A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Swine Farms’ Characteristics and Collection of Environmental Samples
2.2. Particulate Matter Assessment
2.3. Bioburden Sampling and Analysis by Culture-Based Methods
2.4. Fungal Sampling and Molecular Detection by Real-Time PCR
2.5. Statistical Analysis
3. Results
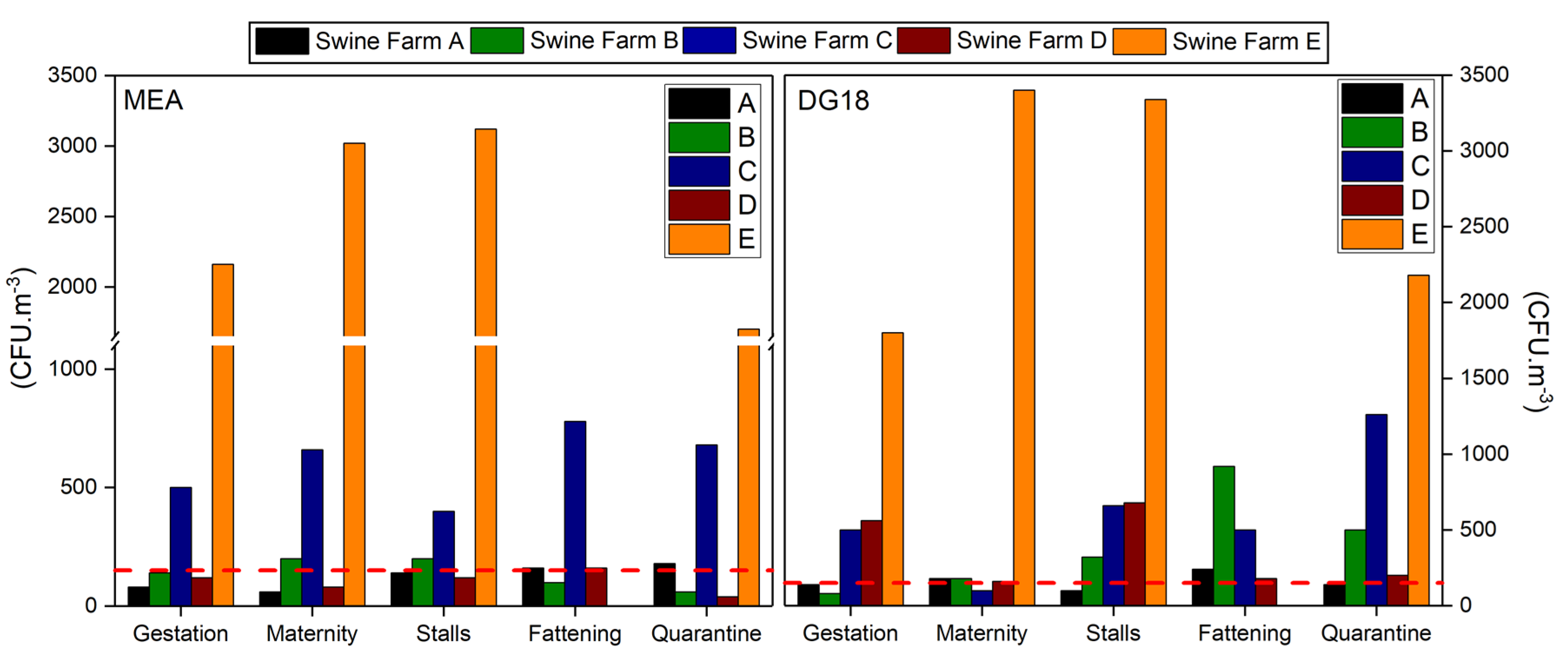
3.1. Particulate Matter
3.2. Bioburden: Bacterial Contamination
3.3. Bioburden: Fungal Contamination
3.4. Correlation and Comparison Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Donham, K.; Haglind, P.; Peterson, Y.; Rylander, R.; Belin, L. Environmental and health studies of farm workers in Swedish swine confinement buildings. Br. J. Ind. Med. 1989, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Purdy, B.M.; Langemeier, M.R.; Featherstone, A.M. Financial performance, risk, and specialization. J. Agric. Appl. Econ. 1997, 29, 149–161. [Google Scholar] [CrossRef]
- Attwood, P.; Ruigewaard, R.; Versloot, P.; Dewit, R.; Heederik, D.; Boleij, J. A study of the relationship between airborne contaminants and environment factors in Dutch swine confinement buildings. Am. Ind. Hyg. Assoc. J. 1987, 48, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.; Martins, H.M.; Santos, S.; Costa, J.M.; Bernardo, F. Co-occurrence of mycotoxins in swine feed produced in Portugal. Mycotox. Res. 2011, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Carolino, E.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal Contamination in Swine: A Potential Occupational Health Threat. J. Toxicol. Environ. Health A 2013, 76, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, J.; Hartung, J.; Schröder, M.; Linkert, K.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Pedersen, P.; et al. Concentrations and Emissions of Airborne Endotoxins and Microorganisms in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 97–109. [Google Scholar] [CrossRef]
- Adhikari, A.; Reponen, T.; Lee, S.A.; Grinshpun, S.A. Assessment of human exposure to airborne fungi in agricultural confinements: Personal inhalable sampling versus stationary sampling. Ann. Agric. Environ. Med. 2004, 11, 269–277. [Google Scholar] [PubMed]
- Viegas, S.; Veiga, L.; Verissimo, C.; Sabino, R.; Figueiredo, P.; Almeida, A.; Carolino, E.; Viegas, C. Occupational Exposure to Aflatoxin B1 in Swine Production and Possible Contamination Sources. J. Toxicol. Environ. Health A 2013, 76, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Mateus, V.; Almeida-Silva, M.; Carolino, E.; Viegas, C. Occupational Exposure to Particulate Matter and Respiratory Symptoms in Portuguese Swine Barn Workers. J. Toxicol. Environ. Health A 2013, 76, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ko, H.; Kim, Y.; Kim, C. Assessment of Korean farmer’s exposure level to dust in pig buildings. Ann. Agric. Environ. Med. 2008, 15, 51–58. [Google Scholar] [PubMed]
- Pearson, C.; Sharples, T. Airborne dust concentrations in livestock buildings and the effect of feed. J. Agric. Eng. Res. 1995, 60, 145–154. [Google Scholar] [CrossRef]
