Protein Oxidation and Sensory Quality of Brine-Injected Pork Loins Added Ascorbate or Extracts of Green Tea or Maté during Chill-Storage in High-Oxygen Modified Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extracts and Chemicals
2.2. Total Phenolic Content in Extracts
2.3. Preparation and Storage of Injected Pork Loins
- (1)
- Phenol content in brine: 0.10% extract in brine gives 0.1 g/100 mL·23.8% = 0.238 mg/mL phenol in brine;
- (2)
- Phenol in loin (2.02 kg) when weight-gain was 193 g (mL): 193 mL·0.238 mg/mL phenol = 44.4 mg phenol;
- (3)
- Phenol (ppm) in loin: 44.4 mg phenol/2.02 kg meat = 22.3 mg phenol/kg meat = 22.3 ppm.
2.4. Salt Analysis
2.5. Color Analysis
2.6. Isolation of Myofibrillar Proteins (MPI)
2.7. Protein Thiol Concentration
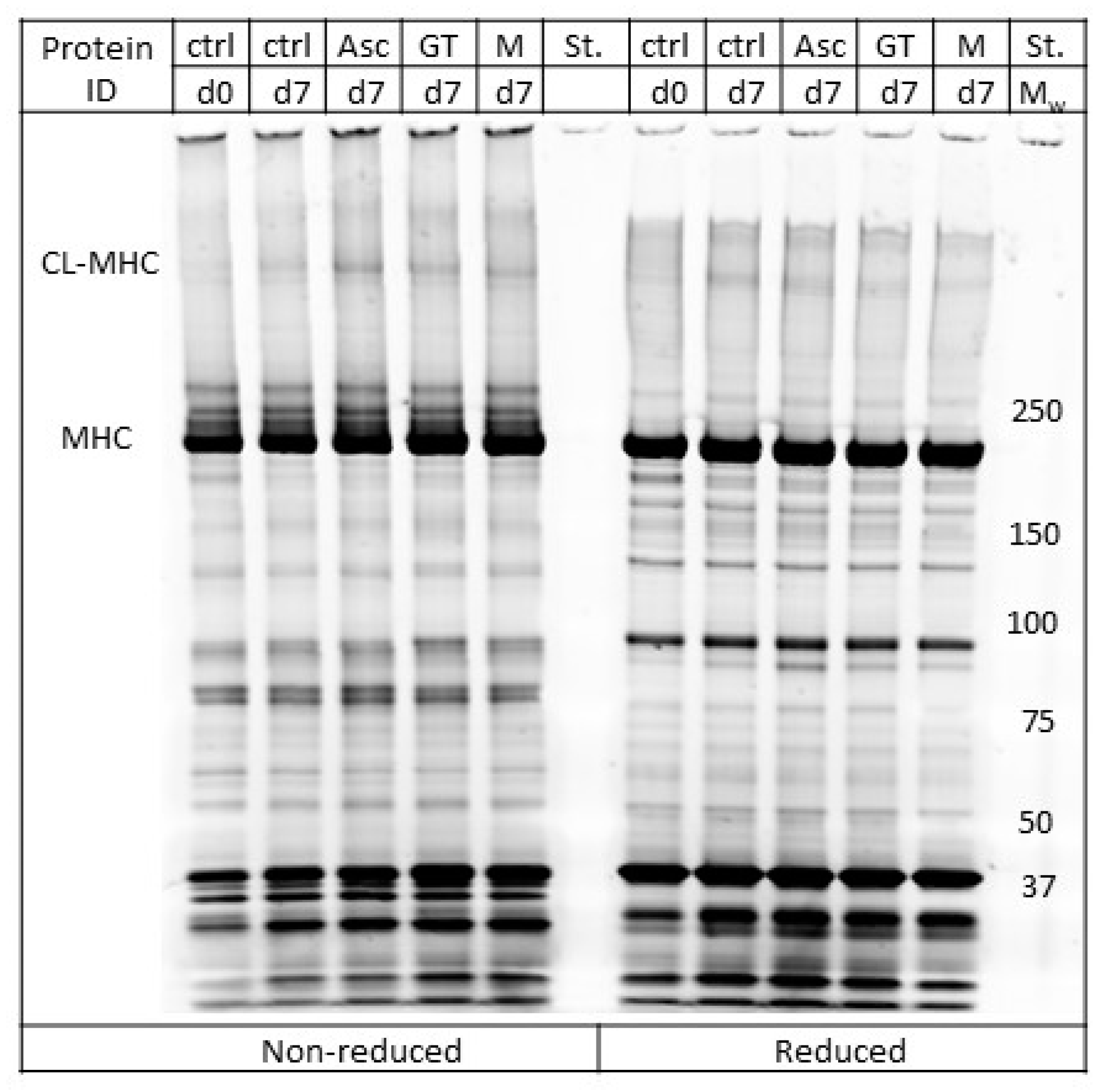
2.8. SDS-PAGE
2.9. Determination of α-Aminoadipic Semialdehyde (AAS)
2.10. Sensory Analysis
2.11. Statistical Data Analysis
3. Results
3.1. Product Analyses
3.2. Color Changes
3.3. Sensory Quality
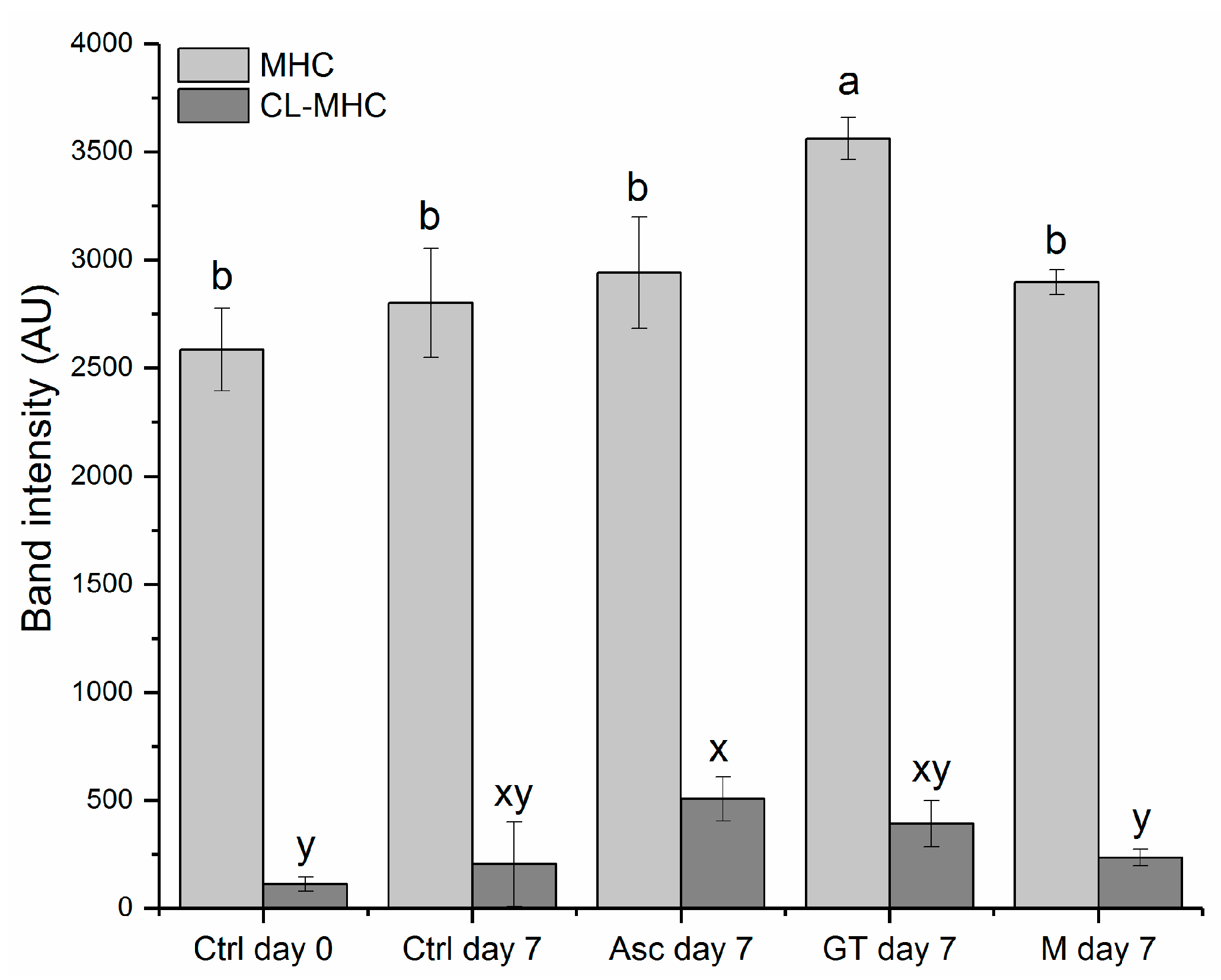
3.4. Oxidative Protein Modifications
3.5. Ascorbate in Brine-Injected Pork
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sheard, P.R.; Tali, A. Injection of salt, tripolyphosphate and bicarbonate marinade solutions to improve the yield and tenderness of cooked pork loin. Meat Sci. 2004, 68, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Walsh, H.; Martins, S.; O’Neill, E.E.; Kerry, J.P.; Kenny, T.; Ward, P. The effects of different cooking regimes on the cook yield and tenderness of non-injected and injection enhanced forequarter beef muscles. Meat Sci. 2010, 84, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Grobbel, J.P.; Dikeman, M.E.; Hunt, M.C.; Milliken, G.A. Effects of different packaging atmospheres and injection-enhancement on beef tenderness, sensory attributes, desmin degradation, and display color. J. Anim. Sci. 2008, 86, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Hunt, M.C.; Nair, M.N.; Rentfrow, G. Improving beef color stability: Practical strategies and underlying mechanisms. Meat Sci. 2014, 98, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.H.; Ockerman, H.W. Effect of ascorbic acid with tumbling on lipid oxidation of precooked roast beef 1. J. Muscle Food 2004, 15, 83–93. [Google Scholar] [CrossRef]
- Sato, K.; Hegarty, G.R. Warmed-over flavor in cooked meats. J. Food Sci. 1971, 36, 1098–1102. [Google Scholar] [CrossRef]
