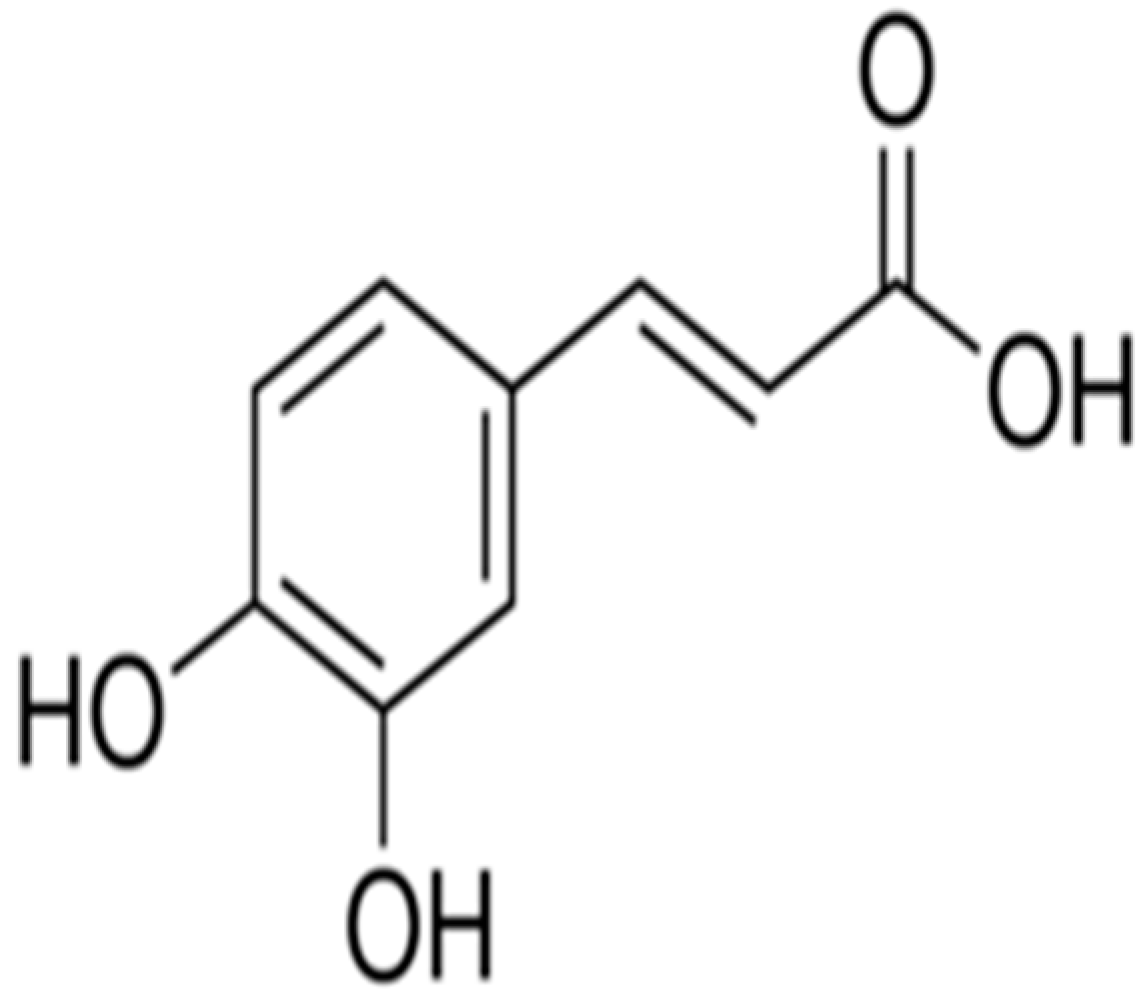
Ameliorative Effect of Caffeic Acid on Capecitabine-Induced Hepatic and Renal Dysfunction: Involvement of the Antioxidant Defence System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animals
2.3. Animal Grouping and Drug Treatments
2.4. Collection of Blood and Liver Samples
2.5. Preparation of Plasma and Cytosolic Fractions
2.6. Determination of Plasma and Liver Protein Content
2.7. Assay of Biomarkers of Hepatic and Renal Toxicity
2.8. Assay for Non-Enzymatic Antioxidants in the Liver
2.8.1. Hepatic Reduced Glutathione Level
2.8.2. Hepatic Ascorbic Acid (AA) Level
2.9. Assay of Hepatic Antioxidant Enzymes
2.9.1. Hepatic Glutathione S-Transferase (GST) Activity
2.9.2. Hepatic Superoxide Dismutase (SOD) Activity
2.9.3. Hepatic Catalase Activity
2.10. Assay of Hepatic Level of Lipid Peroxidation
2.11. Statistical Analysis
3. Results
3.1. Influence of Caffeic Acid on Capecitabine-Induced Changes in Hepatic and Renal Function Markers in the Plasma of Rats
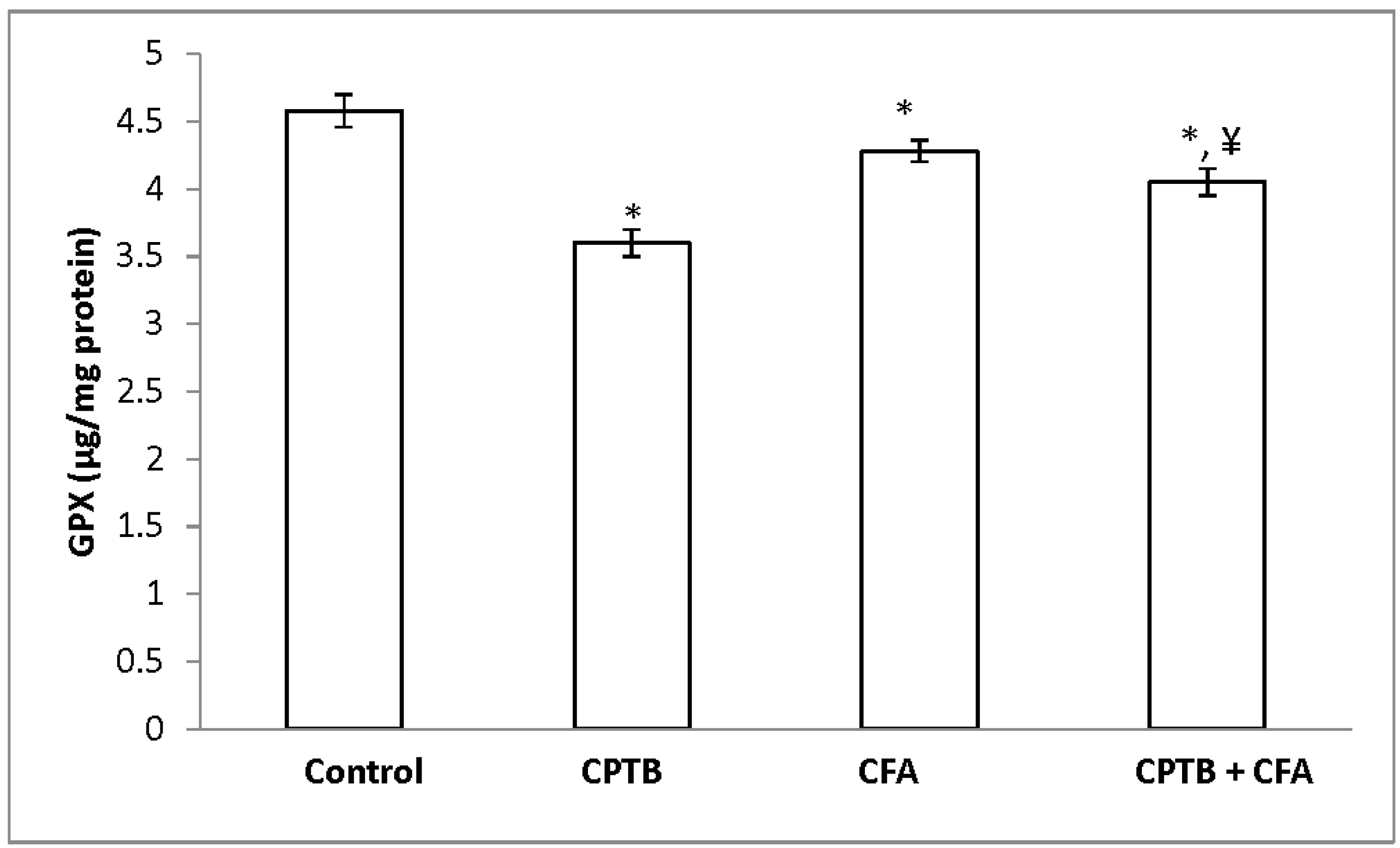
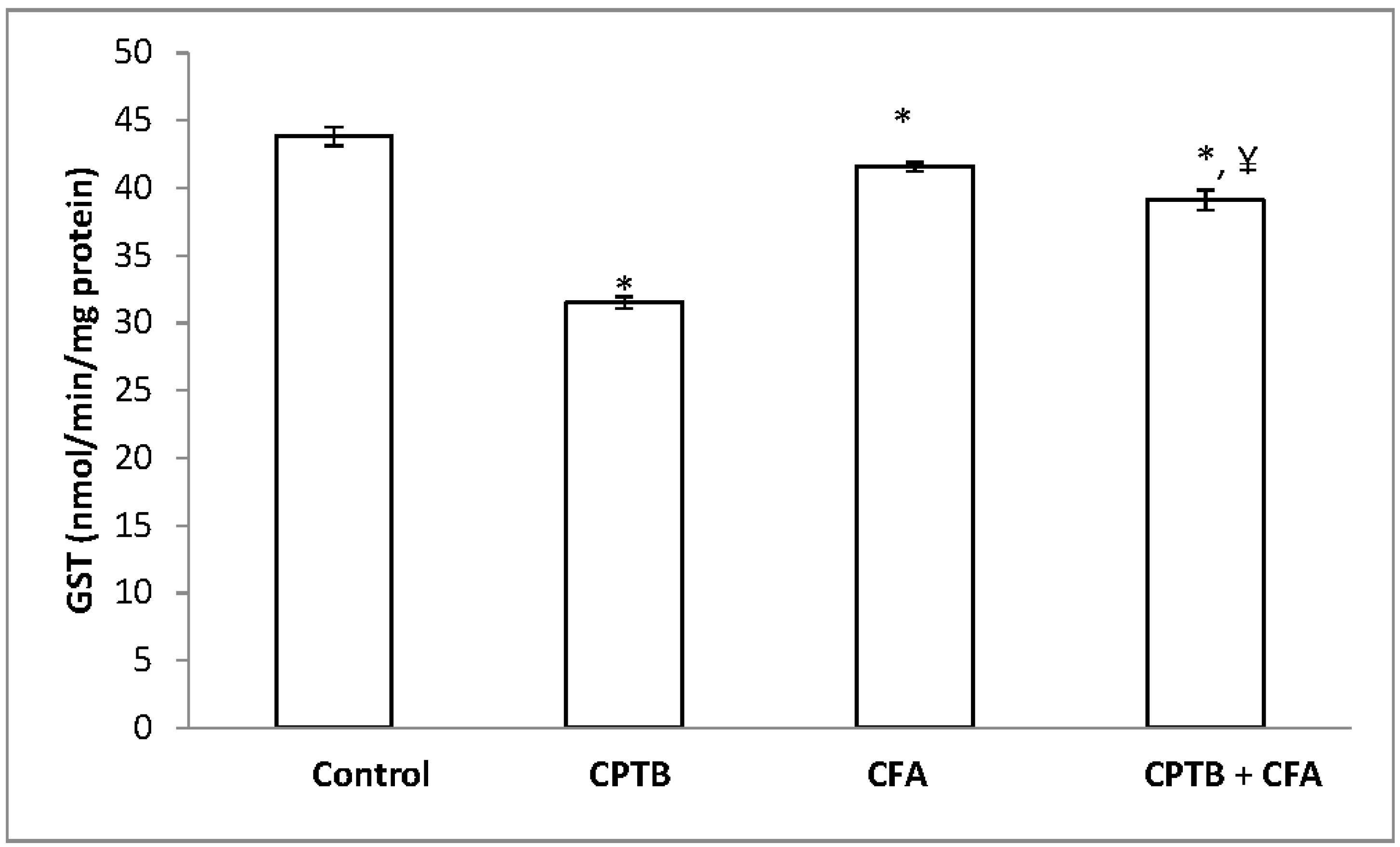
3.2. Effect of Caffeic Acid on Capecitabine-Induced Changes in the Activities of Enzymatic Antioxidants in the Liver of Rats
3.3. Effect of Caffeic Acid on Capecitabine-Induced Changes in the Levels of Non-Enzymatic Antioxidant in the Liver of Rats
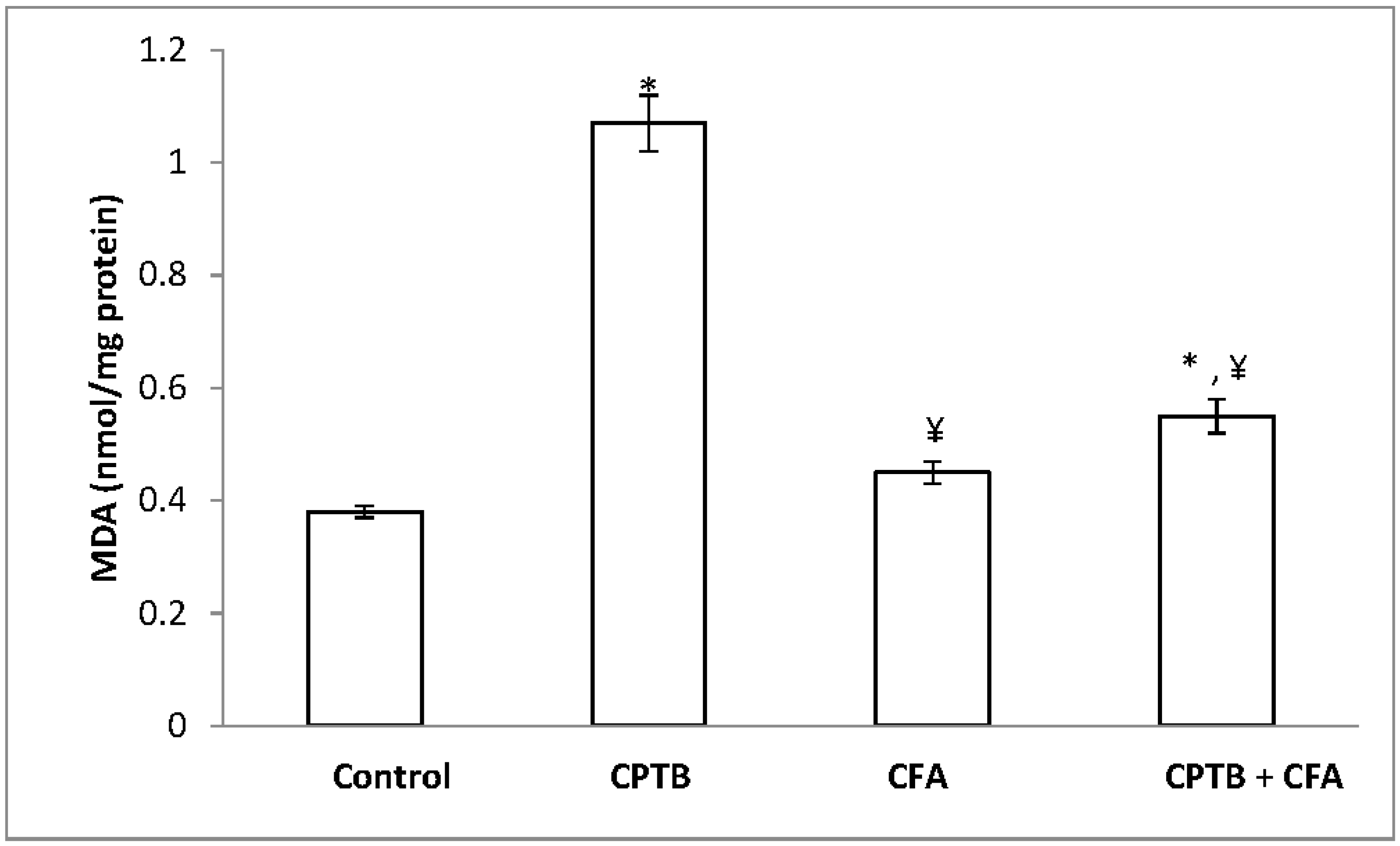
3.4. Influence of Caffeic Acid on Capecitabine-Induced Hepatic Lipid Peroxidation in Rats
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Premkumar, K.; Pachiappan, A.; Abraham, S.K.; Santhiya, S.T.; Gopinath, P.M.; Ramesh, A. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia 2001, 72, 906–911. [Google Scholar] [CrossRef]
