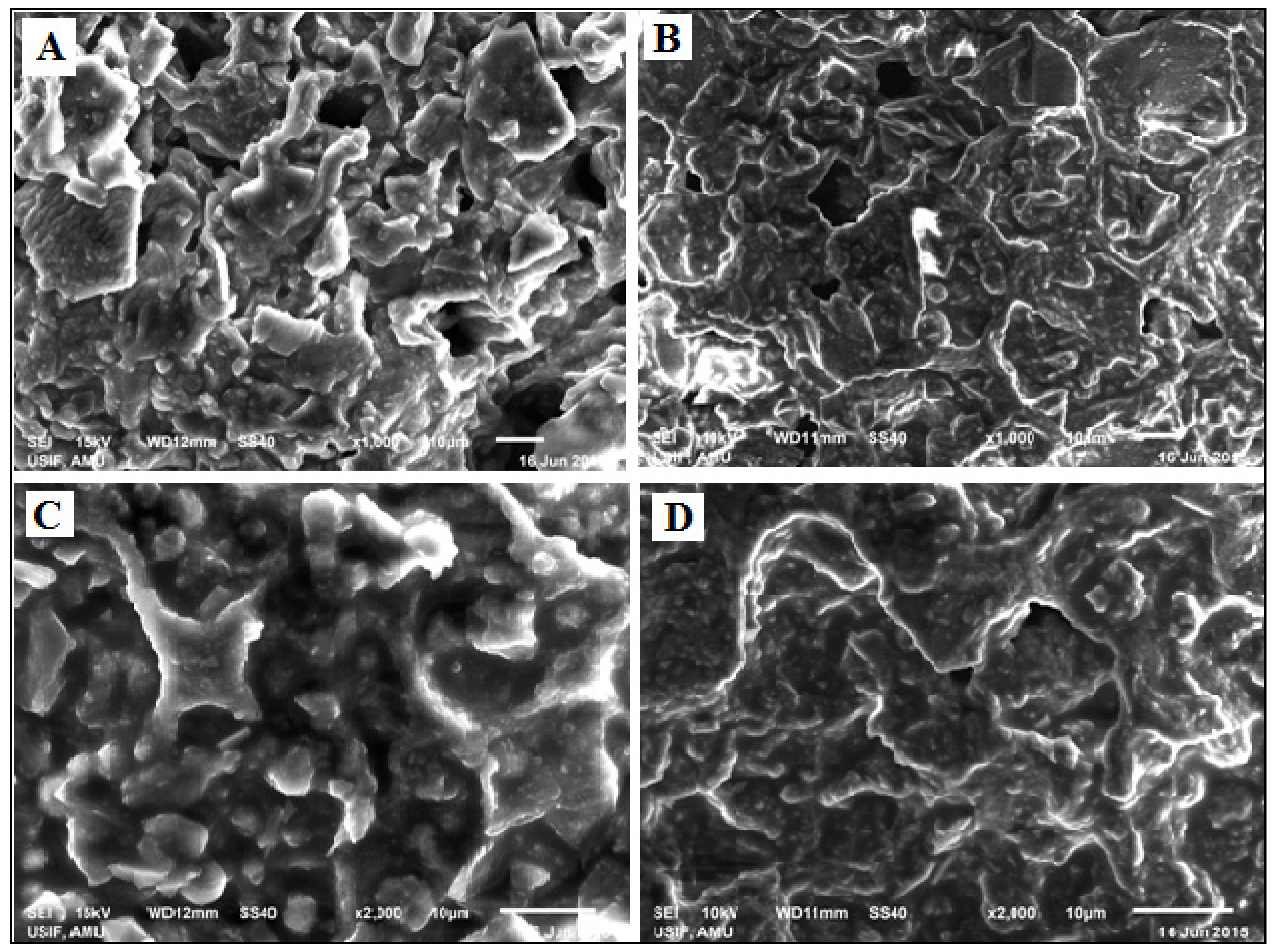
Figure 1.
SEM images of T. ammi L. seed biomass: (A) before SC-CO2, (B) after SC-CO2 at 1000× magnification, and (C) before SC-CO2, (D) after SC-CO2 at 2000× magnification.
Figure 1.
SEM images of T. ammi L. seed biomass: (A) before SC-CO2, (B) after SC-CO2 at 1000× magnification, and (C) before SC-CO2, (D) after SC-CO2 at 2000× magnification.
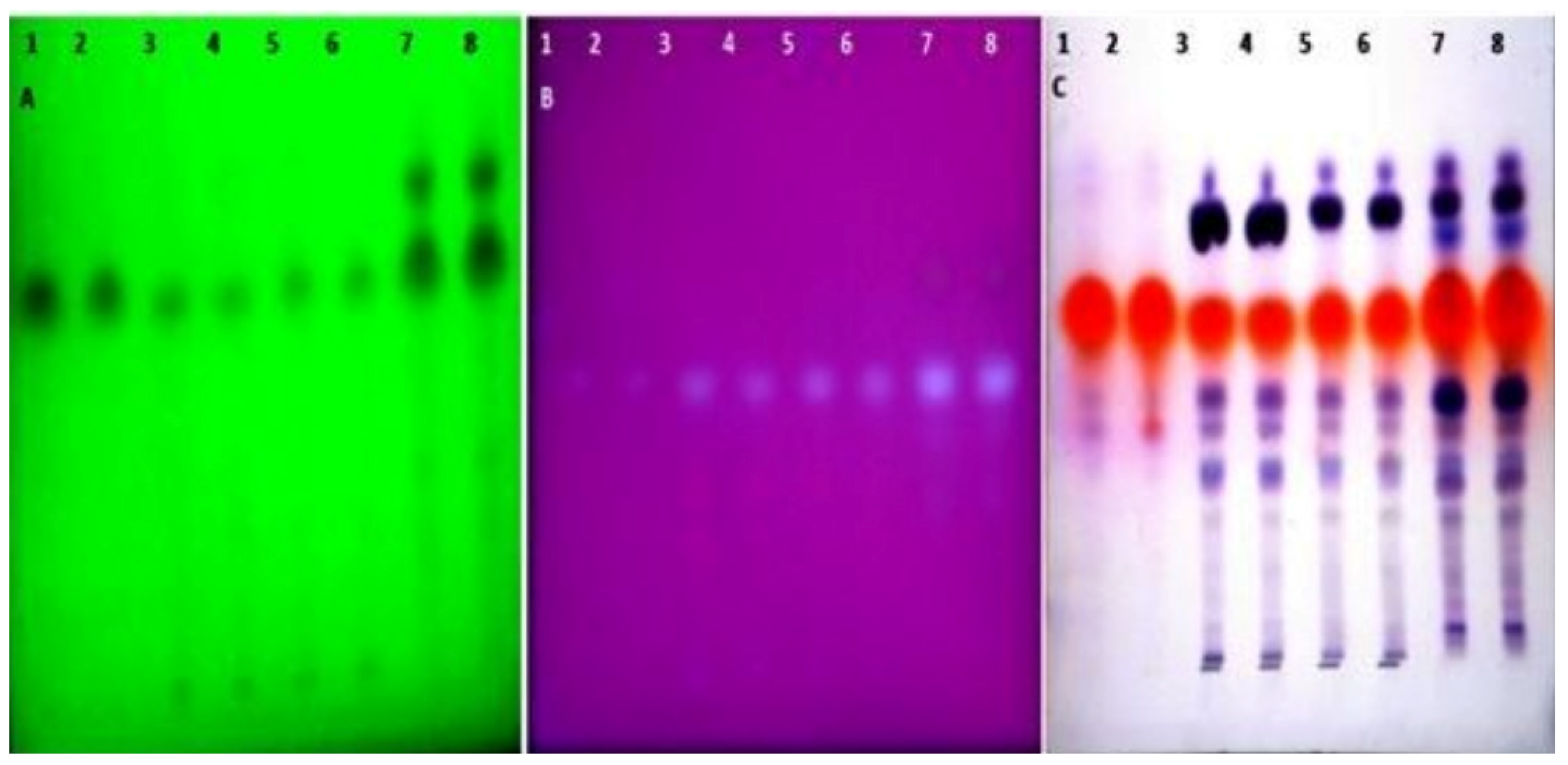
Figure 2.
HPTLC fingerprint of different essential oils of seeds of T. ammi L. extracted using different extraction techniques (track 1–2: Hydrodistilled oil, 3–4: