Comparative Evaluation of Oxidative Stress Modulating and DNA Protective Activities of Aqueous and Methanolic Extracts of Acacia catechu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Determination of Phenolic Content
2.3. Determination of Flavonoid Content
2.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. 2,2′-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS•+) Radical Scavenging Assay
2.7. Superoxide Radical Scavenging Assay
2.8. Inhibition of Lipid Peroxidation
2.9. DNA Protection Assay
2.10. Statistical Analysis
3. Results
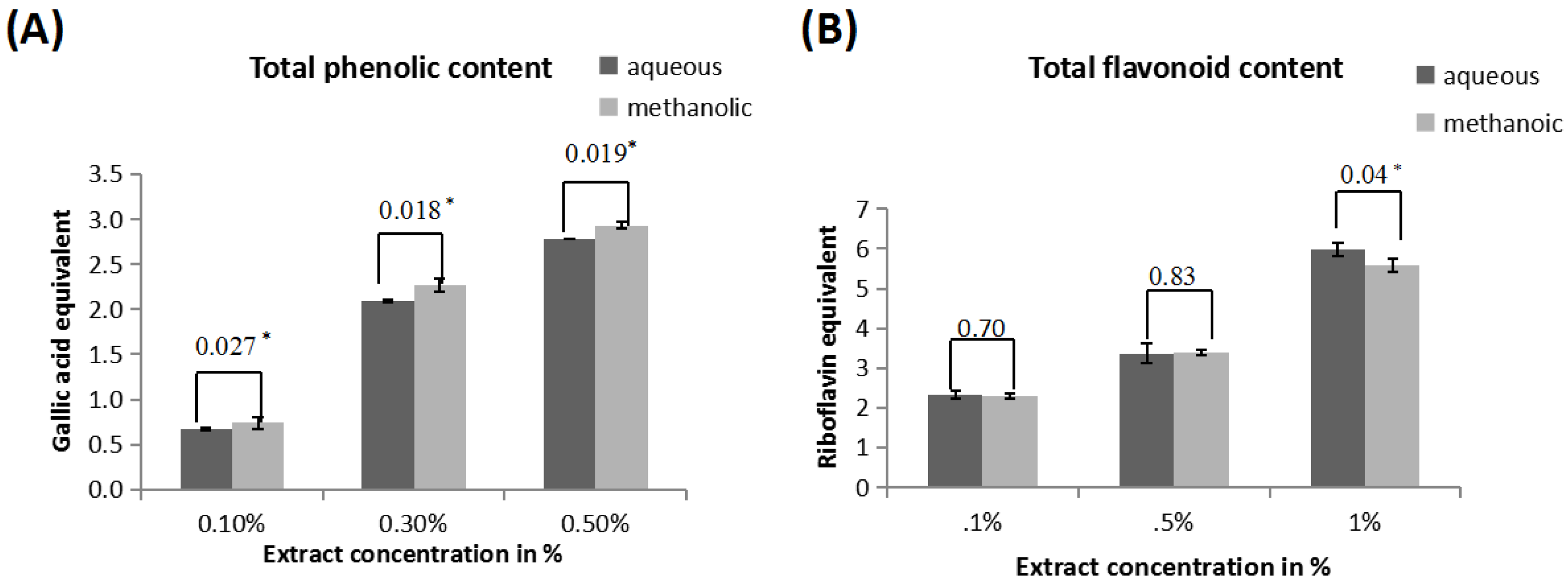
3.1. Determination of Phenolic Content
3.2. Determination of Flavonoid Content
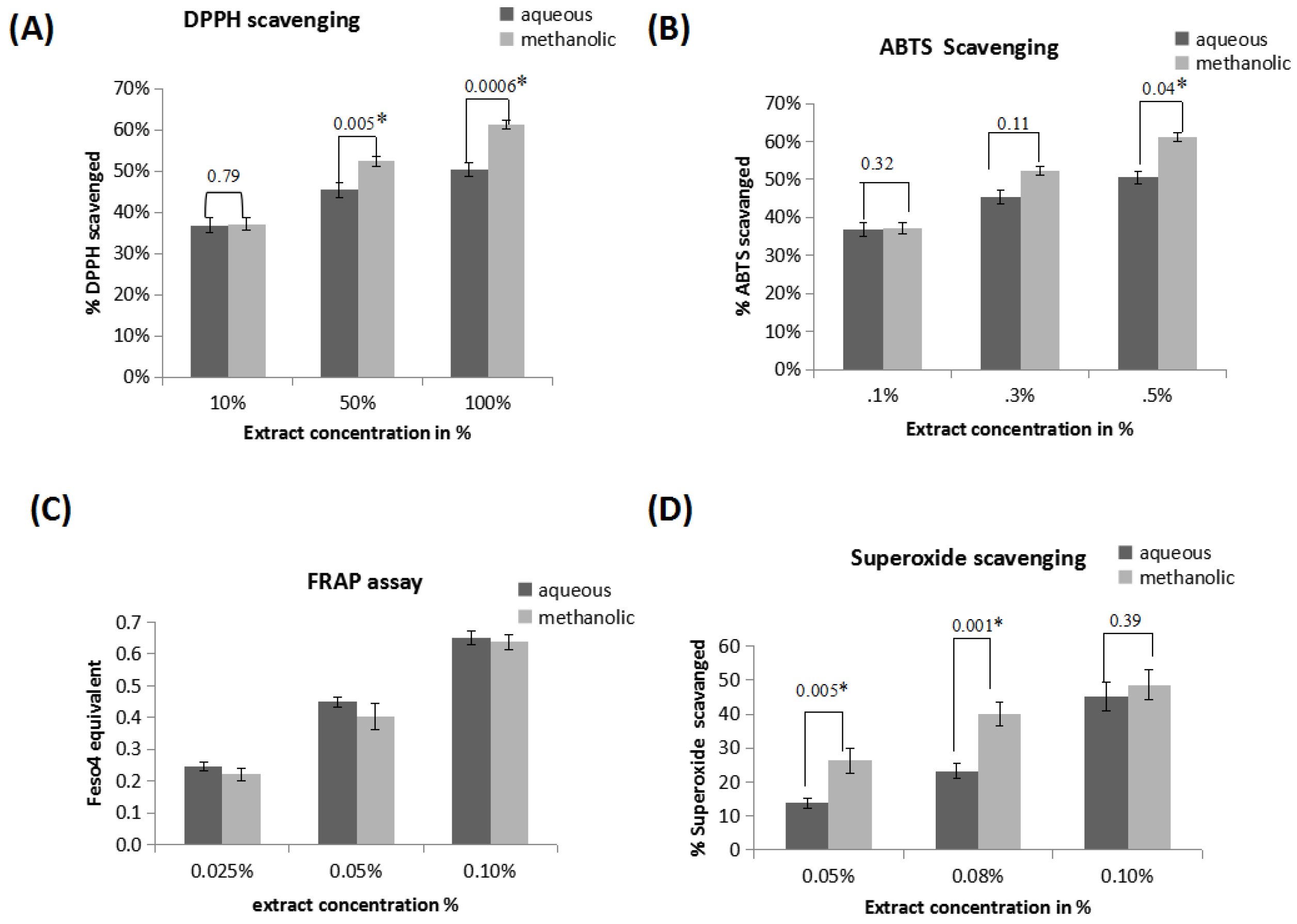
3.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
3.4. 2,2′-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS•+) Radical Scavenging Assay
3.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Superoxide Radical Scavenging Assay
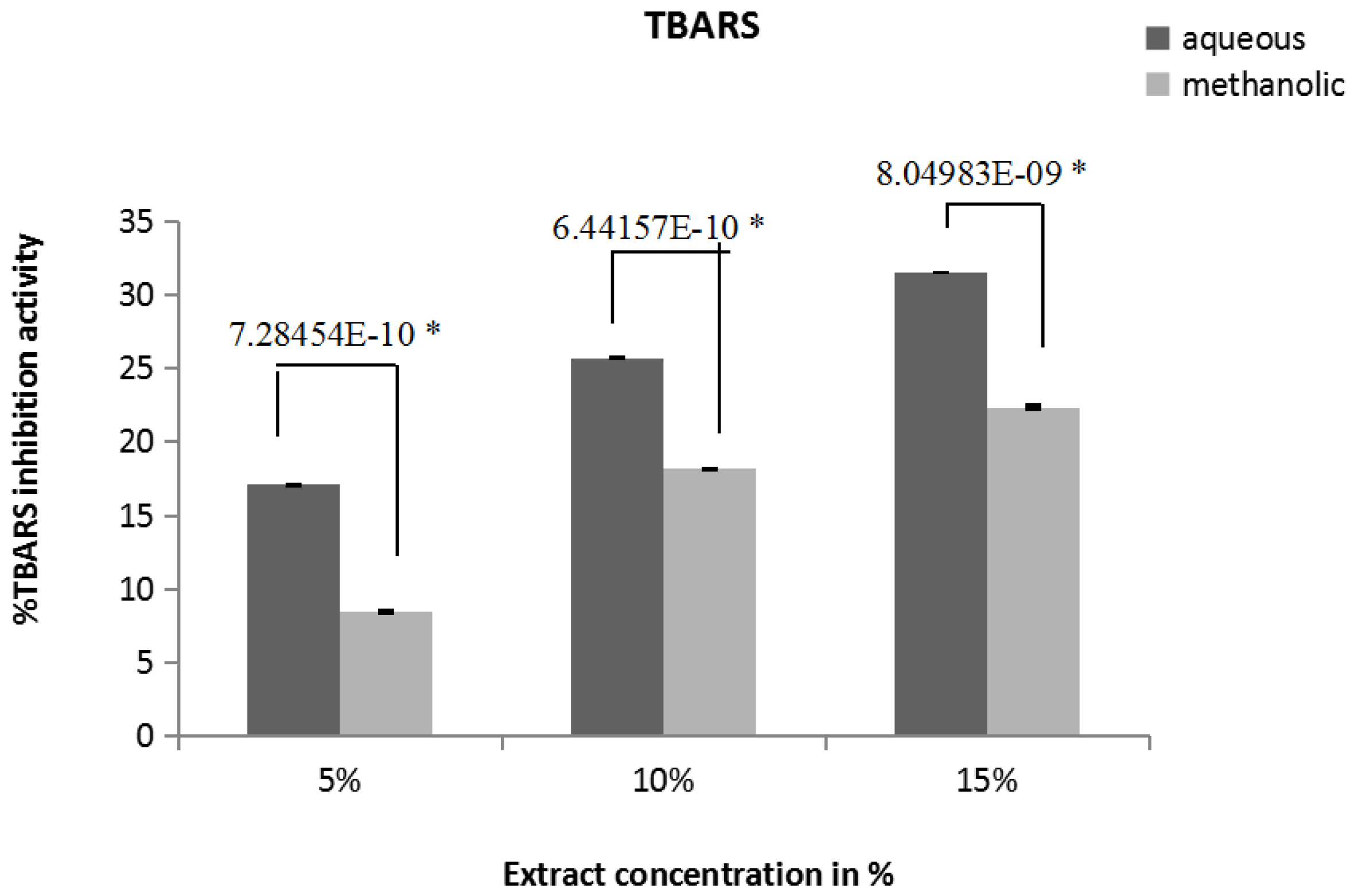
3.7. Inhibition of Lipid Peroxidation
3.8. DNA Damage—Protection Assay
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trigui, M.; Ben Hsouna, A.; Tounsi, S.; Jaoua, S. Efficacy of Lawsoniainermis leaves extract and its phenolic compounds against olive knot and crown gall diseases. Ind. Crops Prod. 2013, 45, 83–88. [Google Scholar] [CrossRef]
- Arya, A.; Mahmood, A.A.; Batoul, S.H.; Mustafa, A.M. Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: In vivo evaluation. Eur. J. Chem. 2012, 9, 1196–1205. [Google Scholar]
- Liang, X.; Fan, Q. Application of Sub-Critical Water Extraction in Pharmaceutical Industry. J. Mater. Sci. Chem. Eng. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, Oxidative Stress, and Antioxidants: A Review. J. Biochem. Mol. Toxicol. 2003, 17, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Antioxidant & DNA Protective Property of Acacia catechu. J. Complement. Integr. Med. 2010, 7, 1. [Google Scholar]
