Understanding Chinese Medicine Patterns of Rheumatoid Arthritis and Related Biomarkers
Abstract
:1. Introduction
2. Methods
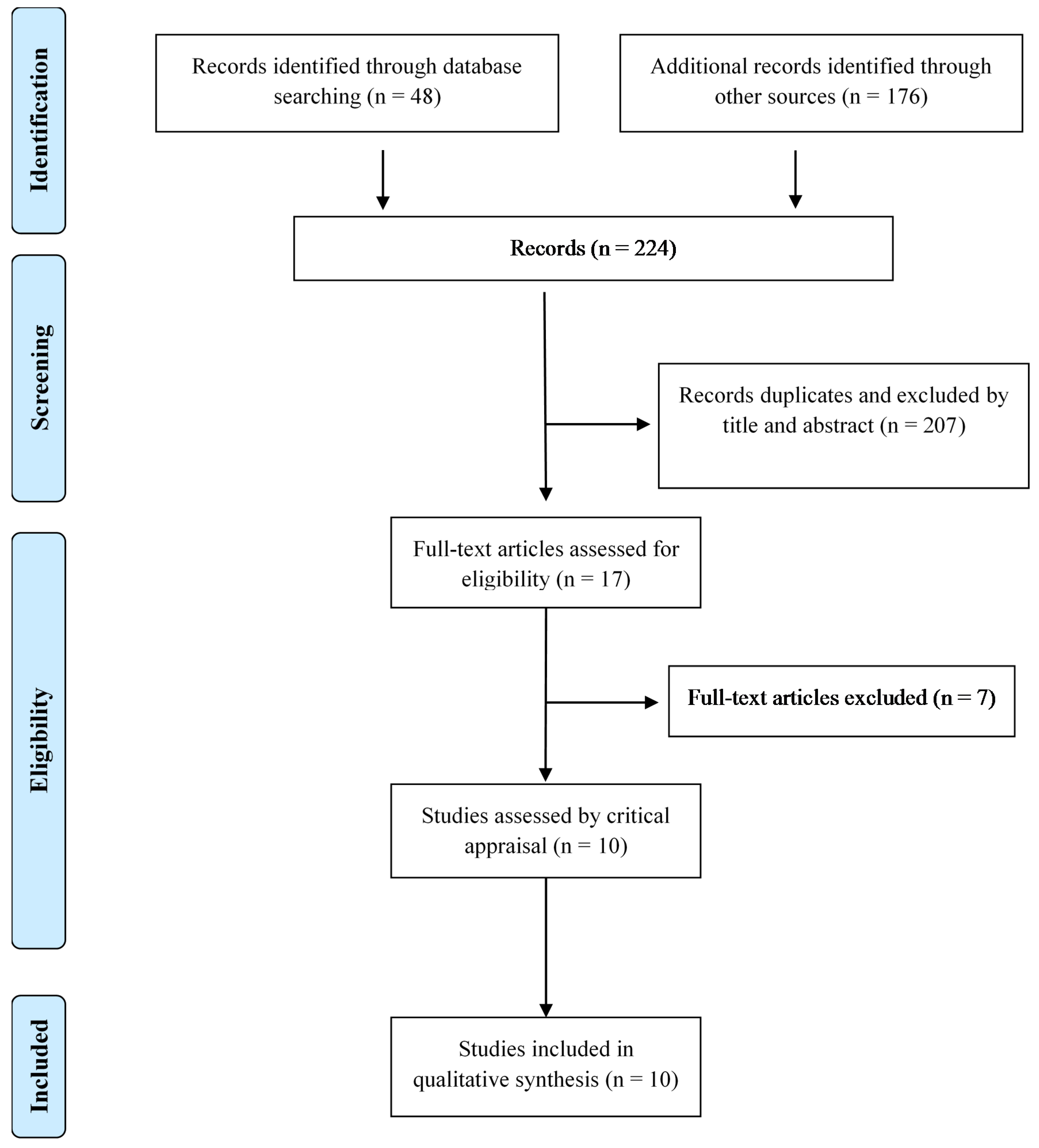
2.1. Search Strategy and Screening
2.2. Inclusion Criteria
2.3. Assessment of Methodological Quality and Data Extraction
3. Results
4. Discussion
4.1. Limitations of the Review
4.2. Implications for Practice
4.3. Implications for Research
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Greten, H.J. Kursbuch Traditionelle Chinesische Medizin: TCM Verstehen und Richtig Anweden; Georg Thieme: Stuttgart, Germany, 2017; ISBN 978-3-13-121663-2. [Google Scholar]
- Zhou, J.; Chen, J.; Hu, C.; Xie, Z.; Li, H.; Wei, S.; Wang, D.; Wen, C.; Xu, G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 127, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zha, Q.; Chang, A.; He, Y.; Lu, A. Pattern differentiation in traditional Chinese medicine can help define specific indications for biomedical therapy in the treatment of rheumatoid arthritis. J. Altern. Complement. Med. 2009, 15, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Van der Greef, J.; van Wietmarschen, H.; Schroën, J.; Wang, M.; Hankemeier, T.; Xu, G. Systems Biology-Based Diagnostic Principles as Pillars of the Bridge between Chinese and Western Medicine. Planta Med. 2010, 76, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Ptri, H.; Maldonato, D.; Robinson, N.J. Data-driven identification of co-morbities associated with rheumatoid arthritis in a large US health plan claims database. BMC Musculoskelet. Disord. 2010, 11, 24. [Google Scholar] [CrossRef]
- Horsten, N.C.A.; Ursum, J.; Roorda, L.D.; Schaardenburg, D.; Dekker, J.; Hoeksma, A.F. Prevalence of hand symptoms, impairments and activity limitations in rheumatoid arthritis in relation to disease duration. J. Rehabil. Med. 2010, 42, 916–921. [Google Scholar] [PubMed]
- Gavrilă, B.; Ciofu, C.; Stoica, V. Biomarkers in Rheumatoid Arthritis, what is new? J. Med. Life 2016, 9, 144–148. [Google Scholar] [PubMed]
- Fang, J.; Zheng, N.; Wang, Y.; Cao, H.; Sun, S.; Dai, J.; Li, Q.; Zhang, Y. Understanding Acupuncture Based on ZHENG Classification from System Perspective. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Qv, X.-Y.; Jiang, J.-G. Proteomics and syndrome of Chinese medicine. J. Cell. Mol. Med. 2010, 14, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lu, C.; Zhang, C.; Yang, J.; Tan, Y.; Lu, A.; Chan, K. Syndrome differentiation in modern research of traditional Chinese medicine. J. Ethnopharmacol. 