- Larsson, K.; Eklund, A.; Malmberg, P.; Belin, L. Alterations in bronchoalveolar lavage fluid but not in lung function and bronchial responsiveness in swine confinement workers. Chest 1992, 101, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zejda, J.E.; Hurst, T.S.; Rhodes, C.S.; Barber, E.; McDuffie, H.H.; Dosman, J.A. Respiratory health of swine producers: Focus on young workers. Chest 1993, 103, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Choudat, D.; Goehen, M.; Korobaeff, M.; Boulet, A.; Dewitte, J.; Martin, M. Respiratory symptoms and bronchial reactivity among pig and dairy farmers. Scand. J. Work Environ. Health 1994, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, P.F.; Van der Gulden, J.W.; Tielen, M.J.; Folgering, H.; Van Schayck, C.P. Health-based selection for asthma, but not for chronic bronchitis, in pig farmers: An evidence-based hypothesis. Eur. Respir. J. 1999, 13, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Monso, E.; Riu, E.; Radon, K.; Magarolas, R.; Danuser, B.; Iversen, M. Chronic obstructive pulmonary disease in never-smoking animal farmers working inside confinement buildings. Am. J. Ind. Med. 2004, 46, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Holness, D.L.; O’Blenis, E.L.; Sass-Kortsak, A.; Pilger, C.; Nethercott, J.R. Respiratory effects and dust exposures in hog confinement farming. Am. J. Ind. Med. 1987, 11, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Holness, D.L.; Nethercott, J.R. Respiratory status and environmental exposure of hog confinement and control farmers in Ontario. In Principles of Health and Safety in Agriculture; Dosman, J.A., Cockroft, D.W., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 69–71. [Google Scholar]
- Vogelzang, P.; Van der Gulden, J.; Preller, L.; Tielen, M.; Van Schayck, C.; Folgering, H. Bronchial hyperresponsiveness and exposure in pig farmers. Int. Arch. Occup. Environ. Health 1997, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Millner, P.D. Bioaerosols associated with animal production operations. Bioresour. Technol. 2009, 100, 5379–5385. [Google Scholar] [CrossRef] [PubMed]
- Tsapko, V.; Chudnovets, A.; Sterenbogen, M.; Papach, V.; Dutkiewicz, J.; Skórska, C.; Krysinska-Traczyk, E.; Golec, M. Exposure to bioaerosols in the selected agricultural facilities of the Ukraine and Poland—A review. Ann. Agric. Environ. Med. 2011, 18, 19–27. [Google Scholar] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gebé, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; Centraalbureau Voor Schimmelcultures: Utrecht, The Netherlands, 2000. [Google Scholar]
- Cruz-Perez, P.; Buttner, M.P.; Stetzenbach, L.D. Detection and quantitation of Aspergillus fumigatus in pure culture using polymerase chain reaction. Mol. Cell. Probes 2001, 15, 81–88. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). About the National Exposure Research Laboratory (NERL). 2017. Available online: http://www.epa.gov/nerlcwww/moldtech.htm (accessed on 19 June 2017).