- Pieniążek, A.; Czepas, J.; Piasecka-Zelga, J.; Gwoździński, K.; Koceva-Chyła, A. Oxidative stress induced in rat liver by anticancer drugs doxorubicin, paclitaxel and docetaxel. Adv. Med. Sci. 2013, 58, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, E.T.; Ore, A.; Ola, O.S.; Adeyemo, O.A. Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats. Med. Sci. 2015, 3, 78–92. [Google Scholar] [CrossRef]
- Cassidy, J.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Randomized phase III study of Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.G.; Cassidy, J.; Tabernero, J.; Maroun, F.; De Braud, G.; Price, T.J.; Van Cutsem, E.; Hill, M.; Gilberg, F.; Schmoll, H. Efficacy findings from a randomized phase III trial of Capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): No impact of age on disease-free survival (DFS). J. Clin. Oncol. 2010, 28, 3521–3627. [Google Scholar] [CrossRef]
- Schüller, J.; Cassidy, J.; Dumont, E.; Roos, B.; Durston, S.; Banken, L.; Utoh, M.; Mori, K.; Weidekamm, E.; Reigner, B. Preferential activation of Capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother. Pharmacol. 2000, 45, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a novel oral fluoropyrimidine carbamate, Capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Kamal, S.S.; Suresh, V.A.; Monika, L.S.; Ullas, B.; Lakshmaiah, K.C.; Rani, A.; Suresh, T.M. Capecitabine-induced Hand-foot Syndrome. J. Indian Acad. Clin. Med. 2007, 8, 2. [Google Scholar]
- Stathopoulos, G.P.; Koutantos, H.; Lazaki, S.K.; Rigatos, J.; Stathopoulos, D.G. Capecitabine (Xeloda) as Monotherapy in Advanced Breast and Colorectal Cancer: Effectiveness and Side-effects. Anticancer Res. 2007, 27, 1653–1656. [Google Scholar] [PubMed]