2012, 140, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.F.; Chung, K.F.; Poon, M.M.; Ho, F.Y.; Zhang, S.P.; Zhang, Z.J.; Ziea, E.T.; Wong Taam, V. Prescription of Chinese Herbal Medicine and Selection of Acupoints in Pattern-Based Traditional Chinese Medicine Treatment for Insomnia: A Systematic Review. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, A.; Zha, Y.; Yan, X.; Song, Y.; Zeng, S.; Liu, W.; Zhu, W.; Su, L.; Feng, X.; et al. Correlations between symptoms as assessed in traditional chinese medicine (TCM) and ACR20 efficacy response: A comparison study in 396 patients with rheumatoid arthritis treated with TCM or Western medicine. J. Clin. Rheumatol. 2007, 13, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Scott, I.C.; Rayner, L.; Hotopf, M.; Kingsley, G.H.; Norton, S.; Scott, D.L.; Steer, S. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36, a systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Jiang, M.; Chen, G.; Lu, A. Incorporation of traditional Chinese medicine pattern diagnosis in the management of rheumatoid arthritis. Eur. J. Integr. Med. 2012, 4, e245–e254. [Google Scholar] [CrossRef]
- Lo, L.C.; Chen, C.H.; Chiang, J.Y.; Cheng, T.L.; Lin, H.J.; Chang, H.H. Tongue Diagnosis of Traditional Chinese Medicine for Rheumatoid Arthritis. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, G.; Lu, C.; Xiao, C.; Li, L.; Niu, X.; He, X.; Jiang, M.; Lu, A. Rheumatoid Arthritis with Deficiency Pattern in Traditional Chinese Medicine Shows Correlation with Cold and Hot Patterns in Gene Expression Profiles. Evid. Based Complement. Altern. Med. 2013, 2013, 248650. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, C.; Zha, Q.; Xiao, C.; Xu, S.; Ju, D.; Zhou, Y.; Jia, W.; Lu, A. A network-based analysis of traditional Chinese medicine cold and hot patterns in rheumatoid arthritis. Complement. Ther. Med. 2012, 20, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xiao, C.; Chen, G.; Jiang, M.; Zha, Q.; Yan, X.; Kong, W.; Lu, A. Cold and heat pattern of rheumatoid arthritis in traditional Chinese medicine: Distinct molecular signatures identified by microarray expression profiles in CD4-positive T cell. Rheumatol. Int. 2012, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Niu, X.; Xiao, C.; Chen, G.; Zha, Q.; Guo, H.; Jiang, M.; Lu, A. Network-Based Gene Expression Biomarkers for Cold and Heat Patterns of Rheumatoid Arthritis in Traditional Chinese Medicine. Evid. Based Complement. Altern. Med. 2012, 2012, 203043. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Lopes, A.J. Chinese medicine pattern differentiation and its implications for clinical practice. Chin. J. Integr. Med. 2011, 17, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lu, C.; Zha, Q.; Kong, H.; Lu, X.; Lu, A.; Xu, G. Plasma metabonomics study of rheumatoid arthritis and its Chinese medicine subtypes by using liquid chromatography and gas chromatography coupled with mass spectrometry. Mol. Biosyst. 