Herb-Induced Liver Injuries in Developing Nations: An Update
Abstract
:1. Introduction
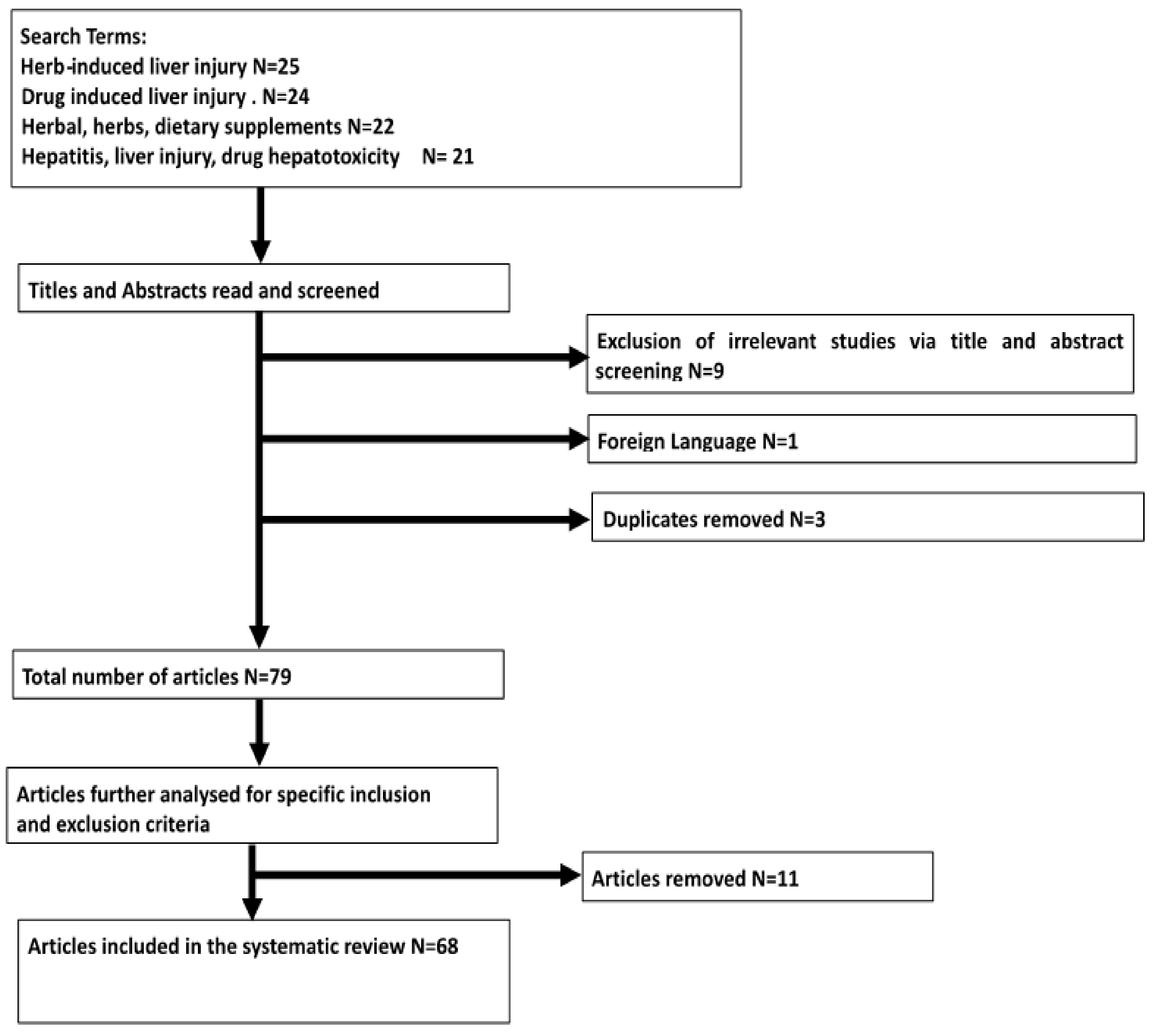
2. Methods
2.1. Database Searching, Search Strategy and Selection Criteria
2.2. Inclusion Criteria and Exclusion Criteria
3. Current Status of Knowledge
3.1. Results and Discussion
Results of Search
3.2. Etiology and Risk Factors Associated with the Development of HILI
3.2.1. Incorrect Identification and Mislabeling of Herbal Products
3.2.2. Co-Morbidities and Drug Interactions
3.2.3. Alcohol Consumption
3.2.4. Adulterants, Impurities and Contaminants
3.2.5. Host-Related Risk Factors
3.3. Diagnosis and Clinical Manifestations of HILI
3.4. Selected Clinical Case Reports of DILI in Sub-Saharan Africa and Other Developing Nations
3.5. Selected Clinical Case Reports of HILI in Sub-Saharan Africa and Other Developing Nations
3.6. Challenges of HILI Management in Sub-Saharan Africa
4. Conclusions
- □
- There is increasing incidence of liver disease in developing nations.