- El-Gerbed, M.S. hepatoprotective effect of vitamin c on Capecitabine-induced liver injury in rats. Egypt. J. Exp. Biol. (Zool.) 2015, 11, 61–69. [Google Scholar]
- Michie, C.O.; Sakala, M.; Rivans, I.; Strachan, M.W.J.; Clive, S. The frequency and severity of Capecitabine-induced hypertriglyceridaemia in routine clinical practice: A prospective study. Br. J. Cancer 2010, 103, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Eleutherios, T.; Andreas, S.; Christodoulos, S. Capecitabine May Induce Coronary Artery Vasospasm. Hell. J. Cardiol. 2011, 52, 320–323. [Google Scholar]
- John, M.L.; Allyson, H. Living in a Box or Call of the Wild? Revisiting Lifetime Inactivity and Sarcopenia. Antioxid. Redox Signal. 2011, 15, 2529–2541. [Google Scholar]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.; Bode, A.M.L. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sudina, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V.; Varfolomeev, S.D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar] [CrossRef]
- Jaikang, C.; Chaiyasut, C. Caffeic acid and its derivatives as heme oxygenase 1 inducer in Hep G2 cell line. J. Med. Plants Res. 2010, 4, 940–946. [Google Scholar]
- Iwahashi, H.; Ishii, T.; Suguta, R.; Kido, R. The effect of Caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthranilic acid, ferric chloride and hydrogen peroxide. Arch. Biochem. Biophys. 1990, 276, 242–247. [Google Scholar] [CrossRef]
- Challis, B.C.; Bartlett, C.D. Possible cocarcinogenic effects of coffee constituents. Nature 1975, 254, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Joyeux, M.; Lobstein, A.; Anton, R.; Mortier, F. Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflawones from ginkgo and some flavonoids. Planta Med. 1995, 61, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Chang-Ho, J.; Hee, R.J.; Gwi, N.C.; Dae-Ok, K.; Lee, U.; Ho, J.H. Neuroprotective and anti-oxidant effects of Caffeic acid isolated from Erigeron annuus leaf. Chin. Med. 2011, 6, 25. [Google Scholar]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Mshvildadze, V.; Gepdiremen, A. The antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(Bdglucopyranosyl)-hederagenin. Phytother. Res. 2006, 20, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, L.M.; Nikki, J.H. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Tietz, N.W.; Pruden, E.L.; Siggaard-Andersen, O. Liver Function. In Tietz Textbook of Clinical Chemistry; Burtis, A.C., Ashwood, E.R., Eds.; WB Saunders: London, UK, 1994; pp. 1354–1374. [Google Scholar]
- Reltman, S.; Frankel, S.A. Colorimetric method for the determination of serum ALT and AST. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Szasz, G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin. Chem. 1969, 15, 124–136. [Google Scholar] [PubMed]
- Jollow, D.J.; Mitchell, J.R.; Zampaghone, N.; Gillete, J.R. Bromobenzene induced liver necrosis, protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Habig, W.A.; Pabst, M.J.; Jacoby, W.B. Glutathione trsansferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Singha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Varshney, R.; Kale, R.K. Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Michael, P.W. Liver is a Target of Arsenic Carcinogenesis. Toxicol. Sci. 2008, 105, 24–32. [Google Scholar]
- Haldar, P.K.; Adhikari, S.; Bera, S.; Bhattacharya, S.; Panda, S.P.; Kandar, C.C. Hepatoprotective Efficacy of Swietenia Mahagoni L. Jacq. (Meliaceae) Bark against Paracetamol induced Hepatic Damage in Rats. Indan J. Pharm. Educ. Res. 2011, 45, 108. [Google Scholar]