Solvent extracted oil, 5–6: Ultra sonication oil, 7–8: SC-CO2 oil) observed at (A) 254 nm, (B) 366 nm, (C) In daylight after derivatization by using the anisaldehyde sulphuric acid reagent.
Figure 2.
HPTLC fingerprint of different essential oils of seeds of T. ammi L. extracted using different extraction techniques (track 1–2: Hydrodistilled oil, 3–4: Solvent extracted oil, 5–6: Ultra sonication oil, 7–8: SC-CO2 oil) observed at (A) 254 nm, (B) 366 nm, (C) In daylight after derivatization by using the anisaldehyde sulphuric acid reagent.
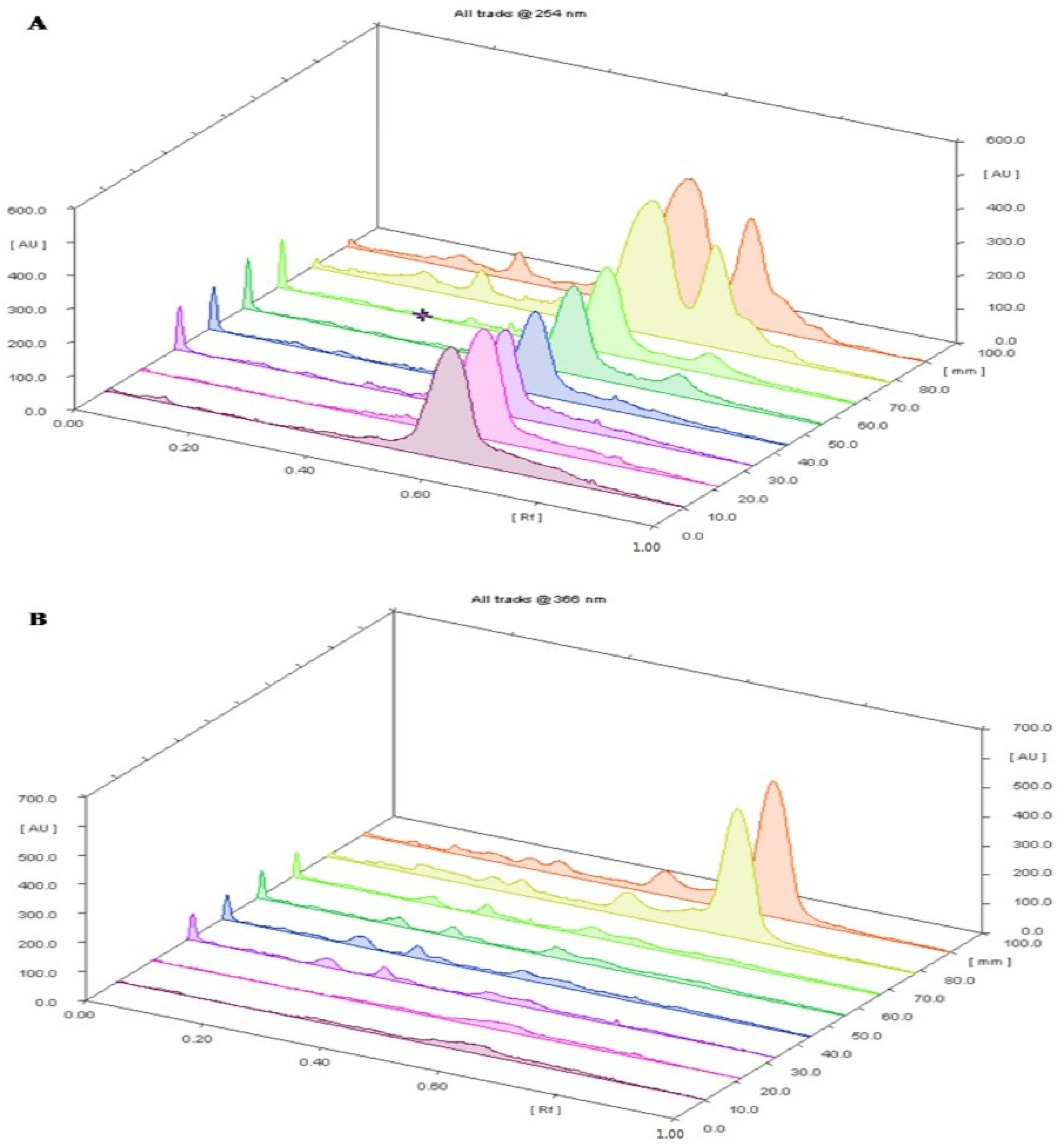
Figure 3.
