In Vitro and In Vivo Evaluation of Essential Oil from Artemisia absinthium L. Formulated in Nanocochleates against Cutaneous Leishmaniasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil from A. absinthium
2.2. Reference Drugs
2.3. Nanocochleate Preparation
2.4. Parasites
2.5. Animals
2.6. In Vitro Studies
2.7. In Vivo Studies
2.8. Statistical Analysis
3. Results
3.1. Preparation of Nanocochleates
3.2. In Vitro Antileishmanial Activity
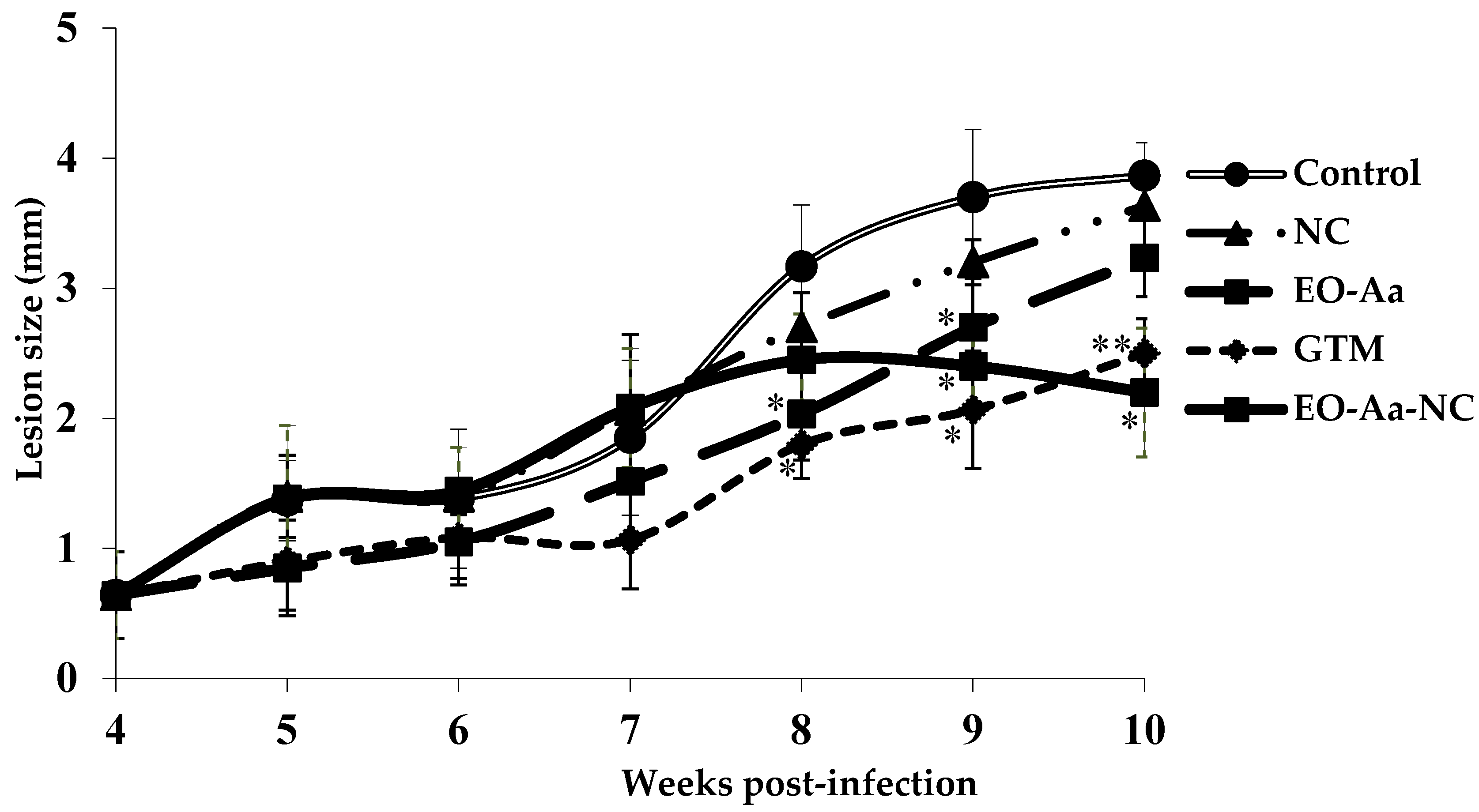
3.3. In Vivo Antileishmanial Activity
4. Discussion
4.1. Preparation of Nanocochleates
4.2. In Vitro Antileishmanial Activity
4.3. In Vivo Antileishmanial Activity
4.4. Future Directions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schroeder, J.; Aebischer, T. Vaccine for leishmaniasis from proteome to vaccine candidates. Hum. Vaccins 2011, 7, 6. [Google Scholar] [CrossRef]
- Diniz, S.A.; Silva, F.L.; Carvalho, A.V.; Bueno, R.; Guerra, R.M.; Abreu-Silva, A.L.; Santos, R.L. Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J. Infect. Dev. Ctries 2008, 2, 24–33. [Google Scholar] [PubMed]
- Ashford, R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; denBoer, M.; The WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- De Paiva-Cavalcanti, M.; de Morais, R.K.S.; Pessoa-e-Silva, R.; Trajano-Silva, L.A.M.; Gonçalves-de-Albuquerque, S.C.; Tavares, D.H.C.; Brelaz-de-Castro, M.C.A.; Silva, R.F.; Pereira, V.R.A. Leishmaniases diagnosis: An update on the use of immunological and molecular tools. Cell Biosci. 2015, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New world and old world Leishmania infections a practical review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Schriefer, A.; Wilson, M.E.; Carvalho, E.M. Recent developments leading toward a paradigm switch in the diagnostic and therapeutic approach to human leishmaniasis. Curr. Opin. Infect. Dis. 2008, 21, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, P.; Konidas, P.; Durkin-Konidas, M. New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment. J. Am. Acad. Dermatol. 2010, 63, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Kedzierski, L.; Sakthianandeswaren, A.; Curtis, J.M.; Andrews, P.C.; Junk, P.C.; Kedzierska, K. Leishmaniasis: Current treatment and prospects for new drugs and vaccines. Curr. Med. Chem. 2009, 16, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, M.; Singh, R.K. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac. J. Trop. Med. 2012, 5, 485–497. [Google Scholar] [CrossRef]
- Fournet, A.; Muñoz, V. Natural products as trypanocidal, antileishmanial and antimalarial drugs. Curr. Top. Med. Chem. 2002, 2, 1215–1237. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011, 15, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Piñón, A.; Scull, R.; Setzer, W.N. Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba. Nat. Prod. Commun. 2014, 9, 1799–1804. [Google Scholar] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. eCAM 2014, 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Mena, L.; Tamargo, B.; Salas, E.; Plaza, L.E.; Blanco, Y.; Otero, A.; Sierra, G.V. Determinación de saponinas y otros metabolitos secundarios en extractos acuosos de Sapindus saponaria L. (jaboncillo). Rev. Cuba Plantas Med. 2015, 20, 106–116. [Google Scholar]