- Amacher, D.E. Serum Transaminase Elevations as Indicators of Hepatic Injury Following the Administration of Drugs. Regul. Toxicol. Pharmacol. 1998, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, H.; Bjornsson, E.; Simren, M.; Aldenborg, F.; Almer, S.; Olsson, R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006, 26, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.E.; Stainberg, S.E.; Freese, P.; Marino, E. Liver function tests. J. Dig. Disord. 2004, 6, 1–3. [Google Scholar]
- Romero, G.; Lasheras, B.; Sainz, S.L.; Cenarruzabeitia, E. Protective effects of calcium channel blockers in carbon tetrachloride-induced liver toxicity. Life Sci. 1994, 55, 981–990. [Google Scholar] [CrossRef]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Brandolt, G.L.; Ely, L.S.; Engroff, P.; Almeid, T.W.; Azambuja, A.A.; Gomes, I. Toxicity of Capecitabine compared with 5-fluorouracil in elderly patients with breast or gastrointestinal tract cancer. Appl. Cancer Res. 2011, 31, 131–137. [Google Scholar]
- Yang, S.Y.; Hong, C.O.; Lee, G.P.; Kim, C.T.; Lee, K.W. The hepatoprotection of Caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Studies on the protective effects of Caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine 2004, 11, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Saumya, R.P.; Satyaranjan, M.; Sabuj, S.; Prasana, K.P. Nephroprotective effect of Bauhinia variegata (Linn.) whole stem extract against cisplatin-induced nephropathy in rats. Indian J. Pharmacol. 2011, 43, 200–202. [Google Scholar]
- Adebayo, J.O.; Yakubu, M.T.; Egwim, E.C.; Owoyele, V.B.; Enaibe, B.U. Effect of ethanolic extract of Khaya senegalensis on some biochemical parameters of rat kidney. J. Ethnopharmacol. 2003, 88, 69–72. [Google Scholar] [CrossRef]