(A) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at A: 254 nm; (B) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at B: 366 nm; (C) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at B: 580 nm.
Figure 3.
(A) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at A: 254 nm; (B) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at B: 366 nm; (C) 3D Chromatogram of 8 tracks of T. ammi L. oil obtained by different extraction techniques at B: 580 nm.
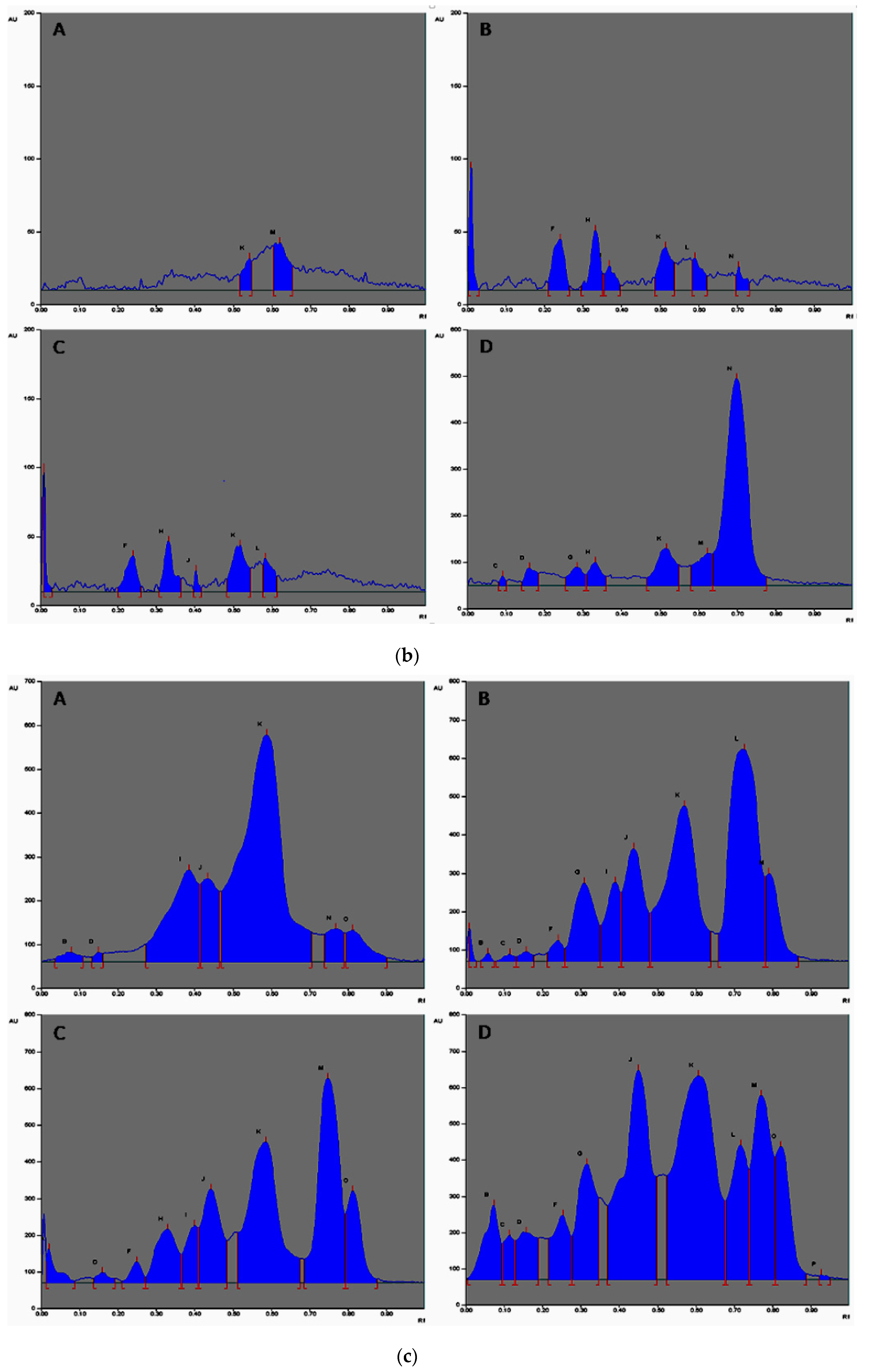
Figure 4.
(a) HPTLC fingerprint of various essential oils of seeds of T. ammi L. extracted via diverse extraction methods. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonicated oil, D: SC-CO2 oil observed at 254 nm with assigned substance; (b) HPTLC fingerprint of various essential oils of seeds of T. ammi L. extracted using different extraction methods. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonicated oil, D: SC-CO2 oil observed at 366 nm with assigned substance; (c) HPTLC chromatograms of different oils of T. ammi L. Extracted using different extraction techniques. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonication oil, D: SC-CO2 oil visualized at 580 nm with assigned substance.
Figure 4.
(a) HPTLC fingerprint of various essential oils of seeds of T. ammi L. extracted via diverse extraction methods. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonicated oil, D: SC-CO2 oil observed at 254 nm with assigned substance; (b) HPTLC fingerprint of various essential oils of seeds of T. ammi L. extracted using different extraction methods. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonicated oil, D: SC-CO2 oil observed at 366 nm with assigned substance; (c) HPTLC chromatograms of different oils of T. ammi L. Extracted using different extraction techniques. A: Hydrodistilled oil, B: Solvent extracted oil, C: Ultra sonication oil, D: SC-CO2 oil visualized at 580 nm with assigned substance.