- Mitsumoto, M.; O’Grad, M.N.; Kerry, J.P.; Buckley, D.J. Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005, 69, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Racanicci, A.M.C.; Menten, J.F.M.; Alencar, S.M.; Buissa, R.S.; Skibsted, L.H. Mate (Ilex paraguariensis) as dietary additive for broilers: Performance and oxidative stability of meat. Eur. Food Res. Technol. 2011, 232, 655–661. [Google Scholar] [CrossRef]
- Gravador, R.S.; Jongberg, S.; Andersen, M.L.; Luciano, G.; Priolo, A.; Lund, M.N. Dietary citrus pulp improves protein stability in lamb meat stored under aerobic conditions. Meat Sci. 2014, 97, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mielnik, M.B.; Sem, S.; Egelandsdal, B.; Skrede, G. By-products from herbs essential oil production as ingredient in marinade for turkey thighs. LWT Food Sci. Technol. 2008, 41, 93–100. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jin, S.K.; Park, W.Y.; Kim, B.W.; Joo, S.T.; Yang, H.S. The effect of garlic or onion marinade on the lipid oxidation and meat quality of pork during cold storage. J. Food Qual. 2010, 33, 171–185. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Heck, C.I.; De Mejia, E.G. Yerba Mate tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- De Zawadzki, A.; Arrivetti, L.O.R.; Vidal, M.P.; Catai, J.R.; Nassu, R.T.; Tullio, R.R.; Berndt, A.; Oliveira, C.R.; Ferreira, A.G.; Neves-Junior, L.F.; et al. Mate extract as feed additive for improvement of beef quality. Food Res. Int. 2017, 99, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Jongberg, S.; Mari Ann, T.; Skibsted, L.H. Dose-Dependent Effects of Green Tea or Maté Extracts on Lipid and Protein Oxidation in Brine-Injected Retail-Packed Pork Chops. Medicines 2018. submitted for publication. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Jongberg, S.; Lund, M.N.; Østdal, H.; Skibsted, L.H. Phenolic Antioxidant Scavenging of Myosin Radicals Generated by Hypervalent Myoglobin. J. Agric. Food Chem. 2012, 60, 12020–12028. [Google Scholar] [CrossRef] [PubMed]
- Nordisk Metodikkomite for Levnedsmidler Chloride (Salt). Determination in Foods by Potentiometric Titration; Nordisk Metodikkomite for Levnedsmidler Chloride: Copenhagen, Denmark, 2004. [Google Scholar]
- Sharma, G.; Bala, R. Digital Color Imagine Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Park, D.; Xiong, Y.L.L.; Alderton, A.L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chem. 2007, 101, 1239–1246. [Google Scholar] [CrossRef]
