Evaluating the Joint Toxicity of Two Benzophenone-Type UV Filters on the Green Alga Chlamydomonas reinhardtii with Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Substances
2.2. Preparation of the Algae Culture
2.3. Experimental Design
2.4. Toxicity Tests
2.5. Measurement of Cell Growth and Photosynthetic Pigments
2.6. Measurement of BP-1 and BP-3
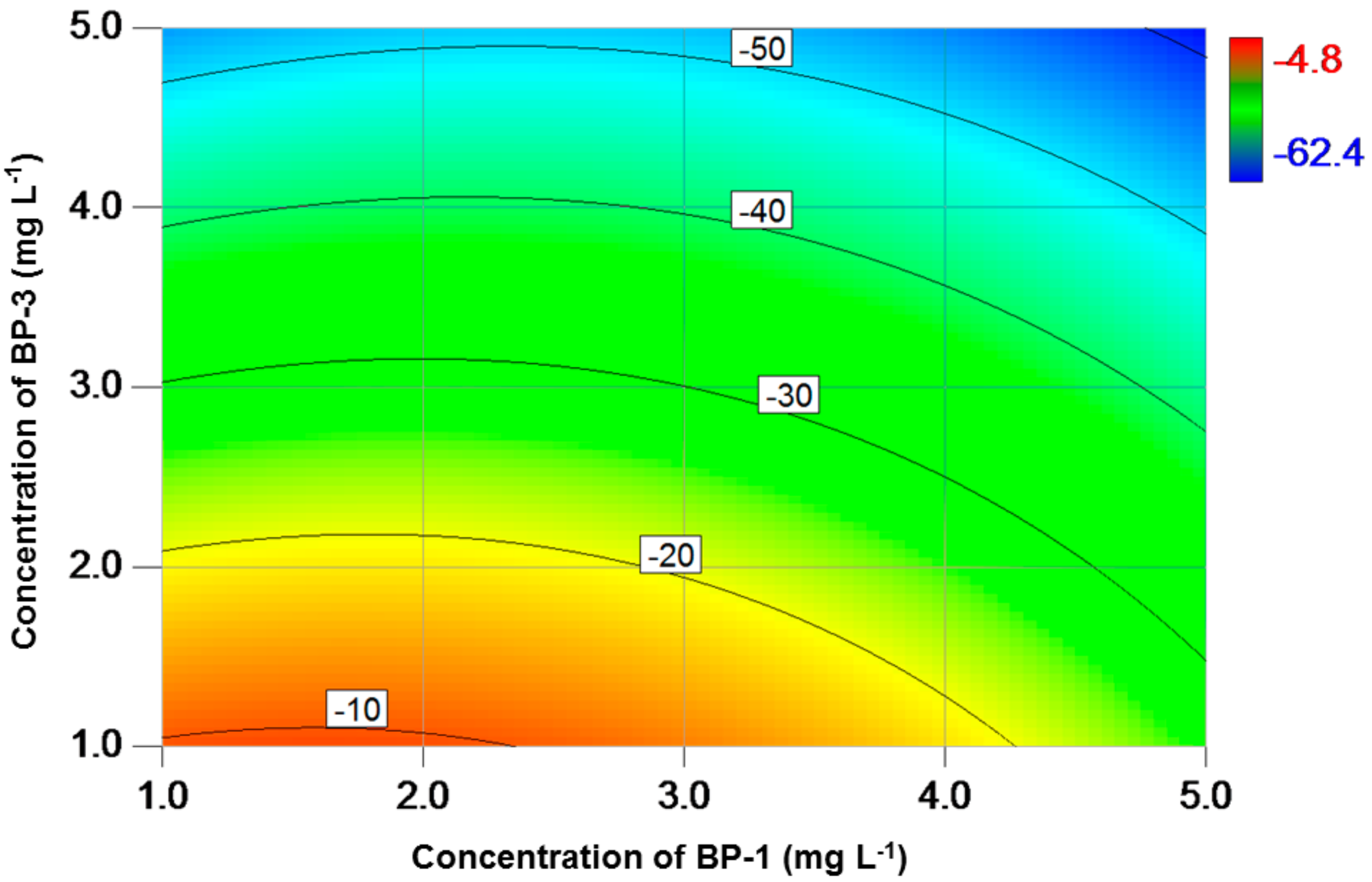
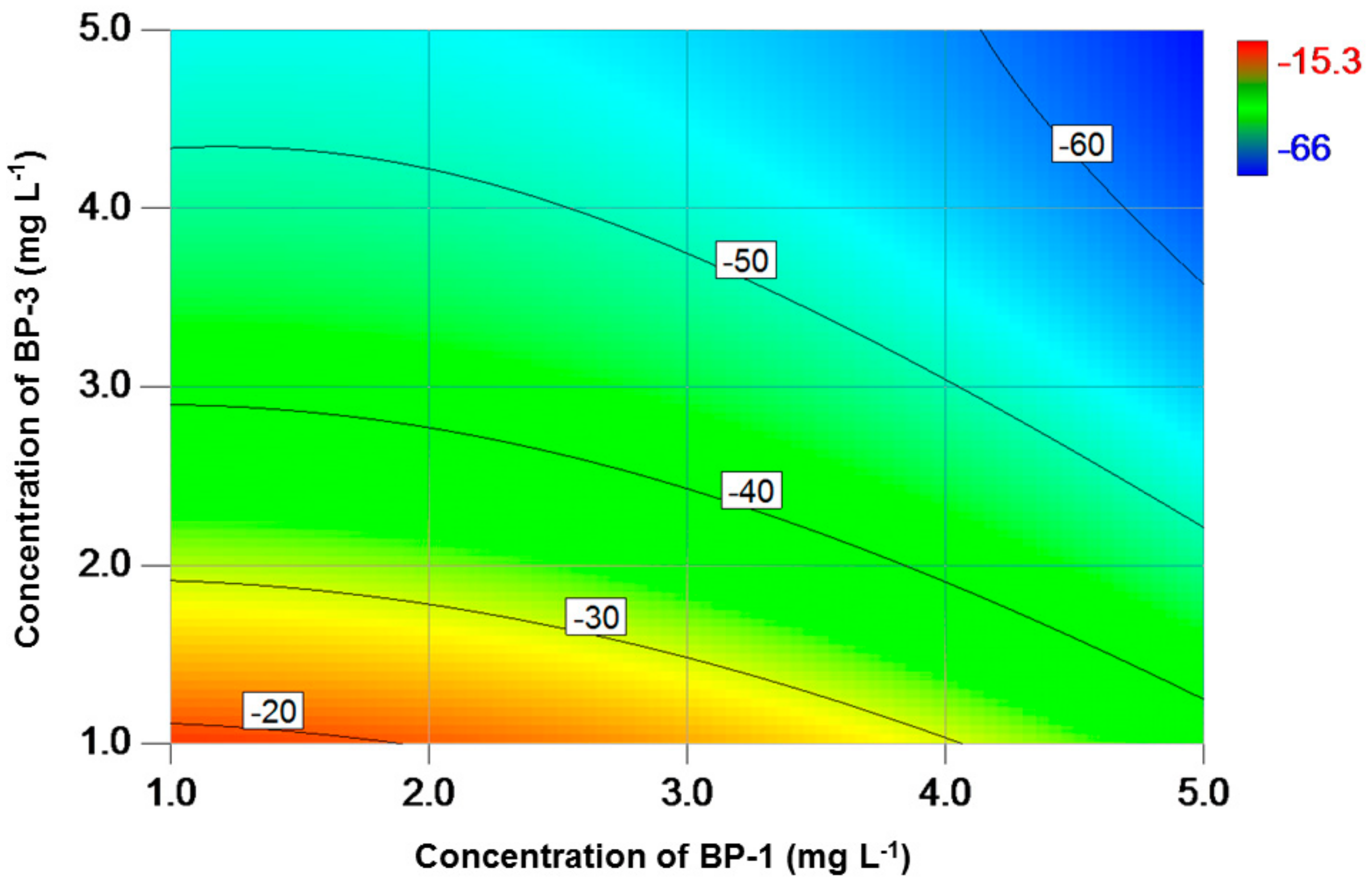
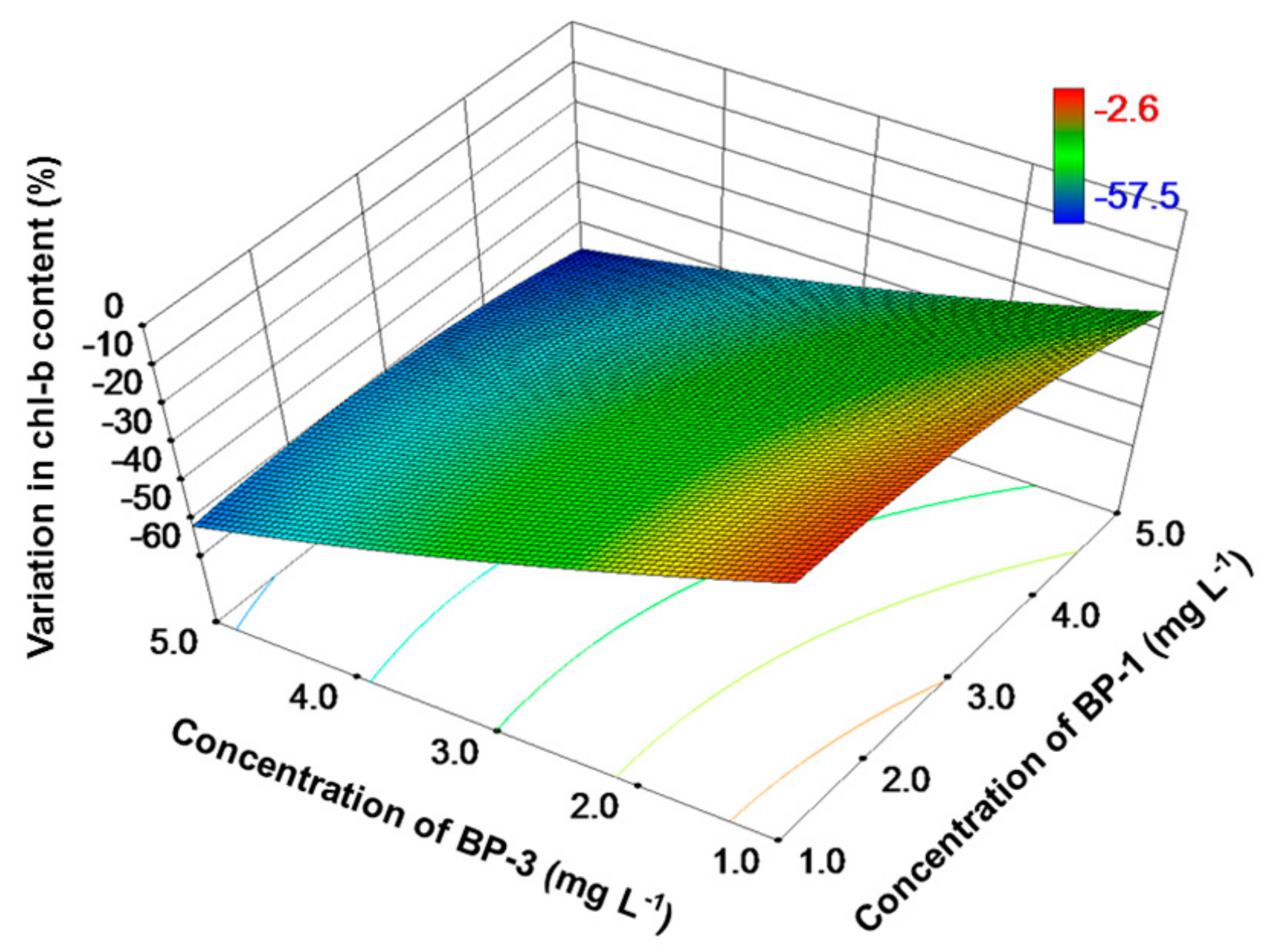
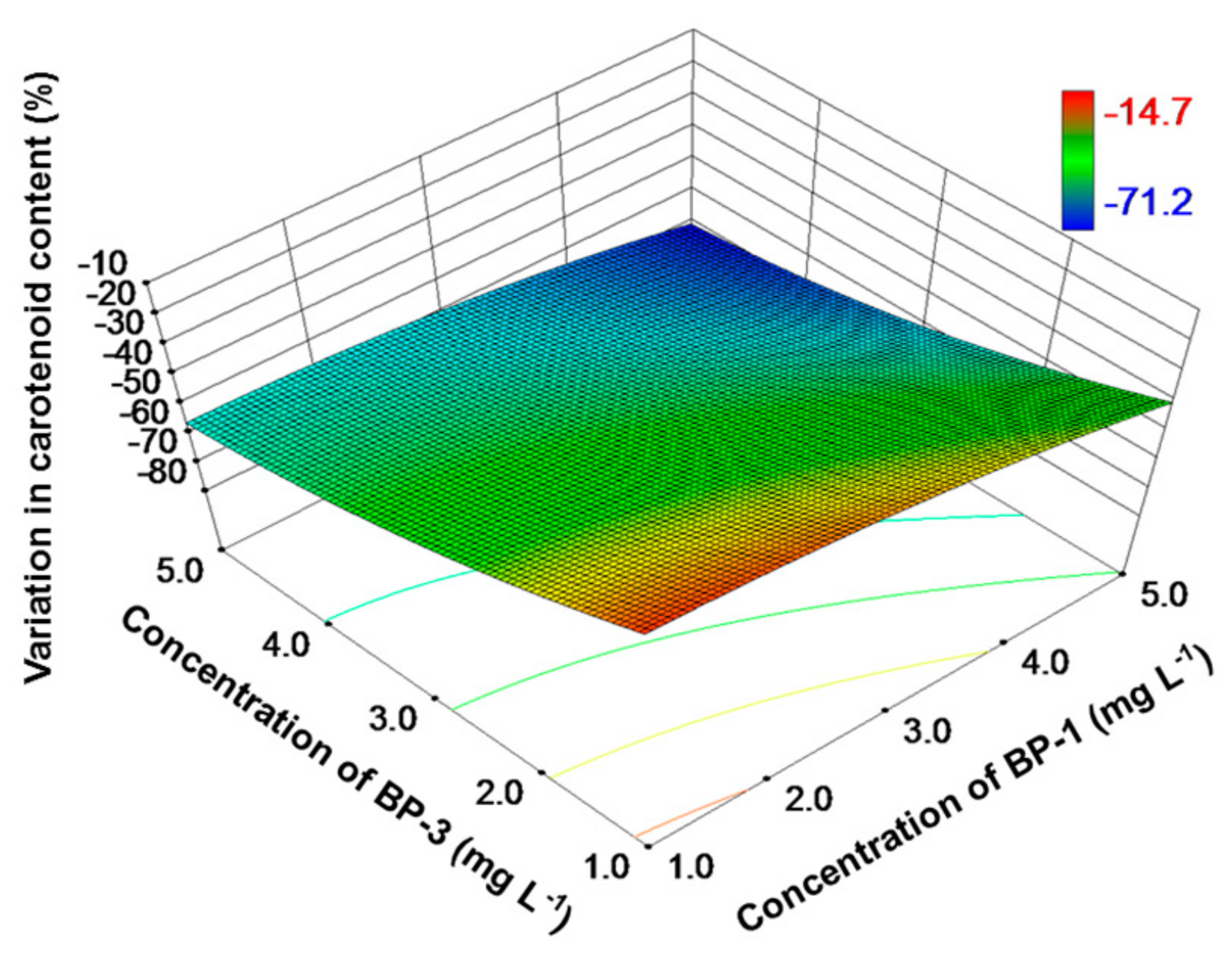
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeon, H.-K.; Chung, Y.; Ryu, J.-C. Simultaneous determination of benzophenone-type UV filters in water and soil by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1131, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ren, N.; Li, Y.-F.; Kunisue, T.; Gao, D.; Kannan, K. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge. Environ. Sci. Technol. 2011, 45, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef] [PubMed]
- ECHA Oxybenzone—Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.004.575#TRADE_NAMEScontainer (accessed on 3 August 2017).