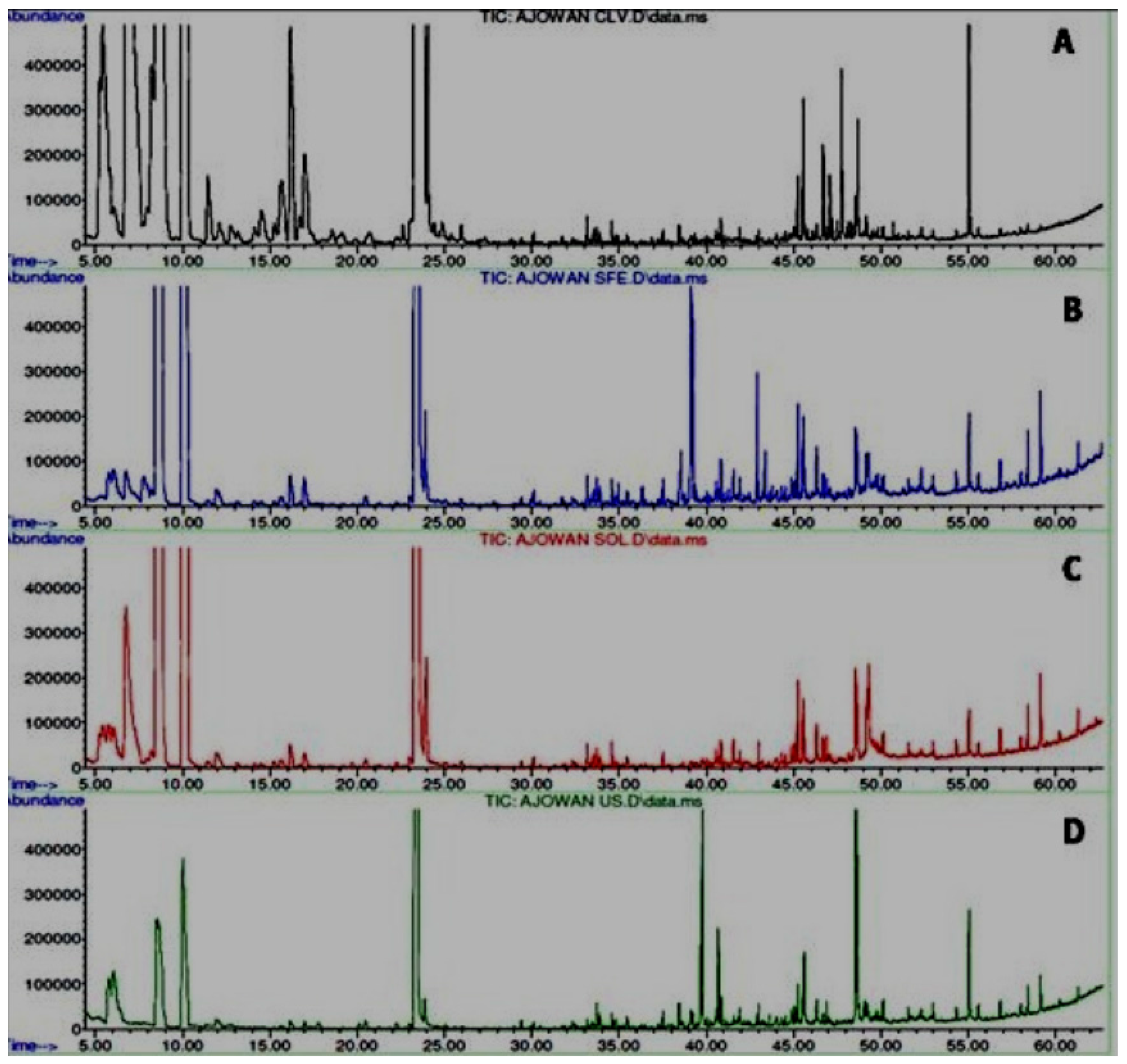
Figure 5.
Comparative GC-MS chromatograms of different oils of T. ammi L. extracted using different extraction techniques. (A) Hydrodistilled oil, (B) Solvent extracted oil, (C) Ultrasonicated oil, (D) SC-CO2 oil.
Figure 5.
Comparative GC-MS chromatograms of different oils of T. ammi L. extracted using different extraction techniques. (A) Hydrodistilled oil, (B) Solvent extracted oil, (C) Ultrasonicated oil, (D) SC-CO2 oil.
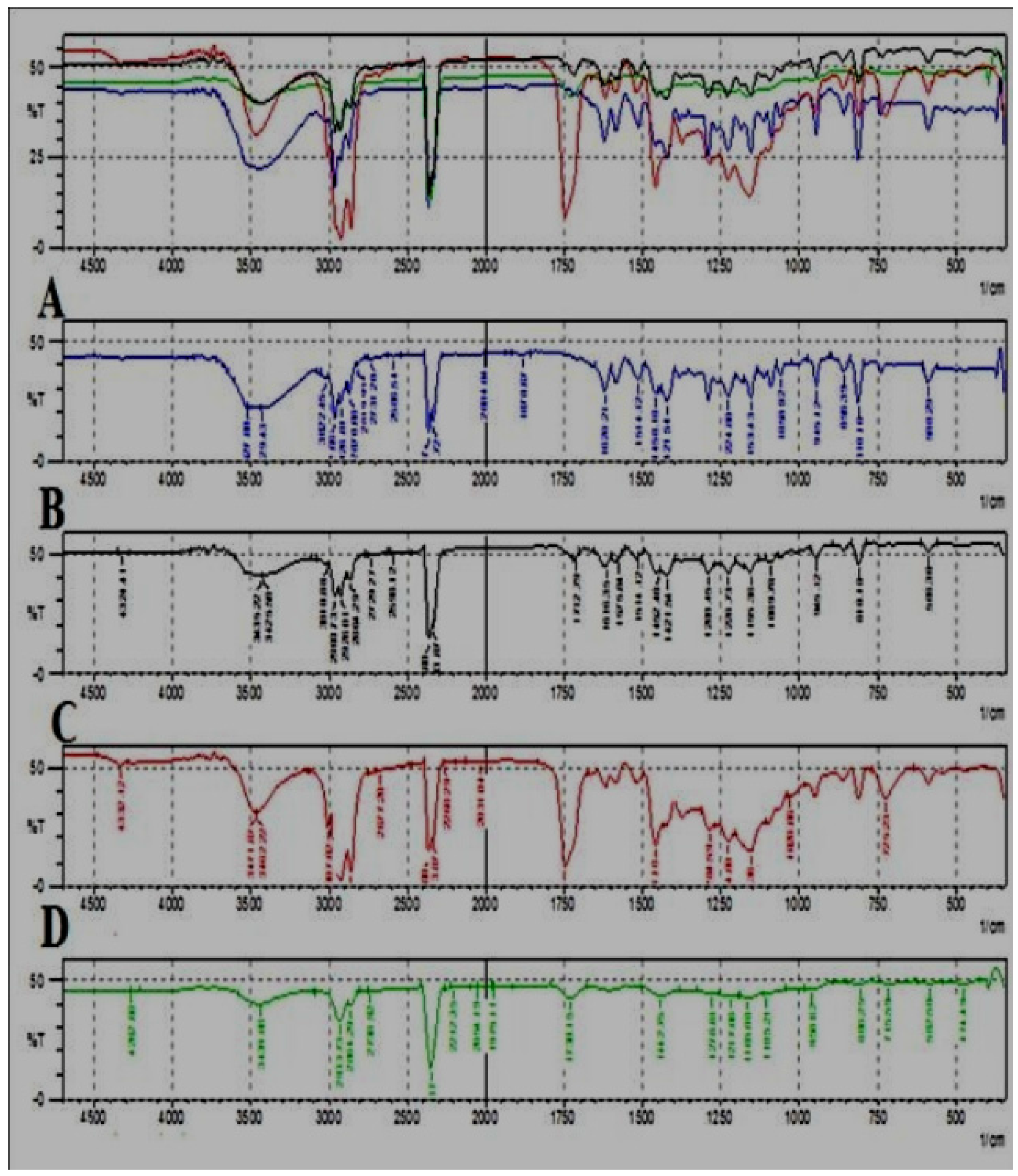
Figure 6.