2012, 8, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Van Wietmarschen, H.A.; Dai, W.; van der Kooij, A.J.; Reijmers, T.H.; Schroën, Y.; Wang, M.; Xu, Z.; Wang, X.; Kong, H.; Xu, G.; et al. Characterization of Rheumatoid Arthritis Subtypes Using Symptom Profiles, Clinical Chemistry and Metabolomics Measurements. PLoS ONE 2012, 7, e44331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Fang, Y.F.; Wang, Y.; Mu, F.X.; Chen, J.; Zou, Q.H.; Zhong, B.; Li, J.Y.; Bo, G.P.; Zhang, R.H. Logistic regression analysis of damp-heat and cold-damp impeding syndrome of rheumatoid arthritis: A perspective in Chinese medicine. Chin. J. Integr. Med. 2012, 18, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xu, X.; Zhou, D.; Wang, L.; Wang, F.; Xu, Z.; Ji, W. Serum proteomic-based analysis by iTRAQ of damp-heat impeding syndrome of rheumatoid arthritis. Eur. J. Integr. Med. 2015, 479–484. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, C.; Chen, G.; Xiao, C.; Zha, Q.; Niu, X.; Chen, S.; Lu, A. Understanding the Molecular Mechanism of Interventions in Treating Rheumatoid Arthritis Patients with Corresponding Traditional Chinese Medicine Patterns Based on Bioinformatics Approach. Evid. Based Complement. Altern. Med. 2012, 2012, 129452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yuanyan, L.; Cheng, X.; Miao, J.; Qinglin, Z.; Aiping, L. Biological Basis of Cold and Heat Pattern of Rheumatoid Arthritis in Traditional Chinese Medicine. World Sci. Technol. 2010, 12, 814–817. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.Q.; Wu, L.J.; Zhang, X.G.; Li, Y.D.; Wang, Y.Y. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst. Biol. 2007, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.J.; de Sá Ferreira, A. Effects of diagnostic errors in pattern differentiation and acupuncture prescription: A single-blinded, interrater agreement study. Evid. Based Complement. Altern. Med. 2015, 2015, 469675. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Zhang, G.B.; Zhang, Y.Y.; Su, S.B. Clinical Applications of Omics Technologies on ZHENG Differentiation Research in Traditional Chinese Medicine. Evid. Based Complement. Altern. Med. 2013, 2013, 989618. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Veronesi, F.; Carina, V.; Costa, V.; Raimondi, L.; De Luca, A.; Alessandro, R.; Fini, M.; Giavaresi, G. Gene therapy for chondral and osteochondral regeneration: Is the future now? Cell. Mol. Life Sci. 2017, 75, 649–667. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Sample Size (m/f) Age (Years) | Methods/Outcomes Tissue Sample Technique Verification/Validation | Remarkable results in RA patients | ||||