- Maheswari, C.; Maryammal, R.; Venkatanarayanan, R. Hepatoprotective activity of “Orthosiphon stami-neus” on lver damage caused by paracetamol in rats. Jordan J. Biol. Sci. 2008, 1, 105–108. [Google Scholar]
- Li, Y.; Chen, L.J.; Jiang, F.; Yang, Y.; Wang, X.X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2004, 48, 6. [Google Scholar] [CrossRef] [PubMed]
- Maddrey, W.C. Drug induced hepatotoxicity. J. Clin. Gastroenterol. 2005, 39, 83–89. [Google Scholar] [CrossRef]
- Proctor, P.H.; McGinness, J.E. The function of melanin. Arch. Dermatol. 1986, 122, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Kocyigrit, A.; Aktepe, S.; Bulut, V. Oxidative stress and antioxidant status of plasma and erythrocytes in patients with malaria. Clin. Biochem. 1997, 30, 631–639. [Google Scholar] [CrossRef]
- Casado, C.J.; Stipek, S.; Crkovska, J.; Ardan, T.; Midelfart, A. Reactive oxygen species (ROS)-generating oxidases in the normal rabbit cornea and their involvement in corneal damage evoked by UVB rays. Histol. Histopathol. 2001, 16, 523–533. [Google Scholar]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxylnitrite: Implication for endothetial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S. Theories of biological aging: Genes, proteins, and free radicals. Free Radic. Res. 2006, 40, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Han, W.K.; Bonventre, J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care 2004, 10, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Touliatos, J.S.; Neitzel, L.; Whitworth, C.; Rybak, L.P.; Malafa, M. Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat. Eur. Arch. Otorhinolaryngol. 2000, 25, 6–9. [Google Scholar] [CrossRef]