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Environmental Working Group. The Trouble with Ingredients in Sunscreens. Available online: http://www.ewg.org/sunscreen/report/the-trouble-with-sunscreen-chemicals/#.WX_xEISGOUk (accessed on 1 August 2017).
- Liao, C.; Kannan, K. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: An assessment of human exposure. Environ. Sci. Technol. 2014, 48, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Shaath, N.A. The Encyclopedia of Ultraviolet Filters; Allured Publishing Corp: Carol Stream, IL, USA, 2007; ISBN 1932633251. [Google Scholar]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.-S. Occurrence, distribution and fate of organic UV filters in coral communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liu, J.; Yang, X.; Peng, Y.; Xu, L. Orthogonal array design for the optimization of ionic liquid-based dispersive liquid-liquid microextraction of benzophenone-type UV filters. J. Sep. Sci. 2011, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Mastroianni, N.; Díaz-Cruz, M.S.; Barceló, D. Fully automated determination of nine ultraviolet filters and transformation products in natural waters and wastewaters by on-line solid phase extraction–liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1294, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-P.; Liu, B.-Y.; Yu, H.-J.; Liu, W.-Q.; Yang, Y.-F. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ. Pollut. 2013, 172, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rosi-Marshall, E.J.; Snow, D.; Bartelt-Hunt, S.L.; Paspalof, A.; Tank, J.L. A review of ecological effects and environmental fate of illicit drugs in aquatic ecosystems. J. Hazard. Mater. 2015, 282, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Smith, V.H.; deNoyelles, F.; Larive, C.K. Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environ. Sci. Technol. 2003, 37, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.V.; García-Hortigüela, P.; Fernández, C. Acute and chronic toxicity of emerging contaminants, alone or in combination, in Chlorella vulgaris and Daphnia magna. Environ. Sci. Pollut. Res. 2015, 22, 5417–5424. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Heiaas, H.H.; Tollefsen, K.E. Combined effects of pharmaceuticals, personal care products, biocides and organic contaminants on the growth of Skeletonema pseudocostatum. Aquat. Toxicol. 2014, 150, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sieratowicz, A.; Kaiser, D.; Behr, M.; Oetken, M.; Oehlmann, J. Acute and chronic toxicity of four frequently used UV filter substances for Desmodesmus subspicatus and Daphnia magna. J. Environ. Sci. Health Part A 2011, 46, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; He, Y.; Kushmaro, A.; Gin, K.Y.-H. Effects of benzophenone-3 on the green alga Chlamydomonas reinhardtii and the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2017, 193, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jurado, A.; Gago-Ferrero, P.; Vàzquez-Suñé, E.; Carrera, J.; Pujades, E.; Díaz-Cruz, M.S.; Barceló, D. Urban groundwater contamination by residues of UV filters. J. Hazard. Mater. 2014, 271, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, I.; Iribarne-Durán, L.M.; Ocón, O.; Salamanca, E.; Fernández, M.F.; Olea, N.; Barranco, E. Determination of personal care products–benzophenones and parabens–in human menstrual blood. J. Chromatogr. B 2016, 1035, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xie, W.; Chen, J. Assessing the combined effects from two kinds of cephalosporins on green alga (Chlorella pyrenoidosa) based on response surface methodology. Food Chem. Toxicol. 2015, 78, 116–121. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Giloni-Lima, P.C.; Delello, D.; Cremonez, M.L.M.; Éler, M.N.; Lima, V.A.; Espíndola, E.L.G. A study of the effects of chromium exposure on the growth of Pseudokirchneriella subcapitata (Korshikov) hindak evaluated by central composite design and response surface methodology. Ecotoxicology 2010, 19, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Guo, R.; Ren, X.; Ren, H. A new method for analysis of the toxicity of organophosphorus pesticide, dimethoate on rotifer based on response surface methodology. J. Hazard. Mater. 2012, 237, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Seyedkolaei, S.J.; Mirvaghefi, A.; Farahmand, H.; Kosari, A.A.; Gholami-Seyedkolaei, S.J.; Gholami-Seyedkolaei, S.J. Optimization of recovery patterns in common carp exposed to Roundup using response surface methodology: Evaluation of neurotoxicity and genotoxicity effects and biochemical parameters. Ecotoxicol. Environ. Saf. 2013, 98, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-operation and Development (OECD). OECD Guideline for the Testing of Chemicals: Freshwater Alga and Cyanobacteria, Growth Inhibition Test (NO. 201); OECD: Paris, France, 2001. [Google Scholar]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
- Hanrahan, G.; Lu, K. Application of factorial and response surface methodology in modern experimental design and optimization. Crit. Rev. Anal. Chem. 2006, 36, 141–151. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Zenker, A.; Rapp, M. A tentative environmental risk assessment of the UV-filters 3-(4-methylbenzylidene-camphor), 2-ethyl-hexyl-4-trimethoxycinnamate, benzophenone-3, benzophenone-4 and 3-benzylidene camphor. Mar. Environ. Res. 2010, 69, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; Maurya, R.; Trivedi, K.; Patidar, S.K.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Estrogenic activity of ternary UV filter mixtures in fish (Pimephales promelas)—An analysis with nonlinear isobolograms. Toxicol. Appl. Pharmacol. 2009, 234, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, M.; Muusse, M.; van den Berg, M.; Sanderson, J.T. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol. Appl. Pharmacol. 2005, 208, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Estrogenic activity of UV filter mixtures. Toxicol. Appl. Pharmacol. 2006, 217, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Behra, R.; Nestler, H.; Suter, M.J.-F.; Sigg, L.; Schirmer, K. Linking toxicity and adaptive responses across the transcriptome, proteome, and phenotype of Chlamydomonas reinhardtii exposed to silver. Proc. Natl. Acad. Sci. USA 2014, 111, 3490–3495. [Google Scholar] [CrossRef] [PubMed]
- Paredes, E.; Perez, S.; Rodil, R.; Quintana, J.B.; Beiras, R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014, 104, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H. Acute toxicity of benzophenone-type UV filters and paraben preservatives to freshwater planarian, Dugesia japonica. Toxicol. Environ. Chem. 2012, 94, 566–573. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.-Z.; Tam, N.F.Y.; Luan, T.-G.; Shin, P.K.S.; Cheung, S.G.; Liu, Y. Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicol. Environ. Saf. 2009, 72, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kabra, A.N.; Ji, M.-K.; Choi, J.; Kim, J.R.; Govindwar, S.P.; Jeon, B.-H. Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonas mexicana. Environ. Sci. Pollut. Res. 2014, 21, 12270–12278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of pesticides to aquatic microorganisms: A review. Environ. Toxicol. Chem. 2001, 20, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Alla, M.M.N.; Hassan, N.M. Changes of antioxidants levels in two maize lines following atrazine treatments. Plant Physiol. Biochem. 