- Goyer, N.; Lavoie, J.; Lazure, L.; Marchand, G. Bioaerosols in the Workplace: Evaluations, Control and Prevention Guide; Institut de Recherche Robert-Sauvé en Santé et en Sécurité du Travail: Montréal, QC, Canada, 2001. [Google Scholar]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed]
- Cole, D.; Todd, L.; Wing, S. Concentrated Swine Feeding Operations and Public Health: A Review of Occupational and Community health effects. Environ. Health Perspect. 2000, 108, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, J.A.; Heber, A.J.; Murphy, J.P.; Henry, S.C.; May, M.M.; Nolan, D.; Gearhart, S.K.; Cederber, B.L.; Oehme, F.W.; Schonewels, D. Total and respirable dust in swine confinement buildings: The benefit of respiratory protective masks and effect of recirculated air. Vet. Hum. Toxicol. 1995, 37, 430–435. [Google Scholar] [PubMed]
- Lauriere, M.; Gorner, P.; Bouchezmahiout, I.; Wrobel, R.; Breton, C.; Fabrie, J.F.; Choudat, D. Physical and biochemical properties of airborne flour particles involvedin occupational asthma. Ann. Occup. Hyg. 2008, 52, 727–737. [Google Scholar] [PubMed]
- Viegas, S.; Aranha, C.L.; Korkalainen, M.; Faria, T.; Pacífico, C.; Carolino, E.; Gomes, A.Q.; Viegas, C. Cytotoxic and inflammatory potential of air samples from occupational settings with exposure to organic dust. Toxics 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Liebner, L.; Kuhl, K.; Kauppinen, T.; Uuksulainen, S. European Agency for Safety and Health at Work. Exposure to Carcinogens and Work-Related Cancer: A Review of Assessment Methods; European Risk Observatory Report; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Viegas, S. Pilot study regarding vehicles cabinets and elevator: Neglected workstations in occupational exposure assessment? In Occupational Safety and Hygiene IV; Costa, N., Barroso, M.P., Carneiro, P., Baptista, J.S., Melo, R.B., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: London, UK, 2017; pp. 283–287. ISBN 978-1-138-05761-6. [Google Scholar]
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. prevalence in different occupational settings. J. Occup. Environ. Hyg. 2017, 4, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Carolino, E.; Sabino, R.; Quintal Gomes, A.; Viegas, S. Occupational Exposure to Fungi and Particles in Animal Feed Industry. Med. Pracy 2016, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Meneses, M.; Carolino, E.; Viegas, S.; Gomes, A.; Sabino, R. Analysis of surfaces for characterization of fungal burden—Does it matter? Int. J. Occup. Med. Environ. Health 2016, 29, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Pinheiro, C.; Sabino, R.; Viegas, S.; Brandão, J.; Veríssimo, C. (Eds.) Environmental Mycology in Public Health: Fungi and Mycotoxins Risk Assessment and Management; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Viegas, S.; Almeida-Silva, M.; Faria, T.; Dos