Comparative FTIR spectra of different oils of T. ammi L. extracted using different extraction techniques. (A) Hydrodistilled oil, (B) Solvent extracted oil, (C) SC-CO2 oil, (D) Ultrasonicated oil.
Figure 6.
Comparative FTIR spectra of different oils of T. ammi L. extracted using different extraction techniques. (A) Hydrodistilled oil, (B) Solvent extracted oil, (C) SC-CO2 oil, (D) Ultrasonicated oil.
Figure 7.
Comparative dose response curve between percent inhibitions against log concentration by the DPPH method.
Figure 7.
Comparative dose response curve between percent inhibitions against log concentration by the DPPH method.
Figure 8.
Comparative dose response curve between percent inhibitions against log concentration by the superoxide scavenging method.
Figure 8.
Comparative dose response curve between percent inhibitions against log concentration by the superoxide scavenging method.
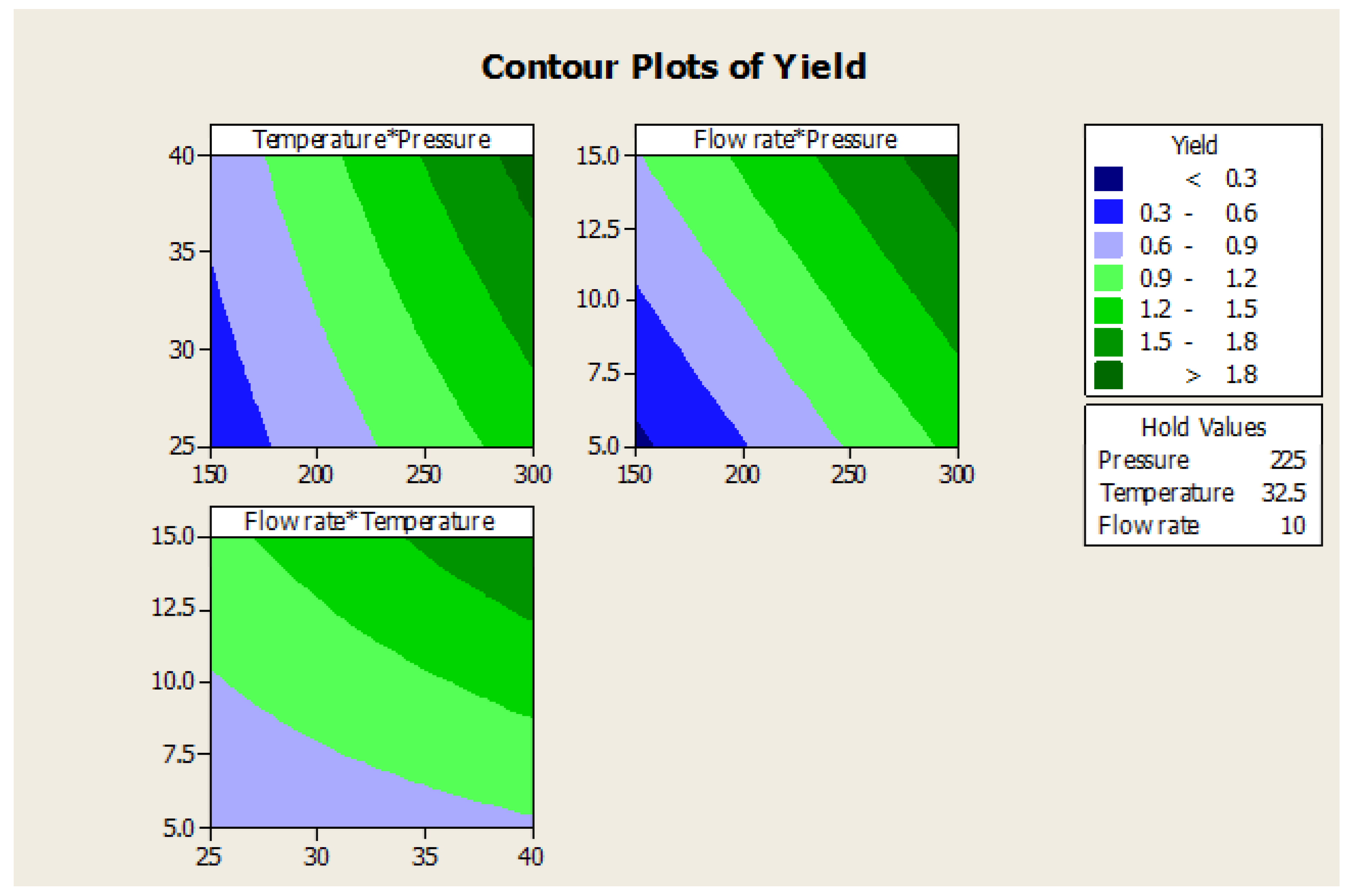
Figure 9.
Contour plots of yield vs. pressure, temperature, and flow rate.
Figure 9.
Contour plots of yield vs. pressure, temperature, and flow rate.