- □
- Herbal medicines are associated with complications such as liver damage with a high incidence of mortalities and morbidities.
- □
- Poor pharmacovigilance programs in sub-Saharan Africa and other developing nations remain a challenge in the documentation of herb-induced liver injury (HILI).
- □
- Although this study has attempted to capture HILI in developing nations, it is feared that the present data is a far cry from the reality, given the increasing incidence of liver disease.
- □
- There should be an introduction of sound regulatory policies predicated on contemporary science and emerging technologies to boost predictive and preventive medicine in developing nations.
Author Contributions
Conflicts of Interest
References
- Crawford, J.M.; Liu, C. Liver and biliary tract. In Robbins Pathologic Basis of Disease, 8th ed.; Kumar, V., Abbas, A.K., Fausto, N., Aster, J.C., Eds.; W.B. Saunders Co.: Philadelphia, PA, USA, 2010; pp. 833–890. [Google Scholar]
- Abdulkareem, E.B.; Banjo, A.A.; Elesha, S.O.; Daramola, A.O. Histopathological study of liver diseases at the Lagos University Teaching Hospital, Nigeria (1989–2000). Niger. Postgrad. Med. J. 2006, 13, 41–46. [Google Scholar] [PubMed]
- Ugiagbe, E.E.; Udoh, M.O. The histopathological pattern of liver biopsies at the University of Benin Teaching Hospital. Niger. J. Clin. Pract. 2013, 16, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Tittarelli, R.; Manocchi, G.; Zaami, S.; Ricci, S.; Giogetti, R.; Terranova, D.; Bustardo, F.P.; Marinelli, E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 2016, 17, 580. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Mannocchi, G.; Marinelli, E.; Gentili, S.; Graziano, S.; Busardò, F.P.; di Luca, N.M. Hepatotoxicity induced by greater celandine (Chelidonium majus L.): A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2146–2152. [Google Scholar]
- Frenzel, C.; Teschke, R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. Int. J. Mol. Sci. 2016, 17, 588. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Liss, G.; Lewis, J.H. Drug-induced liver injury: What was new in 2008? Exp. Opin. Drug Metab. Toxicol. 2009, 5, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Frenzel, C.; Glass, X.; Schulz, J.; Eickoff, A. Herbal hepatotoxicity: A critical review. Br. J. Clin. Pharmacol. 2013, 75, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, V. Traditional herbal remedies used by South African women for gynaecological complaints. J. Ethnopharmacol. 2003, 86, 97–108. [Google Scholar] [CrossRef]
- Akpan, E.E.; Ekrikpo, U.E. Acute Renal Failure Induced by Chinese Herbal Medication in Nigeria. Case Rep. Med. 2015, 2015, 150204. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Engel, A.; Fattinger, K.; Marbet, U.A.; Criblez, D.C.; Reichen, J.; Zimmermann, A.; Oneta, C.M. Herbal does not mean innocuous: Ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J. Hepatol. 2007, 47, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Sticket, F.; Patsenker, E.; Schuppan, D. Herbal hepatotoxicity. J. Hepatol. 2005, 43, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Nwokediuko, S.C.; Osuala, P.C.; Uduma, U.V.; Alaneme, A.K.; Onwuka, C.C.; Mesigo, C. Pattern of liver disease admissions in a Nigerian tertiary hospital. Niger. J. Clin. Pract. 