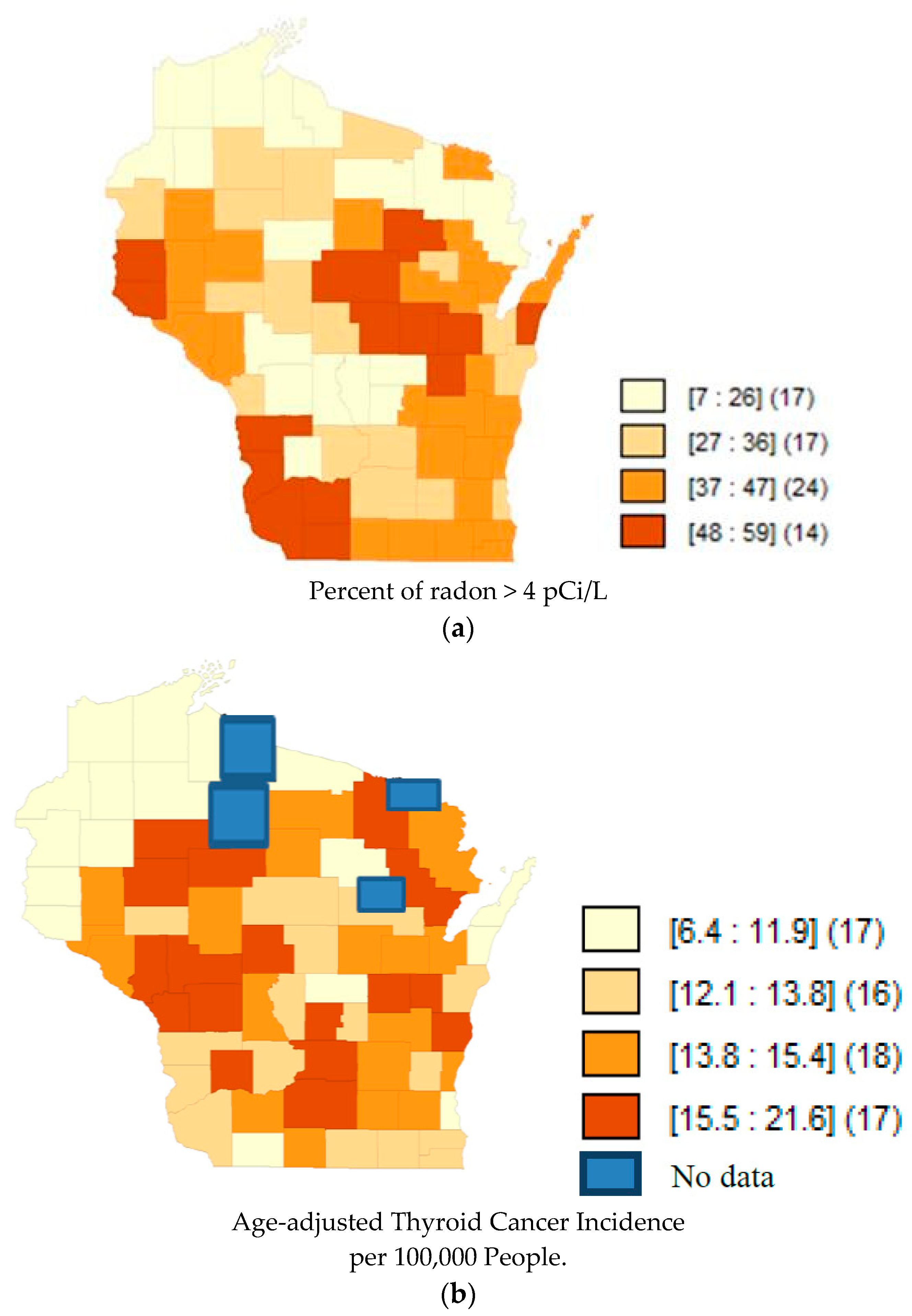
| States | Correlation Coefficient | p-Value |
|---|---|---|
| Iowa | −0.19 | 0.07 |
| New Jersey | 0.28 | 0.23 |
| Wisconsin | −0.03 | 0.78 |
| All 3 States | −0.12 | 0.12 |
| States | Beta Coefficient | p-Value |
|---|---|---|
| Iowa | −0.07 | 0.07 |
| New Jersey | 0.06 | 0.23 |
| Wisconsin | −0.01 | 0.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oakland, C.; Meliker, J.R. County-Level Radon and Incidence of Female Thyroid Cancer in Iowa, New Jersey, and Wisconsin, USA. Toxics 2018, 6, 17. https://doi.org/10.3390/toxics6010017
Oakland C, Meliker JR. County-Level Radon and Incidence of Female Thyroid Cancer in Iowa, New Jersey, and Wisconsin, USA. Toxics. 2018; 6(1):17. https://doi.org/10.3390/toxics6010017
Chicago/Turabian StyleOakland, Caroline, and Jaymie R. Meliker. 2018. "County-Level Radon and Incidence of Female Thyroid Cancer in Iowa, New Jersey, and Wisconsin, USA" Toxics 6, no. 1: 17. https://doi.org/10.3390/toxics6010017