- Ali-Osman, F. Quenching of DNA cross-link precursors of chloroethylnitrosoureas and attenuation of DNA interstrand cross-linking by glutathione. Cancer Res. 1989, 49, 5258–5261. [Google Scholar] [PubMed]
- Masella, R.; Di Benedetto, R.; Vari, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Aniya, Y.; Naito, A. Oxidative stress-induced activation of microsomal glutathione S transferase in isolated rat liver. Biochem. Pharmacol. 1993, 45, 37–42. [Google Scholar] [PubMed]
- Hidir, P.; Murat, O.; Huseyin, O.; Mehmet, F.S.; Neriman, C.; Ilter, K. Ameliorative effect of Caffeic acid phenethyl ester on histopathological and biochemical changes induced by cigarette smoke in rat kidney. Toxicol. Ind. Health 2010, 26, 175–182. [Google Scholar]
- Packer, L.; Cadenas, E. Oxidative stress and disease. In Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; Marcel Dekker, Inc.: New Yok, NY, USA; Basel, Switzerland, 2002; pp. 5–8. [Google Scholar]
- Catala, A. Lipid peroxidation modifies the assembly of biological membranes. The Lipid Whisker Model. Front. Physiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, J. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2014, 6. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Prasanna, A.; Haldar, P.K. Evaluation of antiproliferative activity of Trichosanthes dioica root against Ehrlich ascites carcinoma cells. Acad. J. Cancer Res. 2011, 4, 38–42. [Google Scholar]


| Treatment Groups | Treatment Duration (1–14 Days) |
|---|---|
| A. Control | Distilled water |
| B. Capecitabine (CPTB) | 30 mg/kgbw CPTB |
| C. Caffeic acid (CFA) | 100 mg/kgbw CFA |
| D. CPTB + CFA Co-treated | 100 mg/kg bw CFA + 30 mg/kgbw CPTB |
| Treatment | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|