2013, 16, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.L.; Woo, S.O. Chinese proprietary medicine in Singapore: Regulatory control of toxic heavy metals and undeclared drugs. Drug Saf. 2000, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awodele, O.; Daniel, A.; Popoola, T.D.; Salami, E.F. A study on pharmacovigilance of herbal medicines in Lagos West Senatorial District, Nigeria. Int. J. Risk Saf. Med. 2013, 25, 205–217. [Google Scholar] [PubMed]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: A tabular compilation of reported cases. Liver Int. 2012, 32, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Bonkovsky, H.L.; Hwang, S.I.; Vega, M.; Barnhart, H.; Serrano, J. Catechins in dietary supplements and hepatotoxicity. Dig. Dis. Sci. 2013, 58, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, J.Y.; Li, N.; Li, M.; Gao, H.; Ji, Y.; Zhang, F.; Wang, H.; Zhou, Y.; Ye, Y.; et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, B.J.; Reynolds, S.J.; Lamorde, M.; Merry, C.; Kukunda-Byobona, C.; Ocama, P.; Semeere, A.S.; Ndyanabo, A.; Boaz, I.; Kiggundu, V.; et al. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLoS ONE 2012, 7, e41737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bout-van den Beukel, C.J.; Koopmans, P.P.; van der Ven, A.J.; De Smet, P.A.; Burger, D.M. Possible drug-metabolism interactions of medicinal herbs with antiretroviral agents. Drug Metab. Rev. 2006, 38, 477–514. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: Analysis of cases with initially reported positive re-exposure tests. Dig. Liver Dis. 2014, 46, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An overview of the evidence and mechanisms of herb-drug interactions. Front. Pharmacol. 2012, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Obot, I. Alcohol use and related problems in sub-Saharan Africa. Afr. J. Drug Alcohol Stud. 2006, 5, 17–26. [Google Scholar]
- Moore, A.A.; Whiteman, E.J.; Ward, K.T. Risks of combined alcohol/medication use in older adults. Am. J. Geriatr. Pharmacother. 2007, 5, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ndububa, D.A.; Ojo, O.S.; Adetiloye, V.A.; Aladegbaiye, A.O.; Adebayo, R.A.; Adekanle, O. The contribution of alcohol to chronic liver disease in patients from South-west Nigeria. Niger. J. Clin. Pract. 2010, 13, 360–364. [Google Scholar] [PubMed]
- Okeke, E.N.; Malu, A.O.; Obafunwa, J.O.; Nwana, E.J. Aetiological significance of alcohol in liver cirrhosis on the Jos Plateau. West Afr. J. Med. 2002, 21, 12–14. [Google Scholar] [PubMed]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Awodele, O.; Popoola, T.D.; Amadi, K.C.; Coker, H.A.; Akintowa, A. Traditional medicinal plants in Nigeria—Remedies or risks. J. Ethnopharmacol. 2013, 150, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Obi, E.; Akunyili, D.N.; Expo, B.; Orisakwe, O.E. Heavy metal hazards of Nigerian herbal remedies. Sci. Total Environ. 2006, 369, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Orisakwe, O.E.; Roberts, I.I. Elemental impurities in registered herbal supplements in Nigeria. A look at Mercury, Antimony and Tin. Rasayan J. Chem. 2012, 5, 220–228. [Google Scholar]
- Ernst, E. Adulteration of Chinese herbal medicines with synthetic drugs: A systematic review. J. Int. Med. 2002, 252, 107–113. [Google Scholar] [CrossRef]
- Ogutcu, A.; Suludere, Z.; Kalendar, Y. Dichlorvos-induced hepatotoxicity in rats and the protective effects of vitamins C and E. Environ. Toxicol. Pharmacol. 2008, 26, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sinyangwe, D.M.; Mbewe, B.; Sijumbila, G. Determination of dichlorvos residue levels in vegetables sold in Lusaka, Zambia. Pan Afr. Med. J. 2016, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Voellinger, J.L.; Van Ness, K.P.; Chapron, B.; Shaffer, R.M.; Neumann, T.; White, C.C.; Kavanagh, T.J.; Kelly, E.J.; Eaton, D.L. Characterization of rat or human hepatocytes cultured in microphysiological systems (MPS) to identify hepatotoxicity. Toxicol. In Vitro 2017, 40, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Ladep, N.G. Why is the tumour different in Africa? In Clinical Dilemmas in Primary Liver Cancer, 1st ed.; Williams, R., Taylor-Robinson, S., Eds.; John Wiley and Sons: West Sussex, UK, 2012; pp. 11–17. [Google Scholar]
