The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Apparatus
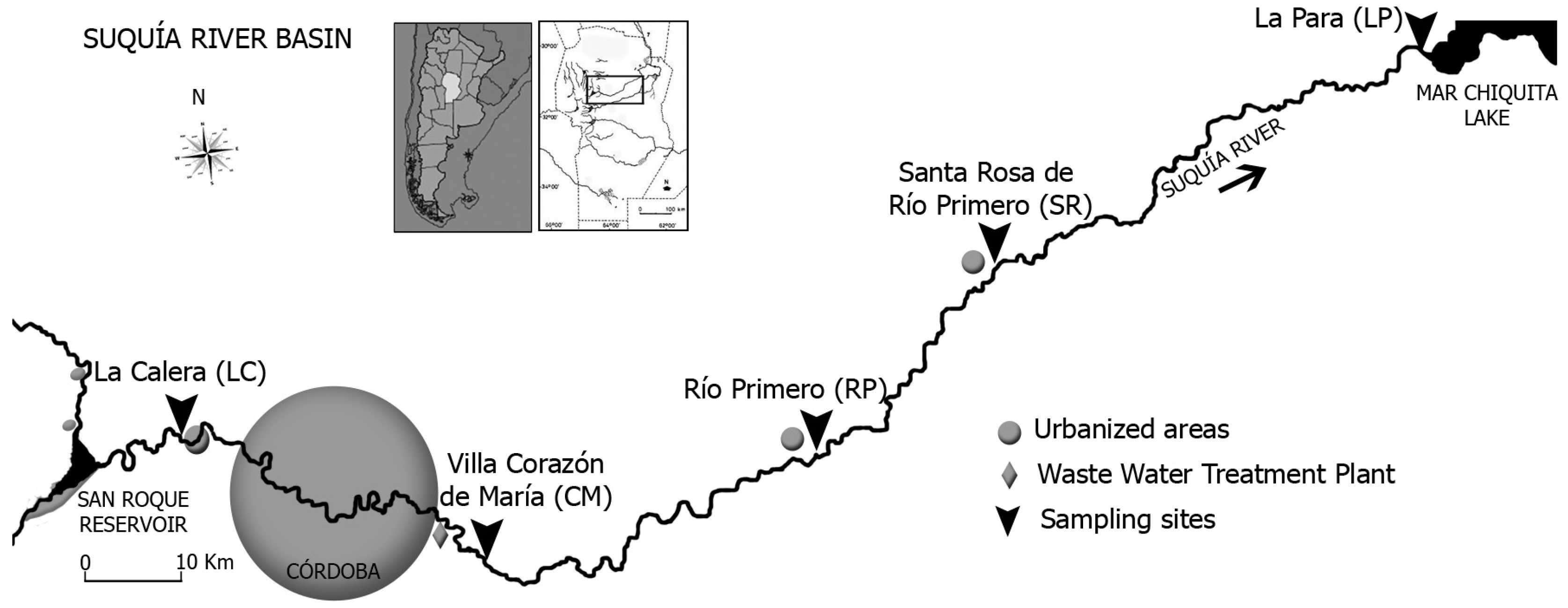
2.3. Study Area and Sampling
2.4. Physical and Chemical Parameters
2.5. Glyphosate and AMPA Analyses
2.5.1. Water Samples
2.5.2. Sediments and Suspended Particulate Matter Samples
2.5.3. Analytical Method
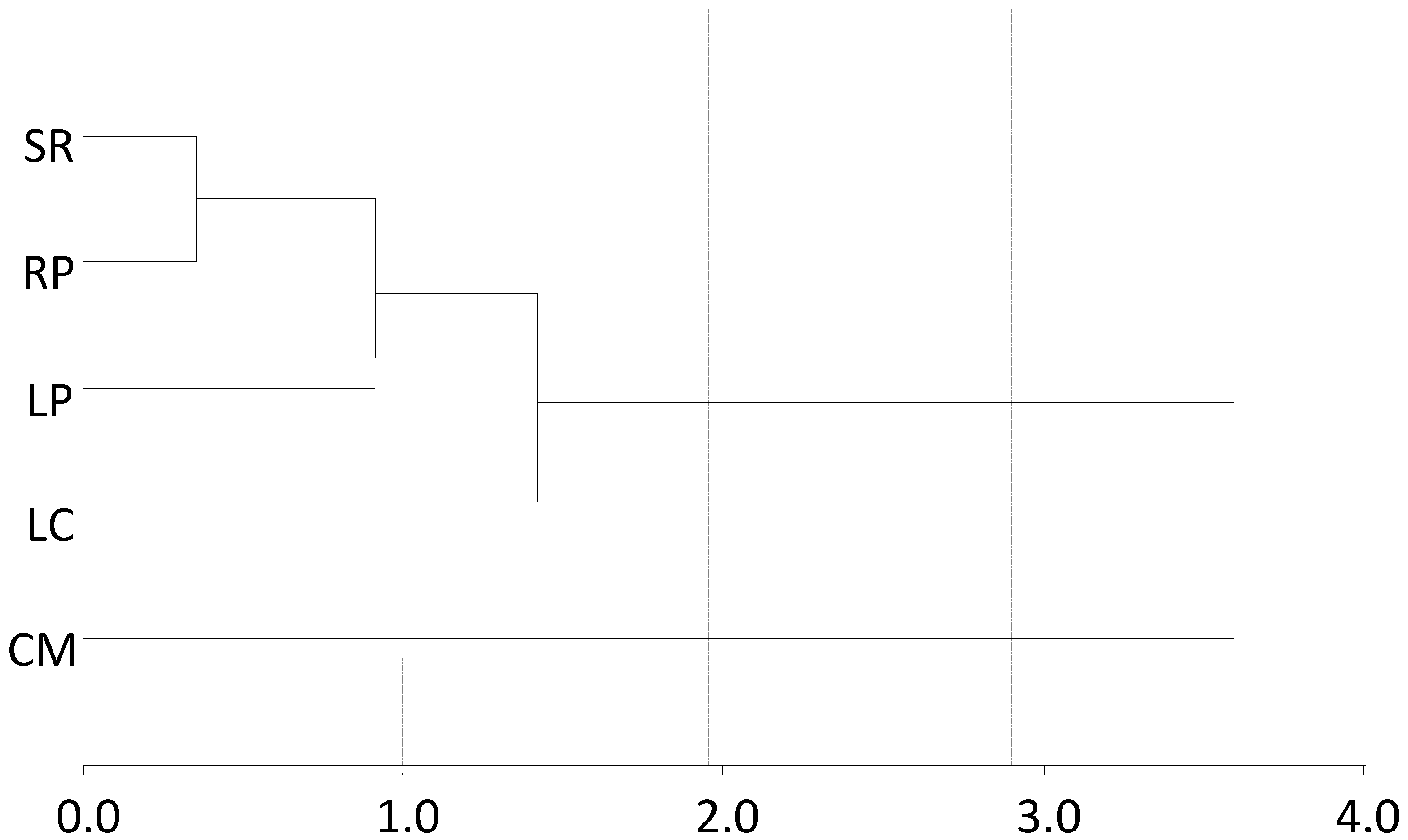
2.6. Statistics
2.7. Ecotoxicological Risk Assessment
3. Results and Discussion
3.1. Water and Sediments Physical and Chemical Characteristics
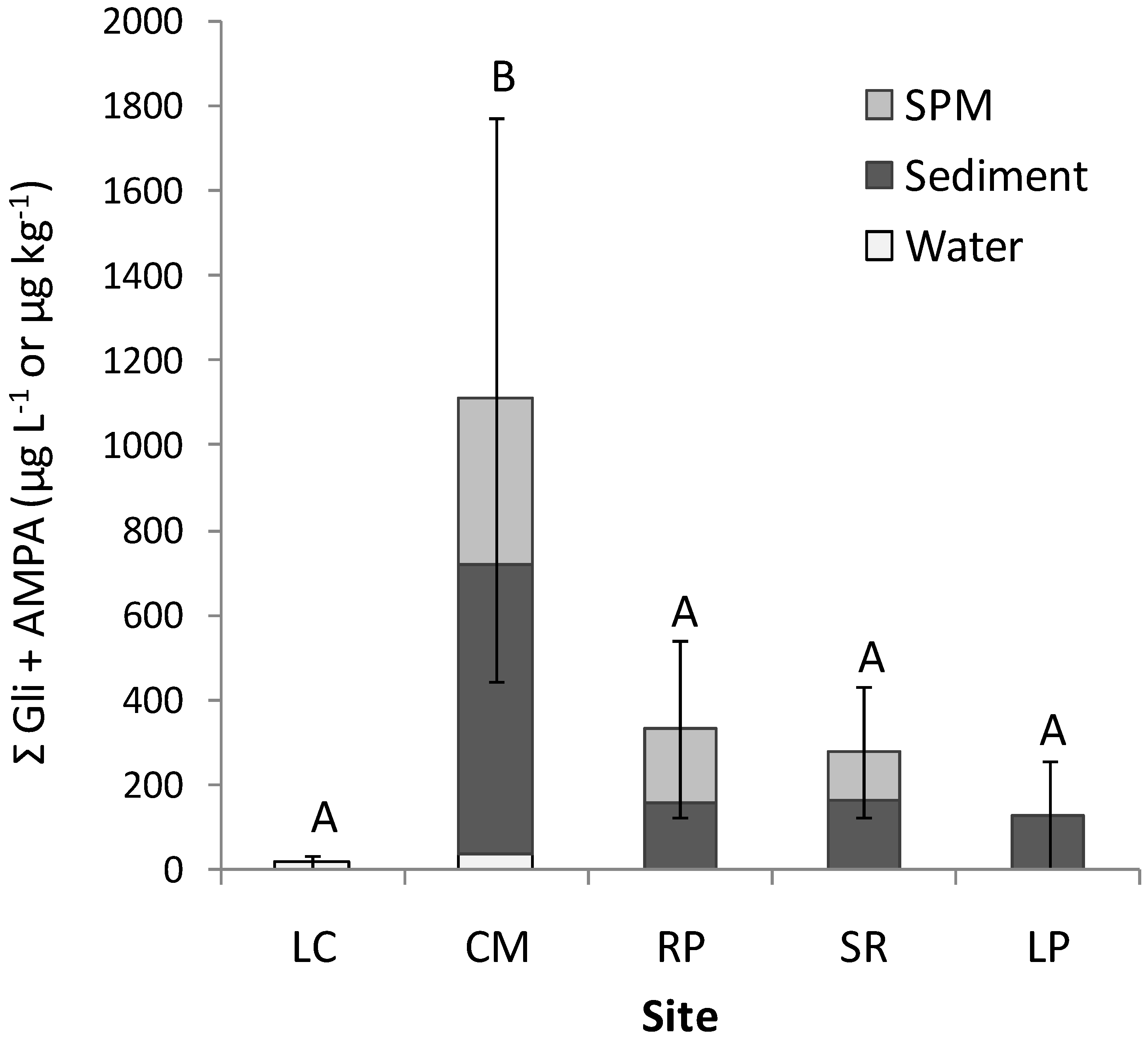
3.2. Occurrence of Glyphosate and AMPA
3.3. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lantieri, M.J.; Butinof, M.; Fernández, R.; Stimolo, M.I.; Blanco, M.; Díaz, M.P. Work Practices, Exposure Assessment and Geographical Analysis of Pesticide Applicators in Argentina. In Pesticides in the Modern World—Effects of Pesticides Exposure; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 115–138. ISBN 978-953-307-454-2. [Google Scholar]
- Lara Flores, S.M. Nuevas Experiencias Productivas y Nuevas Formas de Organización Flexible de Trabajo en la Agricultura Mexicana, 1st ed.; Pablos, J., Ed.; Ciudad de México: México, 1998; pp. 27–277. ISBN 968-6454-91-8. [Google Scholar]
- FAO (Organización de las Naciones Unidas para la Agricultura y la Alimentación). Buenas Prácticas Agrícolas para la Agricultura Familiar. Cadena de las Principales Hortalizas de Hojas en Argentina. Available online: http://www.fcagr.unr.edu.ar/PHR/9%20BPA%20para%20Hortalizas%20de%20Hoja%202010.pdf (accessed on 5 August 2015).