- Koutina, G.; Jongberg, S.; Skibsted, L.H. Protein and lipid oxidation in Parma ham during production. J. Agric. Food Chem. 2012, 60, 9737–9745. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jongberg, S.; Wen, J.; Tørngren, M.A.; Lund, M.N. Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packag. Shelf Life 2014, 1, 38–48. [Google Scholar] [CrossRef]
- Akagawa, M.; Sasaki, D.; Ishii, Y.; Kurota, Y.; Yotsu-Yamashita, M.; Uchida, K.; Suyama, K. New method for the quantitative determination of major protein carbonyls, alpha-aminoadipic and gamma-glutamic semialdehydes: Investigation of the formation mechanism and chemical nature in vitro and in vivo. Chem. Res. Toxicol. 2006, 19, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Suyama, K.; Uchida, K. Fluorescent detection of a-aminoadipic and g-glutamic semialdehydes in oxidized proteins. Free Radic. Biol. Med. 2009, 46, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Morcuende, D.; Ventanas, S. Determination of oxidation. In Handbook of Processed Meats and Poultry Analysis; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Utrera, M.; Morcuende, D.; Rodríguez-Carpena, J.G.; Estévez, M. Fluorescent HPLC for the detection of specific protein oxidation carbonyls-α-aminoadipic and γ-glutamic semialdehydes—In meat systems. Meat Sci. 2011, 89, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Soerheim, O.; Hoey, M. Effects of food ingredients and oxygen exposure on premature browning in cooked beef. Meat Sci. 2013, 93, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Clausen, I.; Jakobsen, M.; Ertbjerg, P.; Madsen, N.T. Modified atmosphere packaging affects lipid oxidation, myofibrillar fragmentation index and eating quality of beef. Packag. Technol. Sci. 2009, 22, 85–96. [Google Scholar] [CrossRef]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Kroll, J.; Hohl, U.C. Model studies on reactions of plant phenols with whey proteins. Mol. Nutr. Food Res. 2001, 45, 72–81. [Google Scholar] [CrossRef]