- Gong, Y.Y.; Egal, S.; Hounsa, A.; Turner, P.C.; Hall, A.J.; Cardwell, K.F.; Wild, C.P. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. Int. J. Epidemiol. 2003, 32, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Ezekwesili-Ofili, J.; Onyemelukwe, N.; Agwaga, P.; Orji, I. The bioload and aflatoxin content of herbal medicines from selected states in Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Lee, C.H.; Lee, M.C.; Wang, J.Y.; Yu, C.J.; Lee, L.N. Hepatotoxicity due to first-line anti-tuberculosis drugs: A five-year experience in a Taiwan medical centre. Int. J. Tuberc. Lung Dis. 2013, 17, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Abboud, G.; Kaplowitz, N. Drug-induced liver injury. Drug Saf. 2007, 30, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Chen, Y.; Li, B.; Zhang, M.; Liu, X.; Li, F.; Chen, C.; Mao, Y.; Chen, J. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. SpringerPlus 2015, 4, 802. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Leise, M.D.; Poterucha, J.J.; Talwalkar, J.A. Drug-induced liver injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.X.; Navarro, V.J. Liver injury from herbal, dietary, and weight loss supplements: A review. J. Clin. Transl. Hepatol. 2015, 3, 93–98. [Google Scholar] [PubMed]
- Davern, T.J.; Chalasani, N.; Fontana, R.J.; Hayashi, P.H.; Protiva, P.; Kleiner, D.E. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011, 141, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Correa, B.; Gómez-Gutiérrez, C.; Uribe, M.; Méndez-Sánchez, N. Herbal Medicine in Mexico: A Cause of Hepatotoxicity. A Critical Review. Int. J. Mol. Sci. 2016, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Iroezindu, M.O.; Agbaji, O.O.; Daniyam, C.A.; Isiguzo, G.C.; Isichei, C.; Akanbi, M.O. Liver function test abnormalities in Nigerian patients with human immunodeficiency virus and hepatitis B virus co-infection. Int. J. STD AIDS 2013, 24, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Eberhart, J.E. Elevated serum Alanine Aminotransferase and gamma glutaryltransferase and mortality in the United States Population. Gastroenterology 2009, 136, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ikuabe, P.O.; Ebuenyi, I.D.; Harry, T.C. Limited elevations in antituberculosis drug-induced serum alanine aminotransferase (ALT) levels in a cohort of Nigerians on treatment for pulmonary tuberculosis and HIV infection in Yenagoa. Niger. J. Med. 2015, 24, 103–107. [Google Scholar] [PubMed]
- Suk, K.T.; Kim, D.J. Drug-induced liver injury: Present and future. Clin. Mol. Hepatol. 2012, 18, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H. Hepatotoxicity the Adverse Effects of Drugs and Other Chemicals on the Liver; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Reuben, A. Hy’s law. Hepatology 2004, 39, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Temple, R. Hy’s law: Predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf. 2006, 15, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E. Drug-induced liver injury: Hy’s rule revisited. Clin. Pharmacol. Ther. 2006, 79, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.; Olsson, R. Suspected drug-induced liver fatalities reported to the WHO database. Dig. Liver Dis. 2006, 38, 33–38. [Google Scholar] [CrossRef] [PubMed]