|---|---|---|---|---|---|---|---|
| TCM Pattern | TCM Signs/Symptoms | Identified Candidate Biomarkers | Implication | Target Disease | |||
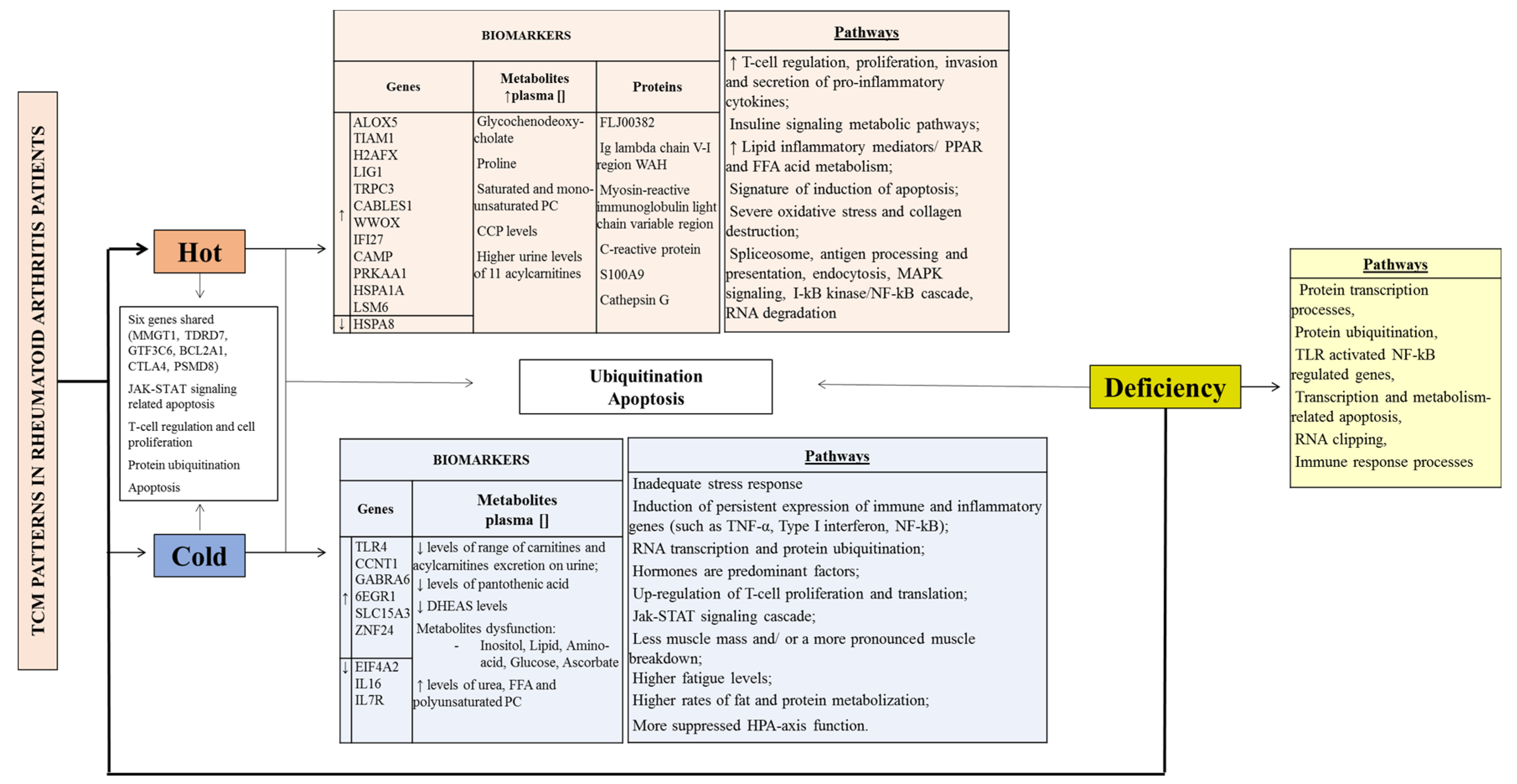
| Lu et al. [18] | 0/20 RA patients [Cold and Hot (1:2)] Age: 12 to 68 | Qualitative study Blood sample (8 ml) RNA isolated from CD4+ T cell Microarray; PPI; IPCA; Cytoscape; BiNGO Databases: BIND, BIOGRID, DIP, HPRD, MINT | Cold |
|
|
| Inflammatory response is more pronounced (higher effective rate of anti-inflammatory drugs) |
| Hot |
| 1.Up-regulated genes: 1.1. TRPC3, CABLES1, VWOX, IFI27 2. Pathways: 2.1. Calcium signalling; CAMs 2.2. PPAR signalling, nuclear hormone receptors 2.3. Fatty acid metabolism | 1. 1.1. Genes participated in proliferation and/or differentiation, cholesterol efflux, regulation of cellular functions, regulation of protein sorting and membrane trafficking, immuno-regulatory processes 2. 2.1. T cell interaction (activation, proliferation and secretion) 2.3. Activated by fatty acids and their derivates | 1. Signature of induction of apoptosis 2.1. Regulation of signal transduction mediated by adhesion molecules on T-lymphocyte interactions (possible effective way to control the pathological inflammatory process) 2.3. PPAR and FFA | |||
| Chen et al. [ 17] | 0/33 RA patients [n = 21 cold, n = 12 hot] Age: 42.8 ± 9.9 | Qualitative study Blood sample RNA isolated from CD4+ T cell Microarray; PPI; IPCA; BiNGO Databases: BIND, BIOGRID, DIP, HPRD, MINT) | Cold | Cold intolerance, cold feeling in the limbs, cold feeling in the joints | 1. Genes highly expressed: 1.1. GABRA6; EGR1; SLC15A3; ZNF24 | Hormones are predominant factors | |
| Hot | Thirst, vexation, fever and turbid urine | 1. Higher expression of: 1.1. TIAM1 1.2. ALOX5 1.3. H2AFX and LIG1 | 1.1. Small G protein signalling pathways activated 1.2. Lipid inflammatory mediators increased/ oxidation-reduction in fatty acid metabolism increased 1.3. T cell proliferation increased | Predominance of immune factors Oxidation-reduction in fatty acid metabolism increased | |||
| Lu et al. [ 19] | 0/45 [RA patients: n = 21 cold, n = 12 hot] [12 healthy volunteers] Age: 18 to 70 | Qualitative study Blood sample RNA isolated from CD4+ T cell Microarray; PPI; IPCA; BiNGO DAVID; GeneSpring Databases (BIND, BIOGRID, DIP, HPRD, IntAct MINT) | Hot and Cold common points | 1. six genes shared (MMGT1, TDRD7, GTF3C6, BCL2A1)
1.1. CTLA4 1.2. PSMD8 1.3. RNA splicing | 1. Five pathways: CAMs, T cell receptor signalling pathway, proteasome