- Jongberg, S.; Lund, M.N.; Waterhouse, A.L.; Skibsted, L.H. 4-Methyl catechol inhibits protein oxidation in meat but not disulfide formation. J. Agric. Food Chem. 2011, 59, 10329–10335. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Wagner, J.R.; Gagnon, H.; Day, R.; Klarskov, K. Modification of Peptide and Protein Cysteine Thiol Groups by Conjugation with a Degradation Product of Ascorbate. Chem. Res. Toxicol. 2013, 26, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Lei, N.; Wang, S.; Xu, X.; Zhou, G.; Li, Z. Emulsifying Properties of Oxidatively Stressed Myofibrillar Protein Emulsion Gels Prepared with (−)-Epigallocatechin-3-gallate and NaCl. J. Agric. Food Chem. 2017, 65, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, L.; Shao, J.; Liu, D.; Kong, B. Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 2017, 127, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jongberg, S.; Terkelsen, L.D.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Elias, R.J. Investigating the hydrogen peroxide quenching capacity of proteins in polyphenol-rich foods. J. Agric. Food Chem. 2011, 59, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; O’Neill, E.E.; Kerry, J.P. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine, M. longissimus dorsi muscle during chilled storage. Food Chem. 2012, 131, 527–532. [Google Scholar] [CrossRef]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Hviid, M.S.; Skibsted, L.H. The combined effect of antioxidants and modified atmosphere packaging on protein and lipid oxidation in beef patties during chill storage. Meat Sci. 2007, 76, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rysman, T.; Van Hecke, T.; De Smet, S.; Van Royen, G. Ascorbate and Apple Phenolics Affect Protein Oxidation in Emulsion-Type Sausages during Storage and in Vitro Digestion. J. Agric. Food Chem. 2016, 64, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Csala, M.; Szarka, A.; Varsányi, M. Role of ascorbate in oxidative folding. Biofactors 2003, 17, 37–46. [Google Scholar] [CrossRef] [PubMed]

| Treatment a | n | NaCl | Ascorbate b | Phenols b | p Time |
|---|---|---|---|---|---|
| (g/100 g) | (ppm) | (ppm) | |||
| Control | 6 | 0.85 ± 0.11 | - | - | 0.0307 (*) |
| Ascorbate (~225 ppm) | 18 | 0.90 ± 0.18 | 225.6 ± 40.0 | - | 0.9878 |
| Green Tea extract (~25 ppm GAE c) | 18 | 0.86 ± 0.12 | - | 24.0 ± 2.2 | 0.1260 |
| Maté extract (~25 ppm GAE c) | 18 | 0.93 ± 0.15 | - | 24.5 ± 3.4 | 0.4670 |
| p Treatment | 0.3909 | - | 0.6141 |
| Treatment x | Storage Time | Lightness | Redness | Yellowness | ΔE |
|---|---|---|---|---|---|
| (days) | (L*) | (a*) | (b*) | ||
| Control (n = 6) | 0 | 60.9 ± 1.5 b | 17.6 ± 0.6 a | 15.3 ± 0.2 a | 0.0 |