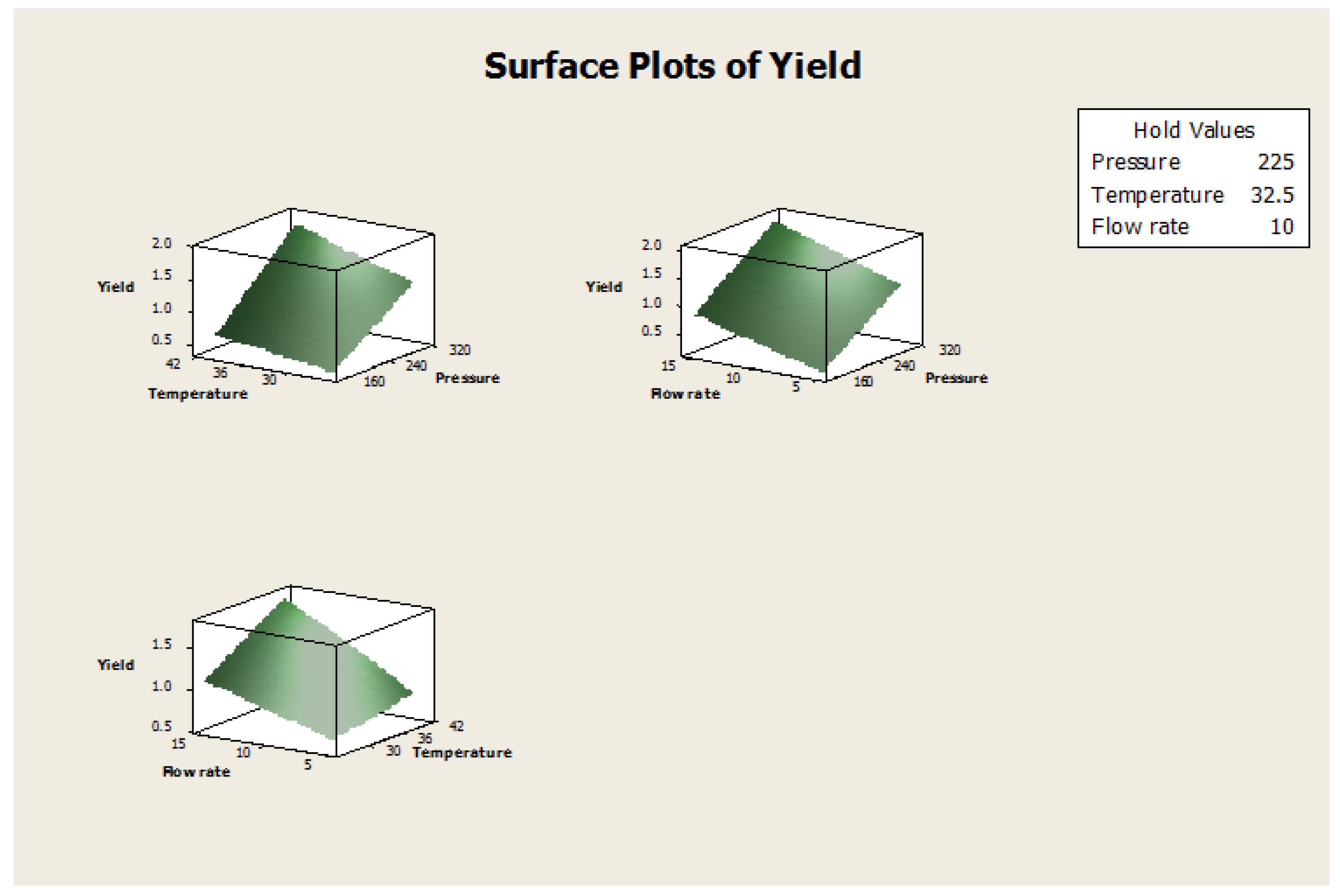
Figure 10.
Surface plots of yield vs. pressure, temperature, and flow rate.
Figure 10.
Surface plots of yield vs. pressure, temperature, and flow rate.
Table 1.
Parameters and levels used in the experimental design.
Table 1.
Parameters and levels used in the experimental design.
| Level | Factors |
|---|
| Pressure (bar) | Temperature (°C) | CO2 Flow Rate (g/min) |
|---|
| 1 | 150 | 25 | 5 |
| 2 | 175 | 30 | 10 |
| 3 | 300 | 40 | 15 |
Table 2.
Solvent system tried for separation of the phytoconstituents of essential oil.
Table 2.
Solvent system tried for separation of the phytoconstituents of essential oil.
| Serial No | Solvent System | Ratio (v/v/v) | Observation |
|---|
| 1 | Hexane:dichloromethane | 95:5 | No separation |
| 2 | Toulene:diisopropyl ether:ethyl acetate | 80:10:10 | No separation |
| 3 | Toulene:diisopropyl ether | 95:5 | No separation |
| 4 | Petroleum ether:ethylacetate:formic acid | 95:5:1 | No separation |
| 5 | Cyclohexane:ethylacetate | 90:10 | No separation |
| 6 | Chloroform:methanol:formic acid | 90:2:1 | Little separation |
| 7 | Chloroform:methanol:formic acid | 90:3:1 | Little separation |
| 8 | Chloroform:methanol:formic acid | 90:1:1 | Little separation |
| 9 | Toulene:methanol:formic acid | 90:7.5:1 | Little separation |
| 10 | Toulene:methanol:formic acid | 90:6:1 | Little separation |
| 11 | Toulene:chloroform:formic acid | 90:2:1 | Little separation |
| 12 | Toulene:acetone | 95:5 | No separation |
| 13 | Toulene:ethyl acetate | 97:5 | Fair |
| 14 | Toulene:ethyl acetate | 97:3 | Fair |
| 15 | Toulene:ethyl acetate:formic acid | 95:5:1 | Fair |
| 16 | Hexane:ethyl acetate:formic acid | 90:1:1 | Good separation |
| 17 | Hexane:ethyl acetate:formic acid | 70:30:1 | Good separation |
| 18 | Hexane:ethyl acetate:formic acid | 70:20:1 | Best separation |
Table 3.
Substance A–Q with their Rf and area percentage of T. ammi essential oil obtained by different extraction techniques.
Table 3.