- Babalola, O.O.; Anetor, J.I.; Adeniyi, F.A.A. Amelioration of carbon tetrachloride induced hepatotoxicity by terpenoid extract from leaves of Vernoniaamygdalina. Afr. J. Med. Sci. 2001, 30, 91–93. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Anticancer and antioxidant activities of Malaysian young ginger (zingiberofficinale Roscoe) varieties grown under different CO2 concentration. J. Med. Plant Res. 2011, 5, 3247–3255. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.; Domina, N.G.; Kirpotina, L.N.; Quinn, M.T. Improved Quantitative Structure-Activity Relationship Models to Predict Antioxidant Activity of Flavonoids in Chemical, Enzymatic and Cellular Systems. Bioorg. Med. Chem. 2007, 15, 1749. [Google Scholar] [CrossRef] [PubMed]
- Wadood, N.; Nisar, M.; Rashid, A.; Wadood, A.; Gul-Nawab; Khan, A. Effect of a compound recipe (medicinal plants) on serum insulin levels of alloxan induced diabetic Rabbits. J. Ayub. Med. Coll. Abbottabad 2007, 19, 32–38. [Google Scholar] [PubMed]
- Hye, M.A.; Taher, M.A.; Ali, M.Y.; Ali, M.U.; Zaman, S. Isolation of (+)-Catechin from Acacia catechu (Cutch Tree) by a Convenient Method. J. Sci. Res. 2009, 1, 300–305. [Google Scholar]
- Patel, J.D.; Kumar, V.; Bhatt, S.A. Antimicrobial screening and phytochemical analysis of the resin part of Acacia catechu. Pharm. Biol. 2009, 47, 34–37. [Google Scholar] [CrossRef]
- Ray, D.; Sharatchandra, K.H.; Thokchom, I.S. Antipyretic, antidiarrhoel, hypoglycemic and hepatoprotective activities oe ethyl acetate extract of Acacia catechu wild. In albino rats. Indian J. Pharmacol. 2006, 38, 408–413. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmaniaparasitised RAW 264.7 cells. Phytochemistry 2005, 66, 2056–2071. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yang, M.; Wen, H.; Chem, J. Estimation of flavonoid total content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Aquino, R.; Morelli, S.; Lauro, M.R.; Abdo, S.; Saija, A.; Tomaino, A. Phenolic constituents and antioxidant activity of an extract of Anthuriumversicolor leaves. J. Natl. Prod. 2001, 64, 1019–1023. [Google Scholar] [CrossRef]
- Alzoreky, N.; Nakahara, K. Antioxidant activity of some edible Yemeni plants evaluated by ferrylmyoglobin/ABTS•+ assay. Food Sci. Technol. Res. 2001, 7, 141–144. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondiaspinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Zwilling, E.; Kaplan, N.O. 3-acetylpyridine: Effects in vitro related to teratogenic activity in chicken embryos. Science 1968, 160, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing antioxidant power assay. J. Agric. Food Chem. 2000, 46, 3396–3402. [Google Scholar] [CrossRef]
- Zhao, C.; Dodin, G.; Yuan, C.; Chen, H.; Zheng, R.; Jia, Z.; Fan, B.T. In vitro protection of DNA from Fenton reaction by plant polyphenol verbascoside. Biochim. Biophys. Acta 2005, 1723, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butter, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Prakash, J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum. Nutr. 2009, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Lahiri, S.S.; Sultana, S.; Tulsawani, R.; Kumar, R. In vitro evaluation of antioxidant and free radical scavenging activities of Rhodiolaimbricate. J. Complement. Integr. Med. 2009, 6, 12. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2 K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilib. 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid. Med. Cell. Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Sharad, S.; Kapur, S. Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. J. Indian Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- Hepsibha, B.T.; Sathiya, S.; Babu, C.S.; Premalakshmi, V.; Sekar, T. In vitro studies on antioxidant and free radical scavenging activities of Azima tetracantha Lam leaf extracts. Indian J. Sci. Technol. 2010, 3, 571–577. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Antioxidant, Anti-inflammatory, and Chemoprotective Properties of Acacia catechu Heartwood Extracts. Phytother. Res. 2015, 29, 818–824. [Google Scholar] [CrossRef] [PubMed]

| Assay | Aqueous Extract IC50 $ | Methanolic Extract IC50 $ |
|---|---|---|
| Total Phenolic Content | 50.28 ± 0.272 | 50.20 ± 0.218 |
| Total flavonoid content | 49.87 ± 0.236 | 40.70 ± 0.482 |
| DPPH radical scavenging | 52.18 ± 0.0686 | 54.44 ± 0.120 |
| FRAP | 52.21 ± 0.043 | 52.02 ± 0.0043 |
| ABTS•+ radical scavenging | 50.21 ± 0.047 | 50.01 ± 0.0042 |
| Superoxide radical scavenging | 50.25 ± 0.399 | 49.74 ± 0.161 |
| Lipid peroxidation | 48.65 ± 0.103 | 49.65 ± 0.241 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, A.; Modak, M. Comparative Evaluation of Oxidative Stress Modulating and DNA Protective Activities of Aqueous and Methanolic Extracts of Acacia catechu. Medicines 2017, 4, 65. https://doi.org/10.3390/medicines4030065
Patil A, Modak M. Comparative Evaluation of Oxidative Stress Modulating and DNA Protective Activities of Aqueous and Methanolic Extracts of Acacia catechu. Medicines. 2017; 4(3):65. https://doi.org/10.3390/medicines4030065
Chicago/Turabian StylePatil, Ashwini, and Manisha Modak. 2017. "Comparative Evaluation of Oxidative Stress Modulating and DNA Protective Activities of Aqueous and Methanolic Extracts of Acacia catechu" Medicines 4, no. 3: 65. https://doi.org/10.3390/medicines4030065