1.1. CTLA4 (up-regulated) participates in the pathways of autoimmune thyroid disease, CAMs and the T cell receptor signalling 1.2. PSMD8 (down-regulated)—imply down-regulation of protein ubiquitination in the cell cycle |
| |
| Cold | Severe fixed pain in a joint or muscle; pain relief upon warming and worse upon cooling; white tongue coating | 1. Significant gene biomarkers: 1.1. EIF4A2 1.2. CCNT1 1.3. IL16 1.4. IL7R | 1. Pathways: up-regulation of cell proliferation, GPI anchor biosynthesis, arachidonic acid metabolism, ABC transporters, pentose and glucoronate interconversions and axon guidance. 1.1. Regulation of translation and cell biosynthetic processes 1.2. RNA transcription and protein ubiquitination (CD4+ T cell and macrophages) 1.3. T cell regulation 1.4. The Jak-STAT signalling cascade; hematopoietic cell lineage; primary immunodeficiency; cytokine-cytokine receptor interaction; T cell regulation. Can block apoptosis and promote the proliferation of CD4+ T cells | Regulation of translation and the Jak-STAT cascade IL7R, candidate marker (simple, minimally invasive pharmacodynamics assay for RA treatments directed at the NF-κB pathway) | |||
| Hot | Severe pain, hot, red, swollen and inflamed joints; pain relief upon cooling and worse upon warming, fever, thirst, restlessness, deep-coloured urine, red tongue with yellow coating. | 1. Significant gene biomarkers: 1.1. CAMP 1.2. PRKAA1 1.3. HSPA1A 1.4. HSPA8 1.5. LSM6 | 1.1. T cell regulation and cell proliferation 1.2. mTOR signalling; adipocytokine signalling; regulation of autophagy; HCM; insulin signalling; FFA metabolism 1.3. spliceosome, antigen processing and presentation, endocytosis, MAPK signalling, T cell regulation; complement and coagulation cascades; I-kB kinase/NF-κB cascade 1.4. spliceosome, antigen processing and presentation, endocytosis, MAPK signalling; I-kB kinase/NF-κB cascade 1.5. Spliceosome; RNA degradation, hematopoietic cell lineage | FFA metabolism and the I-kB kinase/NF-κB cascade | |||
| Gu et al. [ 22] | 0/57 [RA patients: n = 28 cold, n = 29 hot], [n = 23 healthy volunteers] Age: 12 to 68 | Qualitative study Plasma samples; LC-MS GC-MS Database: NIST | Cold | Severe pain in a joint or muscle, pallor, intolerance of cold, absence of thirst, loose stools, clear profuse urine, pale tongue and slow pulse. | 1. Metabolites perturbations in: 1.1. Inositol metabolism 1.2. Lipid metabolism 1.3. Amino acid metabolism 1.4. Glucose metabolism 1.5. Ascorbate metabolism | 1.1. Inositol is up-regulated in RA patients—this could modulate intracellular signalling systems and further induce the production of inflammatory mediators and finally might affect other metabolic pathways. 1.2. Lipid metabolism/ FFA prominently up-regulated (e.g. arachidonic acid) and may reflect the activation of the immune system. | Rates of fat and protein mobilization may be higher |