- Paulino, E.; De Almeida, R. Terra e Território a Questão Camponesa No Capitalismo; Expressão Popular: São Paulo, Brasil, 2010. [Google Scholar]
- World Health Organization (WHO). Organización Panamericana de la Salud (OPS). División Salud y Ambiente. Plaguicidas y Salud en las Américas; OMS/OPS: Washington, DC, USA, 1993. [Google Scholar]
- CASAFE (Cámara De Sanidad Agropecuaria Y Fertilizantes). Available online: http://www.casafe.org/biblioteca/estadisticas/ 2008 (accessed on 15 March 2013).
- Grunewald, K.; Wido Schmidt, C.U.; Gudrun, H. Behavior of Glyphosate and Aminomethylphosphonic Acid (Ampa) in Soils and Water of Reservoir Radeburg Ii Catchment (Saxony/Germany). J. Plant Nutr. Soil Sci. 2001, 164, 65–70. [Google Scholar] [CrossRef]
- Annett, R.; Hamid, R.H.; Hontela, A. Impact of Glyphosate and Glyphosate-Based Herbicides on the Freshwater Environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Gimsing, A.N. Fate of Glyphosate in Soil and the Possibility of Leaching to Ground and Surface Waters: A Review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Thompson, D.G. Ecological Risk Assessment for Aquatic Organisms from over-Water Uses of Glyphosate. J. Toxicol. Environ. Health B Crit. Rev. 2003, 6, 289–324. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other Herbicides, and Transformation Products in Midwestern Streams, 2002. J. Am. Water Resour. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and Its Degradation Product Ampa Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Castro, R.; Rivero-Pérez, N.; Bello-Mendoza, R.; Sánchez, D. Occurrence of Glyphosate in Water Bodies Derived from Intensive Agriculture in a Tropical Region of Southern Mexico. Bull. Environ. Contam. Toxicol. 2014, 93, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Boas, P. Accompanying Experiments on Weed Control on Public Footways Using the Roller Wiper ‘Rotofix’. Nachrichtenbl Deut Pflanzenschutzd 2006, 58, 46–49. [Google Scholar]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological Risk Assessment for Roundup Herbicide. Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer Science + Business Media: New York, NY, USA, 2000; Volume 167, pp. 35–120. ISSN 0179-5953. [Google Scholar]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). Reregistration Elegibility Decision (Red): Glyphosate. Office of Prevention, Pesticides and Toxic Substances. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/0178fact.pdf (accessed on 22 September 2015).
- WHO (World Health Organization). Iarc Monographs: Evaluation of Five Organophosphate Insecticides and Herbicides. Available online: www.who.int/en/ (accessed on 1 January 2016).
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental Fate of Glyphosate and Aminomethylphosphonic Acid in Surface Waters and Soil of Agricultural Basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of Glyphosate in Surface Waters, Sediments and Soils Associated with Direct Sowing Soybean Cultivation in North Pampasic Region of Argentina. Environ. Pollut. 2008, 156, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Primost, J.E.; Marino, D.; Aparicio, V.C.; Costa, J.L.; Carriquiriborde, P. Glyphosate and Ampa, "Pseudo-Persistent" Pollutants under Real-World Agricultural Management Practices in the Mesopotamic Pampas Agroecosystem, Argentina. Environ. Pollut. 2017, 229, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.E.; Marino, D.; Abelando, M.; Almada, P.; Apartin, C.D. Water Quality of the Main Tributaries of the Parana Basin: Glyphosate and Ampa in Surface Water and Bottom Sediments. Environ. Monit. Assess. 2016, 188, 458. [Google Scholar] [CrossRef] [PubMed]
- Peluso, F.; Dubny, S.; Othax, N.; Gonzalez-Castelain, J. Environmental Risk of Pesticides: Applying the Delazulpestrisk Model to Freshwaters of an Agricultural Area of Argentina. Hum. Ecol. Risk Assess. J. 2014, 20, 1177–1199. [Google Scholar] [CrossRef]
- Ramaswamy, B.R.; Shanmugam, G.; Velu, G.; Rengarajan, B.; Joakim Larsson, D.G. Gc–Ms Analysis and Ecotoxicological Risk Assessment of Triclosan, Carbamazepine and Parabens in Indian Rivers. J. Hazard. Mater. 2011, 186, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Loftin, K.A.; Lee, E.A.; Hinshaw, G.H.; Dietze, J.E.; Scribner, E.A. Determination of Glyphosate, its Degradation Product Aminomethylphosphonic Acid, and Glufosinate, in Water by Isotope Dilution and Online Solid-Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry. United States Geological Survey, 2009. Available online: http://pubs.er.usgs.gov/publication/tm5A10 (accessed on 1 January 2015).
