Effects of Cadmium Exposure on Age of Menarche and Menopause
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Statistical Analysis
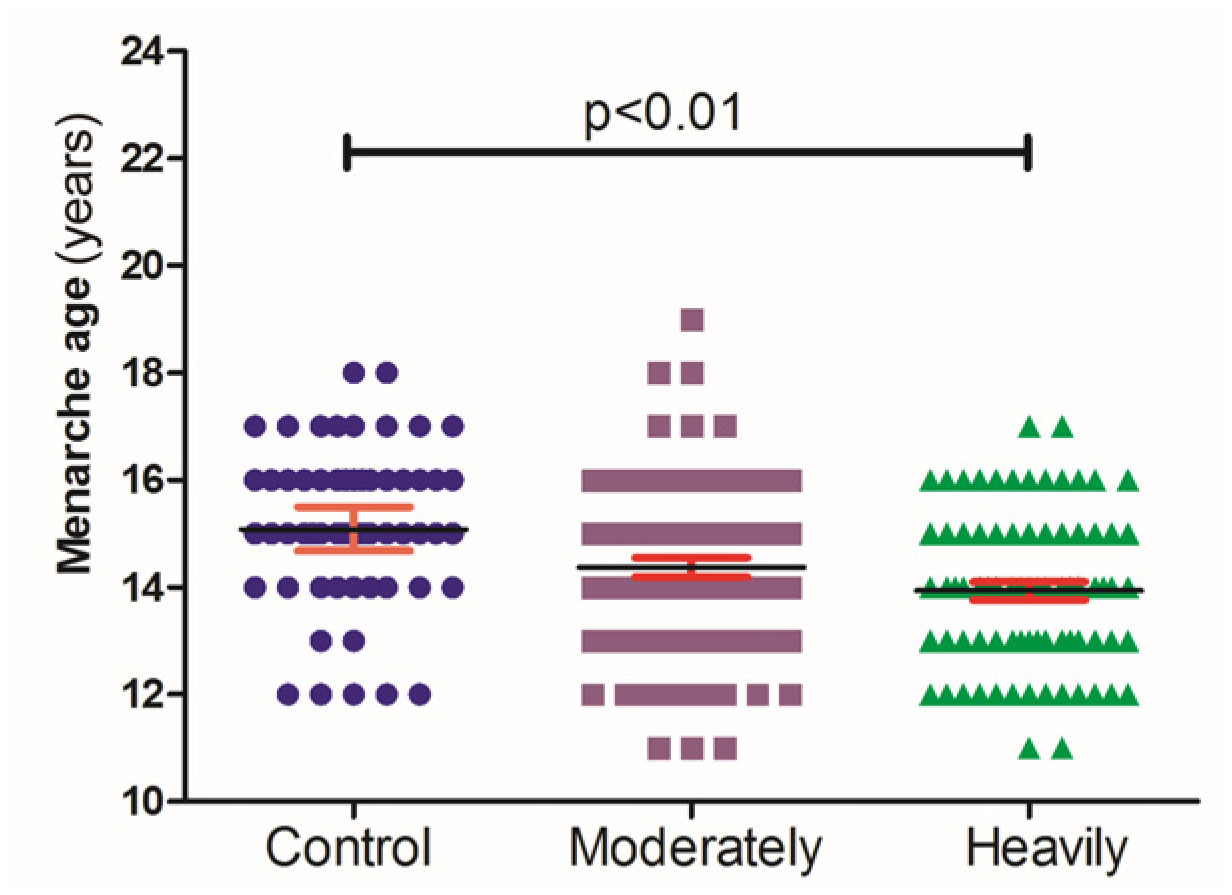
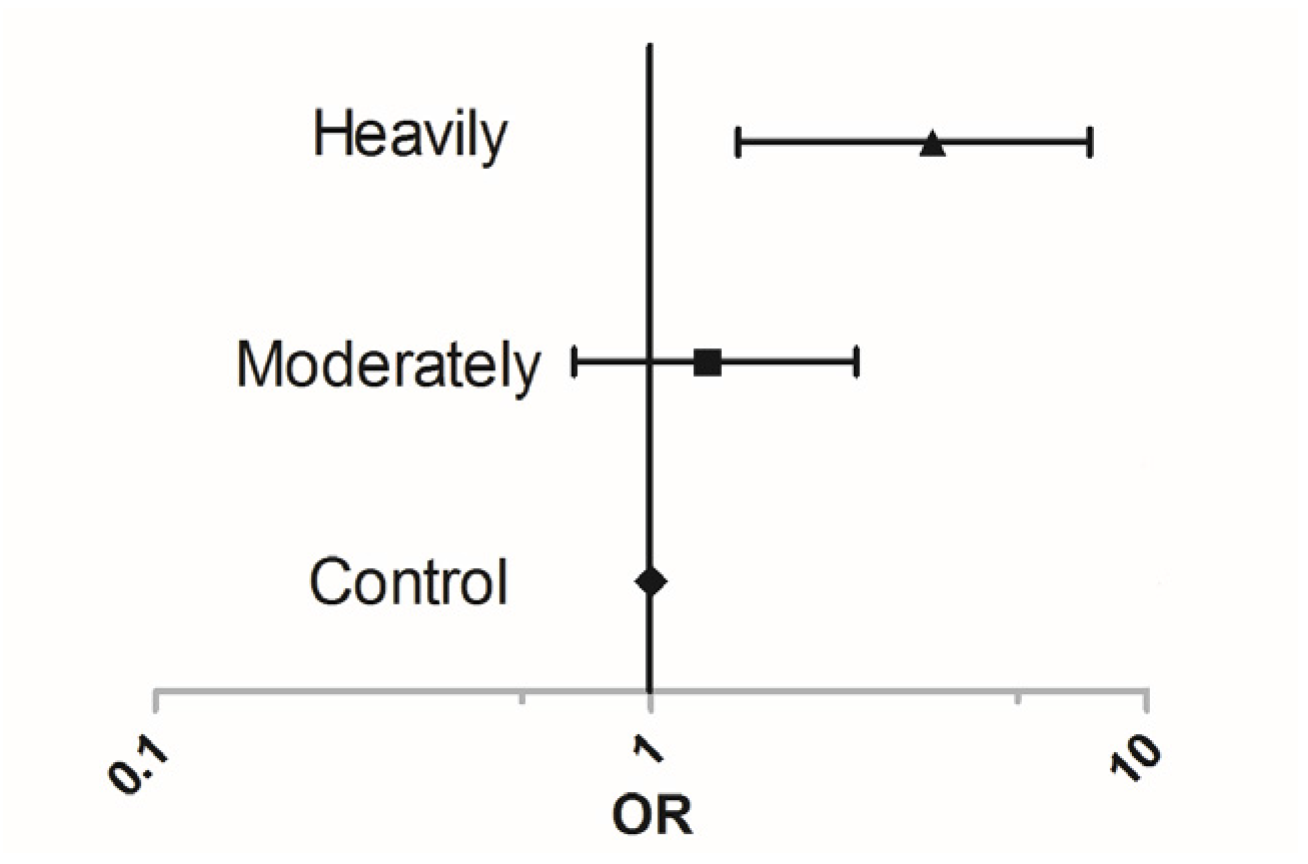
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, M.D.; Kenney, N.; Stoica, A.; Hilakivi-Clarke, L.; Singh, B.; Chepko, G.; Clarke, R.; Sholler, P.F.; Lirio, A.A.; Foss, C.; et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 2003, 9, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Hofer, N.; Diel, P.; Wittsiepe, J.; Wilhelm, M.; Kluxen, F.M.; Degen, G.H. Dose and route-dependent hormonal activity of the metalloestrogen cadmium in the rat uterus. Toxicol. Lett. 2009, 191, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nakagawa, H.; Honda, R.; Tanebe, K.; Saito, S.; Teranishi, H.; Tawara, K. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup. Environ. Med. 2002, 59, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Urrutia, M.; Montenegro, M.; Llanos, M.N. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicol. Lett. 2009, 188, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Ranasinghe, S.; Sjaarda, L.A.; Mumford, S.L. Cadmium and Reproductive Health in Women: A Systematic Review of the Epidemiologic Evidence. Curr. Environ. Health Rep. 2014, 1, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.T.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2017, 5614, 31366–31368. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Lu, Y.; Liang, Y.; Chen, B.; Wu, M.; Li, S.; He, G.; Jin, T. Exposure assessment of dietary cadmium: Findings from Shanghainese over 40 years, China. BMC Public Health 2013, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Gray, L.E., Jr.; Marcus, M.; Ojeda, S.R.; Pescovitz, O.H.; Witchel, S.F.; Sippell, W.; Abbott, D.H.; Soto, A.; Tyl, R.W.; et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 2008, 121, S192–S207. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Buck, G.M.; Mendola, P. Blood lead levels and sexual maturation in U.S. girls: The Third National Health and Nutrition Examination Survey, 1988–1994. Environ. Health Perspect. 2003, 111, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Gollenberg, A.L.; Hediger, M.L.; Lee, P.A.; Himes, J.H.; Louis, G.M. Association between lead and cadmium and reproductive hormones in peripubertal U.S. girls. Environ. Health Perspect. 2010, 118, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Berglund, M.; Akesson, A. Toxic metals and the menopause. J. Br. Menopause Soc. 2004, 10, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rull, R.P.; Canchola, A.J.; Reynolds, P.; Horn-Ross, P.L. Urinary Cadmium and the Timing of Menarche and Pubertal Development in Girls. J. Adolesc. Health 2014, 54, S10–S11. [Google Scholar] [CrossRef]
- Yu, X.; Filardo, E.J.; Shaikh, Z.A. The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol. Appl. Pharmacol. 2010, 245, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Louis, G.M.; Chen, Z.; Peterson, C.M.; Sundaram, R.; Croughan, M.S.; Sun, L.; Hediger, M.L.; Stanford, J.B.; Varner, M.W.; et al. Trace elements and endometriosis: The ENDO Study. Reprod. Toxicol. 2013, 42c, 41–48. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, G.; Jin, T. Effects of Cadmium Exposure on Age of Menarche and Menopause. Toxics 2018, 6, 6. https://doi.org/10.3390/toxics6010006
Chen X, Zhu G, Jin T. Effects of Cadmium Exposure on Age of Menarche and Menopause. Toxics. 2018; 6(1):6. https://doi.org/10.3390/toxics6010006
Chicago/Turabian StyleChen, Xiao, Guoying Zhu, and Taiyi Jin. 2018. "Effects of Cadmium Exposure on Age of Menarche and Menopause" Toxics 6, no. 1: 6. https://doi.org/10.3390/toxics6010006