- De Valle, M.B.; Av Klinteberg, V.; Alem, N.; Olsson, R.; Björnsson, E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment. Pharmacol. Ther. 2006, 24, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Norris, W.; Paredes, A.H.; Lewis, J.H. Drug-induced liver injury in 2007. Curr. Opin. Gastroenterol. 2008, 24, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Benichou, C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J. Hepatol. 1990, 11, 272–276. [Google Scholar] [PubMed]
- Woo, H.J.; Kim, H.Y.; Choi, E.S.; Cho, Y.H.; Kim, Y.; Lee, J.H.; Jang, E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine 2015, 22, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Dierkhising, R.; Kremers, W.K.; Sandeep, M.S.; Karanth, D.; Adarsh, C.K. Single-center experience with drug-induced liver injury from India: Causes, outcome, prognosis, and predictors of mortality. Am. J. Gastroenterol. 2010, 105, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Isa, S.E.; Ebonyi, A.O.; Shehu, N.Y.; Idoko, P.; Anejo-Okopi, J.A.; Simji, G.; Odesanya, R.U.; Abah, I.O.; Jimoh, H.O. Antituberculosis drugs and hepatotoxicity among hospitalized patients in Jos, Nigeria. Int. J. Mycobacteriol. 2016, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T. Presentation of drug-induced liver injury in Singapore. Singapor. Med. J. 2006, 47, 116–120. [Google Scholar]
- Devarbhavi, H.; Singh, R.; Patil, M.; Sheth, K.; Adarsh, C.K.; Balaraju, G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J. Gastroenterol. Hepatol. 2013, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, C.; Liu, W.; Wang, F. Acute liver failure associated with traditional Chinese medicine: Report of 30 cases from seven tertiary hospitals in China*. Crit. Care Med. 2014, 42, e296–e299. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.T.; Wang, H.; Gui, H.L.; Ye, M.Z.; Dai, W.J.; Xiang, X.G.; Zhao, G.D.; Wang, W.J.; Xie, Q. Clinical and pathological features in 138 cases of drug-induced liver injury. Zhonghua Gan Zang Bing Za Zhi 2012, 20, 185–189. [Google Scholar] [PubMed]
- Wai, C.T.; Tan, B.H.; Chan, C.L.; Sutedja, D.S.; Lee, Y.M.; Khor, C.; Lim, S.G. Drug-induced liver injury at an Asian center: A prospective study. Liver Int. 2007, 27, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Bahre, R. Severe hepatotoxicity by Indian Ayurvedic herbal products: A structured causality assessment. Ann. Hepatol. 2009, 8, 258–266. [Google Scholar] [PubMed]
- Tarn, D.M.; Karlamangla, A.; Coulter, I.D.; Paterniti, D.A.; Knox, L.; Khang, P.S.; Hui, K.-K.; Wenger, N.S. A cross-sectional study of provider and patient characteristics associated with outpatient disclosures of dietary supplement use. Patient Educ. Couns. 2015, 98, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.J.; Amata-Kynvi, A.; Dvorkin, L.; Whelan, J.S. Herbs and other dietary supplements: Healthcare professionals’ knowledge, attitudes, and practices. Altern. Ther. Health Med. 2003, 9, 42–49. [Google Scholar] [PubMed]
- Cellini, M.; Attipoe, S.; Seales, P.; Gray, R.; Ward, A.; Stephens, M.; Deuster, P.A. Dietary supplements: Physician knowledge and adverse event reporting. Med. Sci. Sports Exerc. 2013, 45, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, J.; Ladep, N.G.; Crossey, M.M.; Okeke, E.; Duguru, M.; Banwat, E.; Taylor-Robinson, S.D. Reasons why West Africa continues to be a hotbed for hepatocellular carcinoma. Niger. Med. J. 2015, 56, 231–235. [Google Scholar] [PubMed]
- Reuben, A.; Koch, D.G.; Lee, W.M. Drug-induced acute liver failure: Results of a U.S. multicenter, prospective study. Hepatology 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. World Development Indicators. 2013. Available online: http://data.worldbank.org/region/SSA (accessed on 10 September 2017).