Santos, M.; Viegas, C. Occupational exposure assessment to particles with task-based approach. In Occupational Safety and Hygiene IV; Costa, N., Barroso, M.P., Carneiro, P., Baptista, J.S., Melo, R.B., Eds.; Taylor and Francis Group: London, UK, 2016; pp. 1–6. [Google Scholar]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Duchaine, C.; Mériaux, A. The importance of combining air sampling and surface analysis when studying problematic houses for mold biodiversity determination. Aerobiologia 2001, 17, 121–125. [Google Scholar] [CrossRef]
- Bex, V.; Mouilleseaux, A.; Causse, R. A survey of Aspergillus contamination in a hospital during renovation. Healthy Build. 2000, 1, 359–364. [Google Scholar]
- Rodrigues, A.G.; Araújo, R. Comparison of Andersen and Honey Jar methods for monitoring hospital wards. Indoor Built Environ. 2007, 16, 71–78. [Google Scholar] [CrossRef]
- Health and Safety Executive (HSE). Statement of Evidence: Respiratory Hazards of Poultry Dust Health and Safety; Executive 03/09; Health and Safety Executive: Liverpool, UK, 2009; 14p. [Google Scholar]
- Mc Donnell, P.; Coggins, M.; Hogan, V.; Fleming, G. Exposure assessment of airborne contaminants in the indoor environment of irish swine farms. Ann. Agric. Environ. Med. 2008, 15, 323–326. [Google Scholar] [PubMed]
- Kuo, N.W.; Chiang, H.C.; Chiang, C.M. Development and application of an integrated indoor air quality audit to an international hotel building in Taiwan. Environ. Monit. Assess. 2008, 147, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.; Simmons, O.D.; Likirdopulos, C.A.; Worley-Davis, L.; Williams, C.M.; Sobsey, M.D. Endotoxin Levels at Swine Farms Using Different Waste Treatment and Management Technologies. Environ. Sci. Technol. 2010, 44, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Influence of Extreme Seasons on Airborne Pollutant Levels in a Pig-Confinement Building. Arch. Environ. Occup. Health 2007, 62, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Kang, J. Exposure levels of airborne bacteria and fungi in Korean swine and poultry sheds. Arch. Environ. Occup. Health 2005, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Quintal Gomes, A.; Faria, T.; Sabino, R. Prevalence of Aspergillus fumigatus complex in waste sorting and incineration plants: An occupational threat. Int. J. Environ. Waste Manag. 2016, 16, 353–369. [Google Scholar] [CrossRef]
- D’Ovidio, D.; Grable, S.L.; Ferrara, M.; Santoro, D. Prevalence of dermatophytes and other superficial fungal organisms in asymptomatic guinea pigs in Southern Italy. J. Small Anim. Pract. 2014, 55, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.; Faísca, V.M.; Carolino, E.; Veríssimo, C.; Viegas, C. Occupational Exposure to Aspergillus by Swine and Poultry Farm Workers in Portugal. J. Toxicol. Environ. Health A 2012, 75, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, T.; Keller, N. Pathogenesis of Aspergillus fumigatusin Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 447–465. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Loeffler, L.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogenic. Cell. Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Pacífico, C.; dos Santos, M.; Monteiro, A.; Lança, C.; Carolino, E.; Viegas, S.; Cabo Verde, S. Microbiota and particulate matter assessment in Portuguese optical shops providing contact lenses services. Healthcare 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gomes, A.Q.; Abegão, J.; Sabino, R.; Graça, T.; Viega, S. Assessment of fungal contamination in waste sorting and incineration—Case study in Portugal. J. Toxicol. Environ. Heal. Part A 2014, 77, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First Metagenomic Survey of the Microbial Diversity in Bioaerosols Emitted in Waste Sorting Plants. Ann. Work Expo. Health 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Rothman, R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 337–348. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Lees, P.S.J.; Merz, W.G.; Schwab, K.J. Inhibition of quantitative PCR analysis of fungal conidia associated with indoor air particulate matter. Aerobiologia 2007, 23, 35–45. [Google Scholar] [CrossRef]
- Hung, L.L.; Miller, J.D.; Dillon, K.H. (Eds.). Field Guide for the Determination of Biological Contaminants in Environmental Samples, 2nd ed.; AIHA: Fairfax, VA, USA, 2005. [Google Scholar]

| Swine Farms | No. of Air Samples Impaction * | No. of Air Samples Impinger | No. of Surfaces Samples (Walls) | No. of Feed Samples | No. of Floor Cover Samples | Animal Quantity |
|---|---|---|---|---|---|---|
| A | 20 | 5 | 5 | 2 | 1 | 1768 |
| B | 20 # | 5 | 5 | 2 | 1 | 8000 |
| C | 20 | 4 # | 5 | 2 | 1 | 3300 |
| D | 20 | 5 | 5 | 2 | 1 | 6000 |
| E | 16 + | 4 | 4 | 2 | 1 | 7000 |
| Aspergillus Sections Targeted | Sequences | Reference |
|---|---|---|
| Fumigati | ||
| Forward Primer | 5′-CGCGTCCGGTCCTCG-3′ | |
| Reverse Primer | 5′-TTAGAAAAATAAAGTTGGGTGTCGG-3′ | Cruz-Perez et al. 2001 [23] |
| Probe | 5′-TGTCACCTGCTCTGTAGGCCCG-3′ | |
| Versicolores | ||
| Forward Primer | 5′-CGGCGGGGAGCCCT-3′ | |
| Reverse Primer | 5′-CCATTGTTGAAAGTTTTGACTGATcTTA-3′ | |
| Probe | 5′-AGACTGCATCACTCTCAGGCATGAAGTTCAG-3′ | EPA 2017 [24] |
| MEA | DG18 | ||
|---|---|---|---|
| Air | (CFU·m−3) (%; n) | Air | (CFU·m−3) (%; n) |
| Cladosporium sp. | 59.4; 12,100 | Cladosporium sp. | 66.5; 14,120 |
| Fusarium graminearum | 13.2; 2700 | Ulocladium sp. | 14.6; 3100 |
| Alternaria sp. | 5.7; 1160 | Chrysonilia sitophila | 4.7; 1000 |
| Others | 21.7; 4420 | Others | 14.2; 3020 |
| Surfaces | (CFU·m−2) (%; n) | Surfaces | (CFU·m−2) (%; n) |
| Cladosporium sp. | 53.8; 210,000 | Scopulariopsis candida | 50.3; 580,000 |
| Scopulariopsis brevicaulis | 33.3; 130,000 | Aspergillus section Circumdati | 19.9; 230,000 |
| Penicillium sp. | 12.8; 50,000 | Cladosporium sp. | 13; 150,000 |
| Others | 0.1; 500 | Others | 16.7; 193,000 |
| Feed | (CFU·g−1) (%; n) | Feed | (CFU·g−1) (%; n) |