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.H., Eds.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 635–662. ISBN 0-89118-088-5. [Google Scholar]
- Walkley, A.; Armstrong, I.B. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Ibáñez, M.; Pozo, O.J.; Sancho, J.V.; López, F.J.; Hernández, F. Re-Evaluation of Glyphosate Determination in Water by Liquid Chromatography Coupled to Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2006, 1134, 51–55. [Google Scholar] [CrossRef] [PubMed]
- SANTE/11813/2017. European Commission Directorate General for Health and Food Safety; Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; Reverté: Brussel, Belgium, 2017. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Introducción a La Bioestadística; Reverté: Barcelona, Spain, 1999; Volumen 5, ISBN 8429118624, 9788429118629. [Google Scholar]
- ETGDRA. Technical Guidance Document on Risk Assessment. Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf (accessed on 18 November 2015).
- Wunderlin, D.A.; Dı́az, M.P.; Amé, M.V.; Pesce, S.F.; Hued, A.C.; Bistoni, M.A. Pattern Recognition Techniques for the Evaluation of Spatial and Temporal Variations in Water Quality. A Case Study: Suquı́a River Basin (Córdoba–Argentina). Water Res. 2001, 35, 2881–2894. [Google Scholar] [CrossRef]
- Monferran, M.V.; Galanti, L.N.; Bonansea, R.I.; Ame, M.V.; Wunderlin, D.A. Integrated Survey of Water Pollution in the Suquia River Basin (Cordoba, Argentina). J. Environ. Monit. 2011, 13, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Bonansea, R.I.; Ame, M.V.; Wunderlin, D.A. Determination of Priority Pesticides in Water Samples Combining Spe and Spme Coupled to Gc-Ms. A Case Study: Suquia River Basin (Argentina). Chemosphere 2013, 90, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Amé, M.E.; Bistoni, M.A.; Wunderlin, D.A. Occurrence and Bioaccumulation of Pharmaceuticals in a Fish Species Inhabiting the Suquía River Basin (Córdoba, Argentina). Sci. Total Environ. 2014, 472, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Merlo, C.; Abril, A.; Amé, M.V.; Argüello, G.A.; Carreras, H.A.; Chiappero, M.S.; Hued, A.C.; Wannaz, E.; Galanti, L.N.; Monferrán, M.V.; et al. Integral Assessment of Pollution in the Suquía River (Córdoba, Argentina) as a Contribution to Lotic Ecosystem Restoration Programs. Sci. Total Environ. 2011, 409, 5034–5045. [Google Scholar] [CrossRef] [PubMed]
- Byer, J.D.; Struger, J.; Klawunn, P.; Todd, A.; Sverko, E. Low Cost Monitoring of Glyphosate in Surface Waters Using the Elisa Method: An Evaluation. Environ. Sci. Technol. 2008, 42, 6052–6057. [Google Scholar] [CrossRef] [PubMed]
- Wauchope, R.D.; Buttler, T.M.; Hornsby, A.G.; Augustijn-Beckers, P.W.M.; Burt, J.P. The Scs/Ars/Ces Pesticide Properties Database for Environmental Decision-Making. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1992; pp. 1–155. [Google Scholar] [CrossRef]
- Lupi, L.; Miglioranza, K.S.B.; Aparicio, V.C.; Marino, D.; Bedmar, F.; Wunderlin, D.A. Occurrence of Glyphosate and Ampa in an Agricultural Watershed from the Southeastern Region of Argentina. Environ. Sci. Technol. 2015, 536, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.K.; Wang, W.X.; Chu, L.M. Influence of Glyphosate and Its Formulation (Roundup®) on the Toxicity and Bioavailability of Metals to Ceriodaphnia Dubia. Environ. Pollut. 2005, 138, 59–68. [Google Scholar] [CrossRef] [PubMed]
- SRHN (Niveles Guía Nacionales De Calidad De Agua Ambiente). Available online: https://www.mininterior.gov.ar/obras-publicas/rh-calidad-niveles.php (accessed on 17 December 2017).