| Countries and Patient Characteristics | Clinical Cases and Prognosis | Reference |
|---|---|---|
| India (1997–2008), N = 313; liver injury, Single-centre, retrospective | Liver injury resulted in 17% overall mortality | [63] |
| Nigeria (January to June, 2013), N = 110, liver injury, single centre, retrospective | Results revealed symptomatic hepatotoxicity in twenty patients with an incidence of 18% | [64] |
| Singapore (2003–2004), N = 29, liver injury, single centre, prospective | Fifteen patients (52%) had liver injury from TCM, while four patients (14%) had liver injury from anti-tuberculosis drugs. Eighteen patients (62%) had hepatitis, seven patients (24%) had cholestatic injury, and four patients (14%) had mixed injury. Three patients (10%) died and one patient (3%) had liver transplant for liver failure. Chinese herbal medicine was majorly implicated | [65] |
| Countries and Patient Characteristics | Clinical Cases and Prognosis | Reference |
|---|---|---|
| Nigeria (2005–2010), N = 365, HILI, Single centre, retrospective | Consumption of herbs and roots was indicated as a risk factor in 46% of patients with liver diseases | [14] |
| Uganda(1994–1998), 500 HIV-infected and 500 HIV-uninfected participants, Single centre, HILI | For all participants, use of herbs was associated with significant liver fibrosis | [21] |
| China (2011–2014), N = 469, liver injury, Single centre, retrospective | The incidence rate of liver injury was 93 cases per 100,0000 patients. Chinese herbal medicine was highlighted as the major cause of liver injury in 36% of patients | [43] |
| China (2007–2012), N = 30, HILI, Multi-centre, retrospective | Acute liver failure with 60% mortality (18 patients died). Chinese medicinal herbs were implicated | [67] |
| China (2008–2010), N = 138, liver injury, Single centre, retrospective | Chinese herbal medicine was the major cause of liver injury resulting 54% of cases. Higher incidences of inflammation and fibrosis in cholestatic and mixed injury types than in the hepatocellular type | [68] |
| Singapore (2004–2006), N = 31; liver injury, Single centre, prospective | Twenty-three patients (74%) had hepatocellular injury, six patients (19%) had cholestatic injury, and two patients (7%) had mixed injury. Chinese herbal medicine was majorly implicated | [69] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadi, C.N.; Orisakwe, O.E. Herb-Induced Liver Injuries in Developing Nations: An Update. Toxics 2018, 6, 24. https://doi.org/10.3390/toxics6020024
Amadi CN, Orisakwe OE. Herb-Induced Liver Injuries in Developing Nations: An Update. Toxics. 2018; 6(2):24. https://doi.org/10.3390/toxics6020024
Chicago/Turabian StyleAmadi, Cecilia Nwadiuto, and Orish Ebere Orisakwe. 2018. "Herb-Induced Liver Injuries in Developing Nations: An Update" Toxics 6, no. 2: 24. https://doi.org/10.3390/toxics6020024