- Gregoriadis, G. Immunological adjuvants: A role for liposomes. Immunol. Today 1990, 11, 89–97. [Google Scholar] [CrossRef]
- Tamargo, B.; Márquez, Y.; Ramírez, W.; Cedré, B.; Fresno, M.; Sierra, V.G. New proteoliposomic vaccine formulation from N. meningitidis serogroup B, without aluminum hydroxide, retains its antimeningococcal protectogenic potencial as well as Th-1 adjuvant capacity. BMC Immunol. 2013, 14 (Suppl. 1), S12. [Google Scholar] [CrossRef]
- Tamargo, B.; Herrera, L.; Bello, A.; Cuéllar, A.; González, H.; Ortiz, L.; Morales, M.; Sierra, V.G. Obtención de fosfolípidos a partir de la Lecitina de Soya (Glicine max L.), para usos biomédicos. Rev. Cub. Quím. 2011, 23, 5–14. [Google Scholar]
- Tamargo, B.; Rosario, L.A.; Batista, N.; Arencibia, D.F.; Fernández, K.; Villegas, A.; Ayala, J.A.; Sierra, V.G. Protección inducida por nanococleatos derivados de proteoliposomas de Leptospira interrogans serovar Canícola. VacciMonitor 2012, 21, 3–9. [Google Scholar]
- Caio, T.S.E.; Lima, M.D.; Kaplan, M.A.C.; Nazareth, M.M.; Rossi-Bergmann, B. Selective effect of 2’,6’-dihydroxy-4’methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MTT assay. J. Immunol. Method 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial potential of tropical rainforest plant extracts. Medicines 2014, 1, 32–55. [Google Scholar] [CrossRef]
- Buffet, P.A.; Sulahian, A.; Garin, Y.J.F.; Nassar, N.; Derouin, F. Culture microtitration a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995, 39, 2167–2168. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Bothiraja, C.; Shaikh, K.; Mali, A. An insight into cochleates, a potential drug delivery system. RSC Adv. 2015, 5, 81188–81202. [Google Scholar] [CrossRef]
- Ravi, K.M.N. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Manandhar, K.D.; Yadav, T.P.; Prajapati, V.K. Antileishmanial activity of nano-amphotericin B deoxycholate. J. Antimicrob. Chemother. 2008, 62, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.G.; Ribeiro, R.R.; da Silva, S.M.; Ferreira, C.S.; de Souza, L.E.; Ferreira, A.A.; de Oliveira, E.; Castro, R.A.; Demicheli, C.; Rezende, S.A.; et al. Mixed formulation of conventional and pegylated liposomes as a novel drug delivery strategy for improved treatment of visceral leishmaniasis. Expert Opin. Drug Deliv. 2014, 11, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Mattos, C.B.; Argenta, D.F.; Melchiades, G.L.; Cordeiro, M.N.S.; Tonini, M.L.; Moraes, M.H.; Weber, T.B.; Roman, S.; Nunes, R.J.; Teixeira, H.F.; et al. Nanoemulsions containing a synthetic chalcone as an alternative for treating cutaneous leshmaniasis: Optimization using a full factorial design. Int. J. Nanomed. 