- Thurman, E.M.; Fallon, J.D. The Deethylatrazine/Atrazine Ratio as an Indicator of the Onset of the Spring Flush of Herbicides into Surface Water of the Midwestern United States. Int. J. Environ. Anal. Chem. 1996, 65, 203–214. [Google Scholar] [CrossRef]
- Pessagno, R.C.; Torres Sánchez, R.M.; Afonso, M.S. Glyphosate Behavior at Soil and Mineral–Water Interfaces. Environ. Pollut. 2008, 153, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Carballo, M.A.; Kleinsorge, E.C.; Simoniello, M.F. Occupational Exposure to Pesticides Mixtures: Oxidative Balance, Enzymatic Biomarkers and Genetic Damage in an Argentinian Population Study. In The Impact of Pesticides; Academy Publish: Cheyenne, WY, USA, 2012; pp. 78–104. [Google Scholar]
- Thompson, D.G.; Wojtaszek, B.F.; Staznik, B.; Chartrand, D.T.; Stephenson, G.R. Chemical and Biomonitoring to Assess Potential Acute Effects of Vision® Herbicide on Native Amphibian Larvae in Forest Wetlands. Environ. Toxicol. Chem. 2004, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
| Site | % of Urban Construction | % of Industries | % of Agriculture | % of Native Vegetation | % of River | Agricultural Model |
|---|---|---|---|---|---|---|
| LC | 42.2 | 6.7 | 0.0 | 46.0 | 4.8 | None |
| CM | 0.1 | 0.2 | 55.0 | 33.0 | 11.6 | Intensive |
| RP | 12.3 | 4.0 | 66.0 | 3.4 | 14.2 | Extensive |
| SR | 30.4 | 1.0 | 50.5 | 7.4 | 10.8 | Extensive |
| LP | 0.0 | 0.0 | 73.8 | 21.6 | 4.6 | Extensive |
| Site | Water | Sediments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DO (mg L−1) | CE (µS cm−1) | pH | Temp. (°C) | OM % | CE (µS cm−1) | pH | Texture | |||
| Sand % | Silt % | Clay % | ||||||||
| LC | C 10.1 ± 2.5 | A 268 ± 28 | B 8.1 ± 0.2 | 17.2 ± 7.0 | B 8.4 ± 4.7 | 988.0 ± 754.0 | A 6.6 ± 0.2 | 41.3 ± 23.3 | 53.3 ± 23.0 | 5.5 ± 3.4 |
| CM | A 3.2 ± 1.7 | C 1388 ± 156 | A 7.5 ± 0.2 | 20.7 ± 8.3 | AB 5.0 ± 4.0 | 578.5 ± 226.9 | AB 6.9 ± 0.3 | 54.2 ± 22.4 | 40.0 ± 25.2 | 5.3 ± 4.2 |
| RP | B 6.9 ± 1.3 | B 1106 ± 248 | AB 7.7 ± 0.2 | 20.5 ± 9.2 | AB 5.6 ± 2.1 | 740.3 ± 225.5 | B 6.9 ± 0.3 | 36.0 ± 21.5 | 63.0 ± 21.8 | 2.5 ± 0.5 |
| SR | BC 8.8 ± 2.0 | B 992 ± 302 | AB 8.0 ± 0.4 | 20.4 ± 7.7 | A 2.4 ± 2.0 | 922.5 ± 657.4 | BC 7.0 ± 0.5 | 56.5 ± 6.9 | 39.0 ± 4.6 | 4.5 ± 5.7 |
| LP | BC 8.7 ± 1.6 | B 1253 ± 69 | C 8.5 ± 0.2 | 20.1 ± 6.9 | A 3.5 ± 2.7 | 825.5 ± 535.9 | C 7.5 ± 0.5 | 17.0 ± 7.1 | 74.8 ± 5.2 | 8.3 ± 5.3 |