| Hot | Inflamed, red and swollen joints, flushed face, fever or feverishness, thirst, irritability, restlessness, constipation, deep-coloured urine, reddened tongue and rapid pulse. | 1. Metabolites disorders: 1.1. Elevated plasma concentrations of glycochenodeoxycholate, proline, saturated and mono-unsaturated PC 1.2. Decreased levels of urea, FFA and polyunsaturated PE. | Presence of oxidative stress and the excess reactive oxygen species production could disturb the redox status, damage macromolecules and exacerbate inflammation. | Oxidative stress and collagen destruction may be more severe. | |||
| Van Wietmarschen et al. [ 23] | 0/39 [RA patients: n = 20 cold, n = 19 hot] Age: Cold pattern 51 ± 13 Hot pattern 54 ± 11 CH: n = 36 | Qualitative study Clinical symptoms. Blood, urine and plasma. Clinical chemistry measurement, metabolite measurement. Database: HMDB | Cold | Cold feeling, aversion to cold |
| 2. More suppressed HPA axis function (associated with a decreased stress response which results in an inadequate response to stress factors and consequently autoimmune and inflammatory disorders) | 1. Carnitine and acylcarnitine supplementation might be beneficial for Cold RA. CRP and RF showed a low variance accounted between the cold and hot RA sub-type. |
| Hot | Warm feeling, pain worsens with warmth and movement; red, warm, swollen joints; dull pain | CCP levels higher | More joint problems | The most discriminating symptoms in the analysis, “warm joints,” “red joints” and “swollen joints” indicate a difference in inflammatory status. | |||
| Wang et al. [ 24] | 59/247 [n = 148 cold, n = 158 hot] Age: 51.3 ± 13.2 | Blood sample ESR, CRP, WBC, RBC, Hb, PLT, TP, ALB, GLB, TNF-alfa, IL-1beta DAS28 score ELISA | Cold-damp | Cold and constant pain worsened by cold or rainy weather and at night but relieved during warm days; heaviness of the joint. Tongue: fat, pale texture, white greasy coating. Pulse: slow or stagnant. | A highly significant relationship existed between PLT and disease severity and a negatively correlation between the level of Hb and disease severity. | ||
| Hot-damp | Severe pain; redness, swelling and heaviness of joint or muscle. Fever, thirst, difficulty walking, yellow urine, annoyance and unrest. Tongue: red texture, yellow coating. Pulse: slippery and quick. | DAS28, ESR, WBC, CRP, PLT, GLB, ALB differed significantly between hot-damp and cold-damp | DAS28, ESR, WBC, CRP, PLT, GLB, ALB may serve as criteria for discriminating damp-hot from damp-cold syndrome. ESR; WBC; CRP - were hot risk factors | ||||