2015, 10, 5529–5542. [Google Scholar]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.W.; Mannino, R.; Zarif, L.; Perlin, D.S. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Sesana, A.M.; Monti-Rocha, R.; Vinhas, S.A.; Morais, C.G.; Dietze, R.; Lemos, E.M. In vitro activity of amphotericin B cochleates against Leishmania chagasi. Mem. Inst. Oswaldo Cruz 2011, 106, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Bracho, G.; Lastre, M.; Mora, N.; Del Campo, J.; Gil, D.; Zayas, C.; Acevedo, R.; González, D.; López, JA.; et al. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol. Cell Biol. 2004, 82, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zarif, L. Drug delivery by lipid cochleates. Methods Enzymol. 2005, 391, 314–329. [Google Scholar] [PubMed]
- Tamargo, B.; Sierra, V.G.; Fleitas, C.; Infante, J.F.; Ramírez, W.; Marquéz, Y.; Pérez, V.; Torralba, D.; García, L.; Burg, V.; et al. Evaluación inmunotoxicológica, de nuevas formulaciones adyuvantes nanoparticulados sin Al(OH)3, ensayadas como candidatos vacunales contra Neisseria meningitidis. VacciMonitor 2011, 20, 10. [Google Scholar]
- Rodríguez-Hernández, D.; Barbosa, L.C.; Demuner, A.J.; de Almeida, R.M.; Fujiwara, R.T.; Ferreira, S.R. Highly potent anti-leishmanial derivatives of hederagenin, a triperpenoid from Sapindus saponaria L. Eur. J. Med. Chem. 2016, 124, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Etel, S.; Borborema, T. Uptake antileishmanial activity of meglumine antomoniate-containing liposomes in Leishmania (Leishmania) major- infect macrofages. Int. J. Antimicrob. Agents 2011, 38, 341–347. [Google Scholar]
- Rafiee, A.; Riazi-rad, F.; Darabi, H.; Khaze, V.; Javadian, S.; Ajdary, S.; Bahrami, F.; Alimohammadian, A.H. Ferroportin-encapsulated nanoparticles reduce infection and improve immunity in mice infected with Leishmania major. Int. J. Pharm. 2014, 466, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Darole, P.S.; Hegde, D.D.; Nair, H.A. Formulation and evaluation of microemulsion based delivery system for amphotericin B. AAPS Pharm. Sci. Tech. 2008, 9, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jogani, V.; Jinturkar, K.; Vyas, T.; Misra, A. Recent patents review on intranasal administration for CNS drug delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 25–40. [Google Scholar] [PubMed]
- Salmanpour, R.; Razmavar, M.R.; Abtahi, N. Comparison of intralesional meglumine antimoniate, cryotherapy and their combination in the treatment of cutaneous leishmaniasis. Int. J. Dermatol. 2006, 45, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Anwar, A.E. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: A comparative study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 335–340. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, M.D.; da Silva, A.C.; Citó, A.M.; Borges, A.R.; de Lima, S.G.; Lopes, J.A.; Figueiredo, R.C. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Pastor, J.; Scull, R.; Gille, L. Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine 2014, 21, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Fournet, A.; Ferreira, M.E.; Rojas de Arias, A.; Torres de Ortiz, S.; Fuentes, S.; Nakayama, H. In vivo efficacy of oral and intralesional administration of 2-substituted quinolines in experimental treatment of New World cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob. Agents Chemother. 1996, 40, 2447–2451. [Google Scholar] [PubMed]