2006, 44, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Sztatelman, O.; Grzyb, J.; Gabryś, H.; Banaś, A.K. The effect of UV-B on Arabidopsis leaves depends on light conditions after treatment. BMC Plant Biol. 2015, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J.; Li, W.; Gan, J. Biodegradation of naproxen by freshwater algae Cymbella sp. and Scenedesmus quadricauda and the comparative toxicity. Bioresour. Technol. 2017, 238, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Melegari, S.P.; Perreault, F.; Costa, R.H.R.; Popovic, R.; Matias, W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013, 142, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Moradshahi, A.; Jahromi, H.D.; Sheikhi, M.H. Influence of PbS nanoparticle polymer coating on their aggregation behavior and toxicity to the green algae Dunaliella salina. Aquat. Toxicol. 2014, 154, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Broderius, S.J.; Kahl, M.D.; Elonen, G.E.; Hammermeister, D.E.; Hoglund, M.D. A comparison of the lethal and sublethal toxicity of organic chemical mixtures to the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2005, 24, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Ozáez, I.; Morcillo, G.; Martínez-Guitarte, J.-L. The effects of binary UV filter mixtures on the midge Chironomus riparius. Sci. Total Environ. 2016, 556, 154–162. [Google Scholar] [CrossRef] [PubMed]
| Properties | BP-1 | BP-3 |
|---|---|---|
| Synonym | 2,4-Dihydroxybenzophenone | 2-Hydroxy-4-methoxybenzophenone |
| CAS. number | 131-56-6 | 131-57-7 |
| Chemical structure |  |  |
| Molecular formula | C13H10O3 | C14H12O3 |
| Molecular weight (g mol−1) | 214.22 | 228.24 |
| Water solubility (mg L−1) | 413.4 | 2295.40 |
| logKow | 2.96 | 3.79 |
| Treatment | Coded Levels | Actual Levels (mg L−1) a | ||
|---|---|---|---|---|
| BP-1 | BP-3 | BP-1 | BP-3 | |
| 1 | 1 | −1 | 5.0 (5.6 ± 0.3) | 1.0 (0.9 ± 0.04) |
| 2 | −1 | 1 | 1.0 (0.9 ± 0.04) | 5.0 (4.4 ± 0.2) |
| 3 | 1.41 | 0 | 5.8 (5.7 ± 0.3) | 3.0 (3.6 ± 0.2) |
| 4 | 0 | 1.41 | 3.0 (2.7 ± 0.2) | 5.8 (5.4 ± 0.3) |
| 5 | 1 | 1 | 5.0 (4.5 ± 0.2) | 5.0 (4.4 ± 0.2) |
| 6 | 0 | 0 | 3.0 (2.7 ± 0.1) | 3.0 (2.6 ± 0.1) |
| 7 | −1 | −1 | 1.0 (0.8 ± 0.04) | 1.0 (0.8 ± 0.04) |
| 8 | 0 | 0 | 3.0 (2.6 ± 0.1) | 3.0 (2.5 ± 0.1) |
| 9 | −1.41 | 0 | 0.2 (0.2 ± 0.01) | 3.0 (2.6 ± 0.1) |
| 10 | 0 | −1.41 | 3.0 (2.6 ± 0.1) | 0.2 (0.2 ± 0.01) |
| Term | Response | |||
|---|---|---|---|---|
| Specific Growth Rate | Chl-a | Chl-b | Carotenoid | |
| Statistics for the fitted models | ||||
| p value | 0.013 | 0.002 | 0.002 | 0.003 |
| R2 | 94.4% | 97.8% | 98% | 97.6% |
| F value | 13.41 | 35.45 | 38.59 | 31.82 |
| Lack of fit | 0.0501 | 0.150 | 0.052 | 0.058 |
| Statistics for the individual coefficients | ||||
| −29.95 (5.22) | −44.90 (2.80) | −32.28 (3.03) | −47.56 (3.25) | |
| −6.23 (2.61) | −7.76 (1.40) | −5.93 (1.52) | −9.21 (1.63) | |
| −19.88 (2.62) | −15.64 (1.40) | −19.53 (1.52) | −17.09 (1.63) | |
| 2.34 (3.69) | 1.54 (1.98) | 3.36 (2.14) | 1.46 (2.30) | |
| −5.98 (3.45) | −3.73 (1.85) | −4.22 (2.00) | −3.82 (2.15) | |
| −2.08 (3.45) | 5.27 (1.85) | 2.09 (2.00) | 5.44 (2.15) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, F.; He, Y.; Gin, K.Y.-H. Evaluating the Joint Toxicity of Two Benzophenone-Type UV Filters on the Green Alga Chlamydomonas reinhardtii with Response Surface Methodology. Toxics 2018, 6, 8. https://doi.org/10.3390/toxics6010008
Mao F, He Y, Gin KY-H. Evaluating the Joint Toxicity of Two Benzophenone-Type UV Filters on the Green Alga Chlamydomonas reinhardtii with Response Surface Methodology. Toxics. 2018; 6(1):8. https://doi.org/10.3390/toxics6010008
Chicago/Turabian StyleMao, Feijian, Yiliang He, and Karina Yew-Hoong Gin. 2018. "Evaluating the Joint Toxicity of Two Benzophenone-Type UV Filters on the Green Alga Chlamydomonas reinhardtii with Response Surface Methodology" Toxics 6, no. 1: 8. https://doi.org/10.3390/toxics6010008