| Wang et al. [ 16] | 0/45 33 [RA patients: n = 12 cold, n = 21 hot, n = 18 deficiency, n = 15 non-deficiency], [Patients with deficiency pattern: n = 8 cold deficiency, n = 10 hot-deficiency], [n = 12 healthy volunteers]. Age: 18 to 70 | Blood sample RNA isolated from CD4+ T cell Microarray; PPI; IPCA BiNGO Databases BIND, BIOGRID, DIP, HPRD, IntAct MINT) | Cold | Cold feeling in joints, pain relieved with warming | Mainly involved in ubiquitination, RNA clipping and Jak-STAT cascade signalling. | Cold and hot patterns: function of Jak-STAT signalling-related apoptosis | |
| Hot | Hot feeling, pain relieved with cooling | Function of insulin signalling. | |||||
| Deficiency | Deformity, inhibited bending and stretching in limbs, pain occurring or worsening during moodiness and numbness. Symptoms clinically occur in the later stage of disease or subsequent to other common articular symptoms. | 1. Seven significantly, highly connected regions | 1. Mainly involved in protein transcription processes, protein ubiquitination, TLR activated NF-κB regulated gene transcription and apoptosis pathways, RNA clipping, NF-κB signal, nucleotide metabolism-related apoptosis and immune response processes. | Inhibition of NF-κB pathway is believed to be a potential therapeutic target in RA. TLRs may be on the onset of joint deformity and inhibited bending and stretching in limbs symptoms (deficiency syndrome features) | |||
| Sun et al. [ 25] | n = 90 [RA patients: n = 30 Heat-damp group, n = 30 Cold-damp, n = 30 Control group], [n = 30 healthy patients] Aged from 42 to 56 (51.3 ± 4.3) | Serum pools samples Strong cation exchange Chrotography iTRAQLC-MS/MS analysis GO DAVID v6.7, UniProtKB/Swiss-Prot and IPA | Hot | Redness, pain and swelling of the joint, scorching sensation, red tongue with yellow and greasy fur, rapid or slippery pulse | 1. Six proteins overexpressed 1.1. FLJ00382, 1.2. Ig lambda chain V-I region WAH, 1.3. Myosin-reactive immunoglobulin light chain variable region, 1.4. C-reactive protein,1.5. S100A9, 1.6. Cathepsin G | 1. Proteins involved in inflammatory responses; five top significant canonical pathways: including Autoimmune Thyroid Disease Signalling, Hematopoiesis from Pluripotent Stem Cells, Primary Immunodeficiency Signalling, IL-17 Signalling, Allograft Rejection Signalling 1.1. Function of regulation of T cell proliferation and immune response 1.2. Involved in the biological process of regulation of immune response and complementary activationv 1.3. Play a role in the immune response 1.4. Sensitive but nonspecific marker of inflammation 1.5. Positive regulation of inflammatory processes and immune