| 3 | 63.2 ± 1.2 a | 18.0 ± 1.1 a | 14.9 ± 0.6 a | 2.4 | |
| 7 | 63.8 ± 1.5 a | 15.4 ± 1.1 b | 14.3 ± 0.6 b | 3.8 | |
| Ascorbate (~225 ppm, n = 6) | 0 | 61.1 ± 2.0 b | 17.0 ± 0.6 b | 14.9 ± 0.3 a | 0.0 |
| 3 | 63.4 ± 0.6 a | 18.2 ± 0.7 a | 15.3 ± 0.5 a | 2.6 | |
| 7 | 64.1 ± 1.3 a | 16.4 ± 1.0 b | 14.3 ± 0.5 b | 3.1 | |
| Green Tea extract (~25 ppm GAE y, n = 6) | 0 | 60.3 ± 1.4 c | 16.5 ± 0.8 b | 14.7 ± 0.7 a,b | 0.0 |
| 3 | 62.8 ± 1.6 b | 17.8 ± 1.1 a | 15.2 ± 0.5 a | 2.8 | |
| 7 | 64.0 ± 1.0 a | 15.4 ± 0.9 c | 14.3 ± 0.5 b | 3.8 | |
| Maté extract (~25 ppm GAE y, n = 6) | 0 | 60.5 ± 1.2 b | 16.7 ± 0.5 b | 14.6 ± 0.2 a,b | 0.0 |
| 3 | 62.9 ± 1.1 a | 17.9 ± 0.9 a | 14.9 ± 0.6 a | 3.0 | |
| 7 | 62.9 ± 1.3 a | 15.8 ± 0.8 b | 14.1 ± 0.4 b | 2.7 |
| Marker of Protein Oxidation | Storage Time * | Control | Ascorbate | Green Tea Extract | Maté Extract |
|---|---|---|---|---|---|
| (days) | (~225 ppm) | (~25 ppm GAE **) | (~25 ppm GAE **) | ||
| Protein thiols (nmol/mg protein, n = 6) | 0 | 62.2 ± 2.2 | 61.2 ± 1.8 x | 61.5 ± 1.6 x,y | 63.9 ± 2.1 x |
| 3 | 59.4 ± 3.6 a | 56.6 ± 3.4 b,y | 59.0 ± 2.6 a,y | 58.1 ± 2.8 a,b,y | |
| 7 | 60.6 ± 1.7 a | 57.8 ± 1.7 b,y | 61.7 ± 1.7 a,x | 61.0 ± 0.7 a,y,x | |
| AAS (pmol/mg MPI, n = 3) | 0 | 62.5 ± 0.0 b | 61.1 ± 7.3 b,x | 55.4 ± 12.4 b,y | 112.5 ± 10.2 a,x |
| 3 | 82.2 ± 15.3 | 108.9 ± 17.5 x | 104.5 ±3.1 x | 80.5 ± 3.0 y | |
| 7 | 64.2 ± 4.6 b | 114.2 ± 27.5 a,x | 110.9 ± 20.6 a,x | 129.2 ± 9.4 a,x |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jongberg, S.; Tørngren, M.A.; Skibsted, L.H. Protein Oxidation and Sensory Quality of Brine-Injected Pork Loins Added Ascorbate or Extracts of Green Tea or Maté during Chill-Storage in High-Oxygen Modified Atmosphere. Medicines 2018, 5, 7. https://doi.org/10.3390/medicines5010007
Jongberg S, Tørngren MA, Skibsted LH. Protein Oxidation and Sensory Quality of Brine-Injected Pork Loins Added Ascorbate or Extracts of Green Tea or Maté during Chill-Storage in High-Oxygen Modified Atmosphere. Medicines. 2018; 5(1):7. https://doi.org/10.3390/medicines5010007
Chicago/Turabian StyleJongberg, Sisse, Mari Ann Tørngren, and Leif H. Skibsted. 2018. "Protein Oxidation and Sensory Quality of Brine-Injected Pork Loins Added Ascorbate or Extracts of Green Tea or Maté during Chill-Storage in High-Oxygen Modified Atmosphere" Medicines 5, no. 1: 7. https://doi.org/10.3390/medicines5010007