| Control | 46.5 ± 1.90 | 3.15 ± 0.11 | 436 ± 2.34 |
| CPTB | 74.8 ± 1.20 (37.86%) * | 6.86 ± 0.15 (54.13%) * | 655 ± 4.82 (33.40%) * |
| CFA | 50.7 ± 1.21 * | 3.63 ± 0.12 * | 460 ± 7.36 * |
| CPTB + CFA | 59.5 ± 3.10 *,¥ | 4.48 ± 0.16 *,¥ | 540 ± 6.64 *,¥ |
| Treatment | Urea (mg/dL) | Creatinine (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|
| CONTROL | 74.33 ± 2.34 | 0.98 ± 0.02 | 3.15 ± 0.1 |
| CPTB | 104.7 ± 1.03 (28.98%) * | 1.28±0.01 (23.60%) * | 4.83 ± 0.1 (34.83%) * |
| CFA | 78.8 ± 1.47 * | 1.10 ± 0.02 * | 3.48 ± 0.2 * |
| CPTB + CFA | 90.0 ± 2.28 *,a | 1.15 ± 0.01 *,a | 3.75 ± 0.1 *,a |
| Treatment | SOD (Units) | Catalase (µmole H2O2 Consumed/min/mg protein) |
|---|---|---|
| Control | 26.3 ± 1.37 | 1.48 ± 0.02 |
| CPTB | 14.1 ± 0.65 (46.46%) * | 0.79 ± 0.04 (44.67%) * |
| CFA | 23.6 ± 0.89 * | 1.23 ± 0.03 * |
| CPTB + CFA | 19.7 ± 0.82 *,¥ | 1.10 ± 0.06 *,¥ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olayinka, E.T.; Ola, O.S.; Ore, A.; Adeyemo, O.A. Ameliorative Effect of Caffeic Acid on Capecitabine-Induced Hepatic and Renal Dysfunction: Involvement of the Antioxidant Defence System. Medicines 2017, 4, 78. https://doi.org/10.3390/medicines4040078
Olayinka ET, Ola OS, Ore A, Adeyemo OA. Ameliorative Effect of Caffeic Acid on Capecitabine-Induced Hepatic and Renal Dysfunction: Involvement of the Antioxidant Defence System. Medicines. 2017; 4(4):78. https://doi.org/10.3390/medicines4040078
Chicago/Turabian StyleOlayinka, Ebenezer Tunde, Olaniyi Solomon Ola, Ayokanmi Ore, and Oluwatobi Adewumi Adeyemo. 2017. "Ameliorative Effect of Caffeic Acid on Capecitabine-Induced Hepatic and Renal Dysfunction: Involvement of the Antioxidant Defence System" Medicines 4, no. 4: 78. https://doi.org/10.3390/medicines4040078