response, can act as a potent amplifier of autoimmune inflammation 1.6. Significant role in the pathogenesis of RA synovial inflammation as a monocyte chemoattractant. | Hot-damp syndrome of RA has severe inflammatory responses and high RA inflammatory activity. Treatment with anti S100A9 may inhibit amplification of the immune response and help preserve tissue integrity. TNF-α is recognized as a key regulator of inflammatory response. |
| Jiang et al. [ 26] | n = 398 RA patients [TCM therapy n = 204: n = 115 cold, n = 99 non-cold dominant], [Biomedicine therapy n = 194: n = 87 cold, n = 107 non-cold dominant] Age: 18 to 70 | RCT ACR20 response after 24 weeks treatment course. Pharmacological Network Building-Up. Molecular Networks of TCM Cold and Hot Patterns. Databases: TCMGeneDIT; BIND, BIOGRID, DIP, HPRD, IntAct MINT) IPCA;, BiNGO | TCM therapy could target:
| Was better in treating the RA patients with TCM hot pattern. After 24 weeks treatment, the effective rate of the TCM therapy in the patients who showed TCM pattern changes from cold dominant pattern to non-cold dominant pattern were higher (p < 0.05). | Protein ubiquitin pathway involved in the intersections between the cold and hot. Six months after the treatment, the TCM pattern changed in some patients. | ||
Biomedicine therapy targets parts of:
| More effective than TCM therapy in RA cold pattern (p < 0.05). | The regulation of ubiquitin-protein ligase activity during mitotic cell cycle was the pathway affected by MTX + SSZ combination therapy and no similar pathway can be affected with the TCM therapy. | |||||
| Cheng et al. [ 27] | n = 194 [RA patients: cold pattern n = 35 Hot pattern n = 7; Undefined pattern n = 152] | Non-RCT Blood samples ACR20 response at week 12 and 24 Indexes: cytokines, clinical inflammatory, clinical immune | The effective rate of the biomedical combination therapy was higher in the patients with a cold pattern than in the patients with a hot pattern (p < 0.01). | CRP has potential diagnostics value to hot and cold pattern in RA | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seca, S.; Franconi, G. Understanding Chinese Medicine Patterns of Rheumatoid Arthritis and Related Biomarkers. Medicines 2018, 5, 17. https://doi.org/10.3390/medicines5010017
Seca S, Franconi G. Understanding Chinese Medicine Patterns of Rheumatoid Arthritis and Related Biomarkers. Medicines. 2018; 5(1):17. https://doi.org/10.3390/medicines5010017
Chicago/Turabian StyleSeca, Susana, and Giovanna Franconi. 2018. "Understanding Chinese Medicine Patterns of Rheumatoid Arthritis and Related Biomarkers" Medicines 5, no. 1: 17. https://doi.org/10.3390/medicines5010017