- Da Silva, S.A.G.; Costa, S.S.; Mendoça, S.C.F.; Silva, E.M.; Moraes, V.L.G.; Rossi-Bergmann, B. Therapeutic effect of oral Kalanchoe pinnata leaf extract in murine leishmaniasis. Acta Trop. 1995, 60, 201–210. [Google Scholar] [CrossRef]
- Monzote, L.; García, M.; Montalvo, A.M.; Linares, R.; Scull, R. Effect of oral treatment with the essential oil from Chenopodium ambrosioides against cutaneous leishmaniasis in BALB/c mice, caused by Leishmania amazonensis. Res. Complement. 2009, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Gould-Fogerite, S.; Kheiri, M.T.; Zhang, F.; Wang, Z.; Scolpino, A.J.; Feketeova, E; Canki, M.; Mannino, R.J. Targeting immune response induction with cochleate and liposome-based vaccines. Adv. Drug Deliv. Rev. 1998, 32, 273–287. [Google Scholar] [CrossRef]
- Gould-Fogerite, S.; Mannino, R.J. Mucosal and Systemic Immunization using Cochleate and Liposome Vaccine. J. Liposome Res. 1996, 6, 357–379. [Google Scholar] [CrossRef]
- Sacks, D.L.; Lal, S.L.; Shrivastava, S.N.; Blackwell, J.; Neva, F.A. An analysis of T-cell responsiveness in Indian kala-azar. J. Immunol. 1987, 138, 908–913. [Google Scholar] [PubMed]
| Formulation | Size (nm) ± SD | Polydispersity Index ± SD | Z Potential (ζ) (mV) ± SD |
|---|---|---|---|
| EO-Aa-NC | 74.2 ± 21.9 | 0.33 ± 0.005 | −40.8 ± 0.4 |
| NC | 32.1 ± 7.4 | 0.48 ± 0.04 | −31.2 ± 0.08 |
| Essential Oil from A. absinthium | IC50 a ± SD b (μg/mL) | |
|---|---|---|
| Leishmania amazonensis | Peritoneal Macrophages | |
| EO-Aa | 13.4 ± 2.4 | 75.1 ± 2.3 |
| EO-Aa-NC | 21.5 ± 2.5 * | 27.7 ± 5.6 * |
| NC | c | d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamargo, B.; Monzote, L.; Piñón, A.; Machín, L.; García, M.; Scull, R.; Setzer, W.N. In Vitro and In Vivo Evaluation of Essential Oil from Artemisia absinthium L. Formulated in Nanocochleates against Cutaneous Leishmaniasis. Medicines 2017, 4, 38. https://doi.org/10.3390/medicines4020038
Tamargo B, Monzote L, Piñón A, Machín L, García M, Scull R, Setzer WN. In Vitro and In Vivo Evaluation of Essential Oil from Artemisia absinthium L. Formulated in Nanocochleates against Cutaneous Leishmaniasis. Medicines. 2017; 4(2):38. https://doi.org/10.3390/medicines4020038
Chicago/Turabian StyleTamargo, Beatriz, Lianet Monzote, Abel Piñón, Laura Machín, Marley García, Ramón Scull, and William N. Setzer. 2017. "In Vitro and In Vivo Evaluation of Essential Oil from Artemisia absinthium L. Formulated in Nanocochleates against Cutaneous Leishmaniasis" Medicines 4, no. 2: 38. https://doi.org/10.3390/medicines4020038