| Site | Glyphosate | AMPA | |||||
|---|---|---|---|---|---|---|---|
| Water | Sediments | SPM | Water | Sediments | SPM | ||
| (µg L−1) | (µg kg−1) | (µg kg−1) | (µg L−1) | (µg kg−1) | (µg kg−1) | ||
| LC | Mean | 17.5 | A <LOD | <LOD | <LOD | A <LOD | <LOD |
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Max | 70.0 | <LOD | <LOD | <LOD | <LOD | <LOD | |
| CM | Mean | 35.2 | B 615.4 | 392.7 | 0.6 | AB 66.5 | <LOD |
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Max | 125.0 | 1882.3 | 1570.7 | 2.2 | 266.1 | <LOD | |
| RP | Mean | <LOD | AB 61.9 | <LOD | 2.1 | C 97.0 | 171.2 |
| Min | <LOD | <LOD | <LOD | <LOD | 38.5 | <LOD | |
| Max | <LOD | 168.7 | <LOD | 4.8 | 222.2 | 684.9 | |
| SR | Mean | <LOD | B 89.5 | <LOD | <LOD | BC 73.0 | 118.4 |
| Min | <LOD | 23.1 | <LOD | <LOD | 23.9 | <LOD | |
| Max | <LOD | 139.0 | <LOD | <LOD | 196.4 | 473.5 | |
| LP | Mean | <LOD | AB 105.1 | <LOD | <LOD | AB 22.6 | <LOD |
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Max | <LOD | 381.9 | <LOD | <LOD | 90.2 | <LOD | |
| Site | Glyphosate | AMPA | ||
|---|---|---|---|---|
| Aquatic Organisms | Benthic Organisms | Aquatic Organisms | ||
| PNEC = 23 µg a.e. L−1 | PNEC = 280 µg a.e. kg−1 | PNEC = 790 µg a.e. L−1 | ||
| LC | MEC | 70 µg a.e. L−1 | <LOD | <LOD |
| HQ | 3.0 | N/A | N/A | |
| CM | MEC | 125 µg a.e. L−1 | 1882.3 µg kg−1 | 2.2 µg a.e. L−1 |
| HQ | 5.4 | 6.7 | 3.0E-03 | |
| RP | MEC | <LOD | 168.7 µg kg−1 | 222.2 µg a.e. L−1 |
| HQ | N/A | 0.6 | 0.3 | |
| SR | MEC | <LOD | 139.0 µg kg−1 | <LOD |
| HQ | N/A | 0.5 | N/A | |
| LP | MEC | <LOD | 381.9 µg kg−1 | <LOD |
| HQ | N/A | 1.4 | N/A | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.J.G.; Amé, M.V. The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment. Toxics 2018, 6, 3. https://doi.org/10.3390/toxics6010003
Bonansea RI, Filippi I, Wunderlin DA, Marino DJG, Amé MV. The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment. Toxics. 2018; 6(1):3. https://doi.org/10.3390/toxics6010003
Chicago/Turabian StyleBonansea, Rocío Inés, Iohanna Filippi, Daniel Alberto Wunderlin, Damián José Gabriel Marino, and María Valeria Amé. 2018. "The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment" Toxics 6, no. 1: 3. https://doi.org/10.3390/toxics6010003