Substance A–Q with their Rf and area percentage of T. ammi essential oil obtained by different extraction techniques.
| Substance (Rf) | Area Percentage (%) 254 nm | Area Percentage (%) 366 nm |
|---|
| HD | SE | US | SC-CO2 | HD | SE | US | SC-CO2 |
|---|
| A (0.01) | - | 6.57 | 3.55 | 0.44 | - | - | - | - |
| B (0.01) | - | - | - | - | - | 14.93 | 11.36 | - |
| C (0.09) | 1.17 | - | - | 0.66 | - | - | - | 0.78 |
| D (0.15) | - | - | - | - | | - | - | 3.01 |
| E (0.2) | - | - | - | 4.52 | - | - | - | - |
| F (0.24) | - | 1.74 | - | - | - | 20.90 | 18.16 | - |
| G (0.3) | - | - | - | 4.86 | - | - | - | 3.93 |
| H (0.35) | - | 1.88 | 2.00 | - | - | 18.96 | 21.78 | 4.45 |
| I (0.37) | - | - | - | 1.30 | - | 8.11 | - | - |
| J (0.41) | - | - | 1.35 | - | - | | 3.25 | - |
| K (0.44) | - | 0.98 | - | 3.20 | 28.37 | 19.60 | 30.43 | 10.82 |
| L (0.46) | 3.50 | - | 5.09 | - | - | 11.14 | 15.02 | - |
| M (0.58) | 79.34 | 73.80 | 73.62 | 53.11 | 71.63 | - | - | 8.03 |
| N (0.66) | 8.49 | 5.19 | - | - | - | 6.36 | - | 68.99 |
| O (0.71) | - | 3.74 | - | 29.99 | - | - | - | - |
| P (0.76) | 5.31 | 6.10 | 14.39 | - | - | - | - | - |
| Q (0.83) | 2.20 | - | - | 1.91 | - | | - | - |
Table 4.
Substance A–P with their Rf and area percentage of T. ammi essential oil obtained by different extraction techniques.
Table 4.
Substance A–P with their Rf and area percentage of T. ammi essential oil obtained by different extraction techniques.
| Substance (Rf) | Area Percentage (%) 580 nm |
|---|
| HD | SE | US | SC-CO2 |
|---|
| A (0.02) | - | 0.59 | 2.21 | - |
| B (0.06) | 1.20 | 0.28 | - | 4.12 |
| C (0.11) | - | 0.41 | - | 1.66 |
| D (0.16) | 0.52 | 0.66 | 0.92 | 3.33 |
| E (0.18) | 0.82 | - | - | - |
| F (0.25) | - | 1.43 | 1.64 | 3.99 |
| G (0.31) | - | 9.29 | - | 7.98 |
| H (0.33) | - | - | 8.03 | - |
| I (0.39) | 18.80 | 6.42 | 4.88 | - |
| J (0.44) | 9.25 | 12.36 | 12.59 | 21.41 |
| K (0.59) | 61.94 | 27.17 | 30.34 | 28.94 |
| L (0.72) | - | 34.46 | - | 8.68 |
| M (0.76) | - | - | 30.23 | 12.75 |
| N (0.78) | 3.76 | 6.92 | - | - |
| O (0.82) | 4.52 | - | 9.14 | 7.02 |
| P (0.92) | - | - | - | 0.13 |
Table 5.
Results of the GC-MS analysis of Tachyspermum ammi oil extracted by different techniques.
Table 5.
Results of the GC-MS analysis of Tachyspermum ammi oil extracted by different techniques.
| Serial No | Component Name | Area Percent (%) 580 nm |
|---|
| RI | HD | SE | US | SC-CO2 |
|---|
| Monoterpene Hydrocarbon |
| 1 | 3-Thujene | 9052 | 1.20 | 0.51 | - | - |
| 2 | (-)-β-Pinene | 9110 | - | - | 2.31 | 0.62 |
| 3 | l-Phellandrene | 9745 | - | - | - | 0.41 |
| 4 | α-Terpinene | 9016 | 0.39 | 0.16 | - | - |
| 5 | p-Cymene | 9842 | - | 21.62 | 4.83 | 11.06 |
| 6 | γ-Terpinene | 9915 | 23.62 | 20.90 | 5.07 | 11.56 |
| 7 | α-terpinolene | 9004 | 0.15 | - | - | - |
| 8 | Cis-sabinene hydrate | 1195 | - | - | - | 0.21 |
| Oxygenated Monoterpene |
| 9 | Cymol | 9816 | 32.78 | | | |
| 10 | 4-Thujanol | 9732 | - | - | 0.15 | 0.21 |
| 11 | Terpineol | 9438 | - | | 11.01 | - |
| 12 | Thymol | 9998 | 35.63 | 49.33 | 69.94 | 66.25 |
| 13 | Carvacrol | 9350 | 0.29 | - | - | 0.48 |
| Sesquiterpene Hydrocarbons |
| 14 | Trans-Caryophyllene | 1462 | - | - | - | 0.09 |
| 15 | β-Selinene | 1499 | 0.02 | 0.07 | - | 0.11 |
| 16 | α-selinene | 1404 | 0.01 | - | - | 0.10 |
| 17 | Eudesma-3,11-Diene | 1417 | - | 0.06 | - | - |
| 18 | Tumerone | 1437 | - | - | 2.25 | - |
| 19 | Curlone.α | 1415 | - | - | 1.18 | - |
| Oxygenated Sesquiterpenes Derivatives |
| 20 | γ-Eudesmol | 1468 | - | - | - | 0.29 |
| 21 | Eudesm-4(14)-en-11-ol | 1459 | - | - | - | 1.28 |
| 22 | Dill-Apiol | 1138 | 0.02 | - | 0.32 | - |
| Aromatic compound |
| 23 | Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1s)- | 9056 | 3.54 | - | - | - |
| 24 | Mentha-1,4,8-triene 1,5,8-p-menthatriene | 9015 | 0.04 | - | - | - |
| 25 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl) | 9980 | 0.50 | - | 0.15 | 0.25 |
| | Non isoprenoid | | | | | |
| 26 | Linalyl propionate | 1284 | 0.26 | - | - | 0.26 |
| 27 | Heneicosane | 2039 | 0.01 | - | - | - |
| 28 | Pentadecane | 1469 | - | - | 0.13 | 0.06 |
| 29 | Phenol, 2,4-Bis (1,1-Dimethylethyl | 1380 | 0.01 | - | - | - |
| 30 | Dodecanoic acid, methyl ester | 1276 | - | - | - | 0.09 |
| 31 | Dodecane | 1176 | - | - | - | 0.05 |
| 32 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, [S-(Z)]- | 1449 | - | - | - | 0.07 |
| 33 | Hexadecane | 1578 | 0.01 | 0.03 | 0.12 | 0.09 |
| 34 | n-Heptadecane | 1690 | - | - | 0.08 | 0.15 |
| 35 | 3 n -butyl Phthalide | 1159 | - | - | - | 0.16 |
| 36 | Octadecane | 1785 | 0.01 | 0.03 | 0.27 | - |
| 37 | Pentadecanoic acid, 14-methyl-, methyl ester | 1665 | - | - | 0.85 | - |
| 38 | Heneicosane | 2098 | - | - | 0.42 | 0.10 |
| 39 | Docosane | 2145 | - | 0.11 | - | 0.07 |
| 40 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 1896 | - | 0.39 | 4.27 | 0.53 |
| 41 | Octadecanoic acid, methyl ester | 1884 | - | - | 0.64 | - |
| 42 | 9-Octadecenoic acid | 1786 | - | 0.83 | - | - |
| 43 | Nonadecane | 1890 | 0.03 | 0.05 | 0.06 | 0.17 |
| 44 | Hexatriacontane | 3597 | - | - | 0.78 | 0.14 |
| 45 | Eicosane | 1959 | - | 0.08 | 0.44 | 0.22 |
| 46 | Pentacosane | 2494 | - | 0.09 | - | 0.06 |
| 47 | Tricosane | 2389 | - | - | 0.07 | 0.28 |
| 48 | Butyl pthalate | 1575 | 0.18 | - | 0.91 | 0.37 |
| 49 | Squalene | 2997 | - | 0.08 | 0.17 | - |
Table 6.