| Cladosporium sp. | 71.4; 10 | Cladosporium sp. | 82.2; 37 |
| Penicillium sp. | 21.4; 3 | Penicillium sp. | 8.9; 4 |
| Fusarium culmorum | 7.1; 1 | Fusarium culmorum | 8.9; 4 |
| Floor covering | (CFU·g−1) (%; n) | Floor covering | (CFU·g−1) (%; n) |
| Penicillium sp. | 50; 4 | - | - |
| Alternaria sp. | 37.5; 3 | - | - |
| Cladosporium sp. | 12.5; 1 | - | - |
| MEA | DG18 | ||
|---|---|---|---|
| Air | (CFU·m−3) (%; n) | Air | (CFU·m−3) (%; n) |
| Circumdati | 55; 220 | Versicolores | 50; 240 |
| Aspergilli | 25; 100 | Usti | 20.8; 100 |
| Nigri | 10; 40 | Aspergilli | 12.5; 60 |
| Versicolores | 5; 20 | Candidi | 12.5; 60 |
| Flavi | 5; 20 | Nidulantes | 4.2; 20 |
| Bacteria/Fungus | Swine Farming | n | Ranks | Test Statistics a | Kruskal–Wallis Multiple Comparisons | ||
|---|---|---|---|---|---|---|---|
| Mean Rank | Chi-Square | df | p | ||||
| Total Bacteria Surface (CFU·m−2) | A | 5 | 12.00 | 1.936 | 4 | 0.748 | |
| B | 5 | 10.00 | |||||
| C | 5 | 14.10 | |||||
| D | 4 | 10.75 | |||||
| E | 5 | 15.30 | |||||
| Gram Negative Bacteria-Surface (CFU·m−2) | A | 5 | 12.40 | 0.081 | 4 | 0.999 | |
| B | 5 | 12.60 | |||||
| C | 5 | 12.00 | |||||
| D | 4 | 12.75 | |||||
| E | 5 | 12.80 | |||||
| Fungi (MEA)-Surface (CFU·m−2) | A | 5 | 17.90 | 13.699 | 4 | 0.008 * | C ≠ D (p = 0.036) |
| B | 5 | 12.50 | |||||
| C | 5 | 6.00 | |||||
| D | 4 | 19.50 | |||||
| E | 5 | 8.00 | |||||
| Fungi (DG18)-Surface (CFU·m−2) | A | 5 | 18.60 | 8.430 | 4 | 0.077 | |
| B | 5 | 13.60 | |||||
| C | 5 | 10.30 | |||||
| D | 4 | 8.50 | |||||
| E | 5 | 10.70 | |||||
| Total bacteria-Air (CFU·m−3) | A | 5 | 12.10 | 3.676 | 4 | 0.452 | |
| B | 5 | 14.10 | |||||
| C | 5 | 10.40 | |||||
| D | 4 | 17.50 | |||||
| E | 5 | 9.40 | |||||
| Gram Negative Bacteria-Air (CFU·m−3) | A | 5 | 10.20 | 7.132 | 4 | 0.129 | |
| B | 5 | 15.00 | |||||
| C | 5 | 15.00 | |||||
| D | 4 | 16.50 | |||||
| E | 5 | 6.60 | |||||
| Fungi (MEA)-Air (CFU·m−3) | A | 5 | 6.40 | 17.602 | 4 | 0.001 * | A ≠ D (p = 0.007) |
| B | 5 | 8.20 | B ≠ D (p = 0.025) | ||||
| C | 5 | 9.40 | |||||
| D | 4 | 22.50 | |||||
| E | 5 | 18.00 | |||||
| Fungi (DG18)-Air (CFU·m−3) | A | 5 | 11.60 | 12.621 | 4 | 0.013 * | B ≠ D (p = 0.005) |
| B | 5 | 6.10 | |||||
| C | 5 | 10.80 | |||||
| D | 4 | 22.50 | |||||
| E | 5 | 13.50 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Faria, T.; Monteiro, A.; Caetano, L.A.; Carolino, E.; Quintal Gomes, A.; Viegas, S. A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study. Toxics 2018, 6, 5. https://doi.org/10.3390/toxics6010005
Viegas C, Faria T, Monteiro A, Caetano LA, Carolino E, Quintal Gomes A, Viegas S. A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study. Toxics. 2018; 6(1):5. https://doi.org/10.3390/toxics6010005
Chicago/Turabian StyleViegas, Carla, Tiago Faria, Ana Monteiro, Liliana Aranha Caetano, Elisabete Carolino, Anita Quintal Gomes, and Susana Viegas. 2018. "A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study" Toxics 6, no. 1: 5. https://doi.org/10.3390/toxics6010005