Results of the FTIR analysis of T. ammi oil extracted by different techniques.
Table 6.
Results of the FTIR analysis of T. ammi oil extracted by different techniques.
| Serial No | Peak | Functional Group | Intensity | HD | SE | US | SC-CO2 |
|---|
| 1 | 810.1 | =C-H(alkene) | stretch, strong | + | - | - | + |
| 2 | 945.12 | =C-H(alkene) | stretch, strong | + | - | - | + |
| 3 | 1155.36 | C-O(alcohol) | stretch, strong | + | + | - | + |
| 4 | 1224.8 | C-O( aldehyde) | stretch, medium | + | + | - | + |
| 5 | 1284.59 | C-O( aldehyde) | stretch, medium | - | + | - | + |
| 6 | 1458.18 | C=C(aromatic) | stretch, medium | + | + | - | + |
| 7 | 1514.12 | N-O(nitro) | stretch, strong | + | - | - | - |
| 8 | 1616.35 | N-O(nitro) | stretch, strong | + | - | - | + |
| 9 | 2729.27 | =C-H(aldehyde) | stretch, medium | + | + | - | + |
| 10 | 2926.01 | methylene | medium, strong | - | + | - | + |
| 11 | 3007.02 | C-H(aromatic) | stretch, medium | - | - | - | + |
| 12 | 3429.43 | N-H(amine) | stretch, medium | + | - | - | + |
Table 7.
Summary of % inhibition and IC50 values of the T. ammi oil sample by the DPPH method (n = 3).
Table 7.
Summary of % inhibition and IC50 values of the T. ammi oil sample by the DPPH method (n = 3).
| Percentage Inhibition % (Mean ± SD7) |
|---|
| Conc. (µg/mL) | Ascorbic Acid | T. ammi |
|---|
| 100 | 92.5 ± 1.1 | 64.1 ±0.8 |
| 50 | 64.3 ± 0.9 | 56.7 ± 0.8 |
| 25 | 45.7 ± 1.0 | 41.5 ± 0.8 |
| 12.5 | 29.5 ± 0.54 | 34.1 ± 0.8 |
| 6.25 | 20.6 ± 0.8 | 28.9 ± 0.8 |
| 3.125 | 12.1 ± 0.8 | 20.9 ± 4.7 |
| 1.5625 | 8.45 ± 1.0 | 12.9 ± 0.8 |
| 0.781 | 5.85 ± 0.8 | 1.5 ± 0.8 |
| IC50 | 28.09 | 36.41 |
Table 8.
Summary of % inhibition and IC50 values of the T. ammi oil sample by the superoxide anion scavenging method (n = 3).
Table 8.
Summary of % inhibition and IC50 values of the T. ammi oil sample by the superoxide anion scavenging method (n = 3).
| Percentage Inhibition ± SD |
|---|
| Conc. (µg/mL) | Ascorbic Acid | T. ammi |
|---|
| 10 | 38.28 ± 0.8 | 39.18 ± 0.8 |
| 20 | 48.72 ± 1.2 | 43.88 ± 0.8 |
| 40 | 62.33 ± 0.8 | 57.69 ± 0.8 |
| 60 | 71.67 ± 1.2 | 77.03 ± 0.8 |
| 80 | 89.28 ± 1.2 | 85.76 ± 0.8 |
| 100 | 95.22 ± 1.2 | 93.00 ± 0.8 |
| IC50 | 20.14 | 20.55 |
Table 9.
Estimated Effects and Coefficients for the Yield.
Table 9.
Estimated Effects and Coefficients for the Yield.
| Term | Effect | Coef | SE Coef | T | P |
|---|
| Constant | | 1.09433 | 0.05154 | 21.23 | 0.000 |
| Pressure | 1.07339 | 0.53670 | 0.05318 | 10.09 | 0.000 |
| Temperature | 0.42795 | 0.21397 | 0.05414 | 3.95 | 0.000 |
| Flow rate | 0.67875 | 0.33937 | 0.05446 | 6.23 | 0.000 |
| Pressure×Temperature | 0.15887 | 0.07943 | 0.05431 | 1.46 | 0.155 |
| Pressure×Flow rate | 0.04000 | 0.02000 | 0.05446 | 0.37 | 0.716 |
| Temperature×Flow rate | 0.21875 | 0.10938 | 0.05446 | 2.01 | 0.054 |
| Pressure×Temperature×Flow rate | −0.00250 | −0.00125 | 0.05446 | −0.02 | 0.982 |