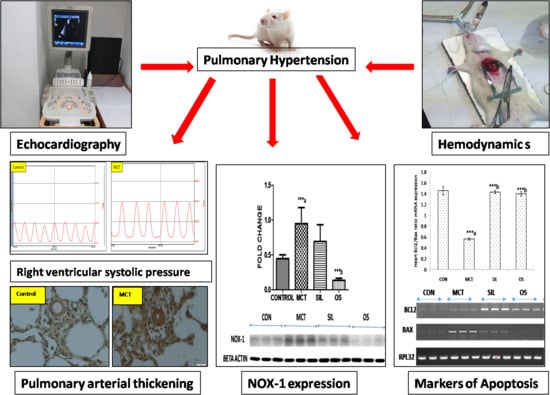
Beneficial Effect of Ocimum sanctum (Linn) against Monocrotaline-Induced Pulmonary Hypertension in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Experimental Animals
2.3. Drugs and Chemicals
2.4. Experimental Design
- Control: Normal saline was administered subcutaneously once in a dose of 1.0 mL/kg and observed for 42 days.
- MCT: Monocrotaline was administered subcutaneously in a single dose of 50 mg/kg on day 1 and observed for 42 days.
- MCT + SIL: Rats were injected MCT (50 mg/kg; sc) followed by sildenafil 175 µg/kg/day orally once daily started from day 29 after MCT administration and continued for 14 days.
- MCT + OS 200: Rats were injected MCT (50 mg/kg; sc) followed by OS (200 mg/kg/day) (the more effective dose taken from previous preventive study) orally started from day 29 after MCT administration and continued for 14 days.
2.5. Parameters Done
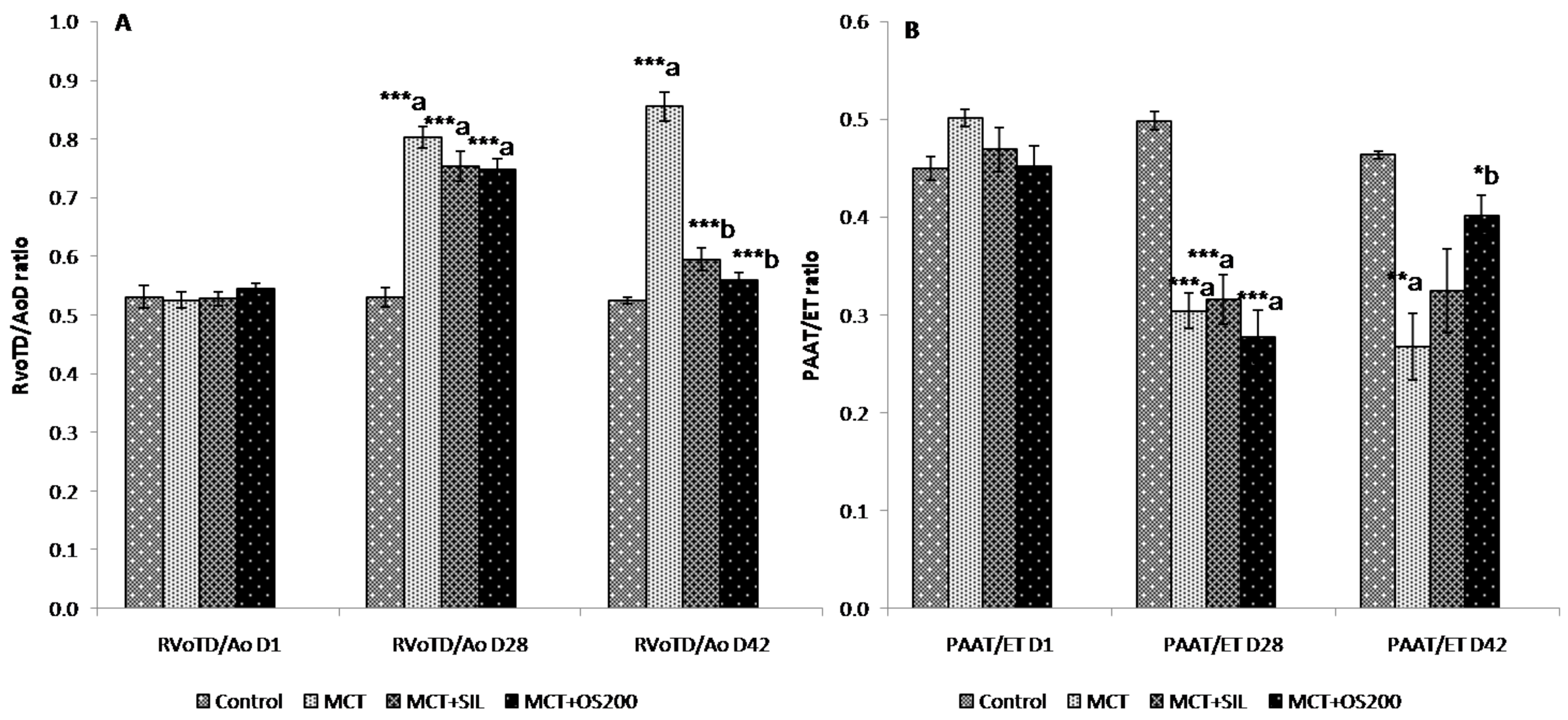
2.5.1. Echocardiography
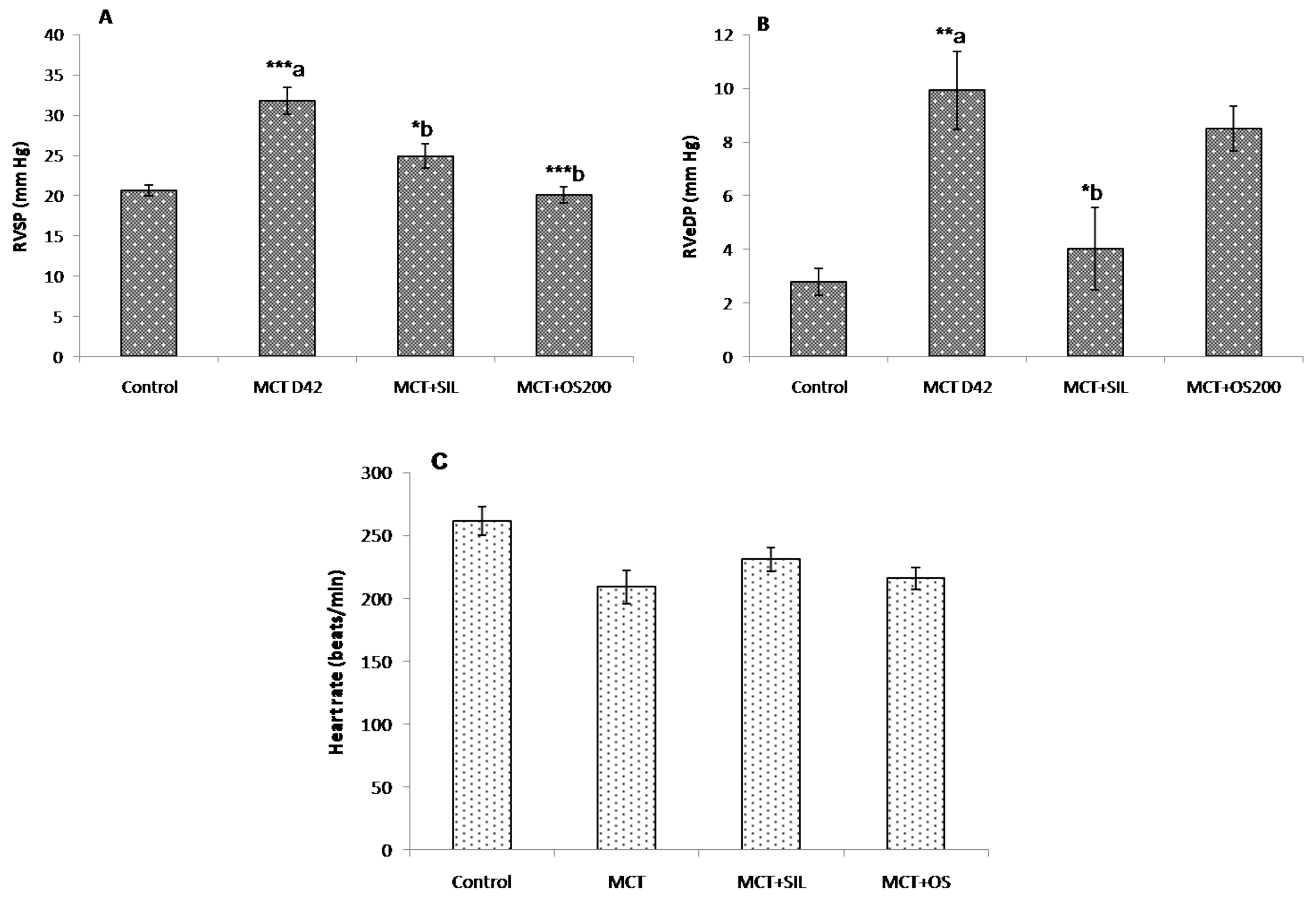
2.5.2. Hemodynamic Parameters
2.5.3. Morphometric Parameters
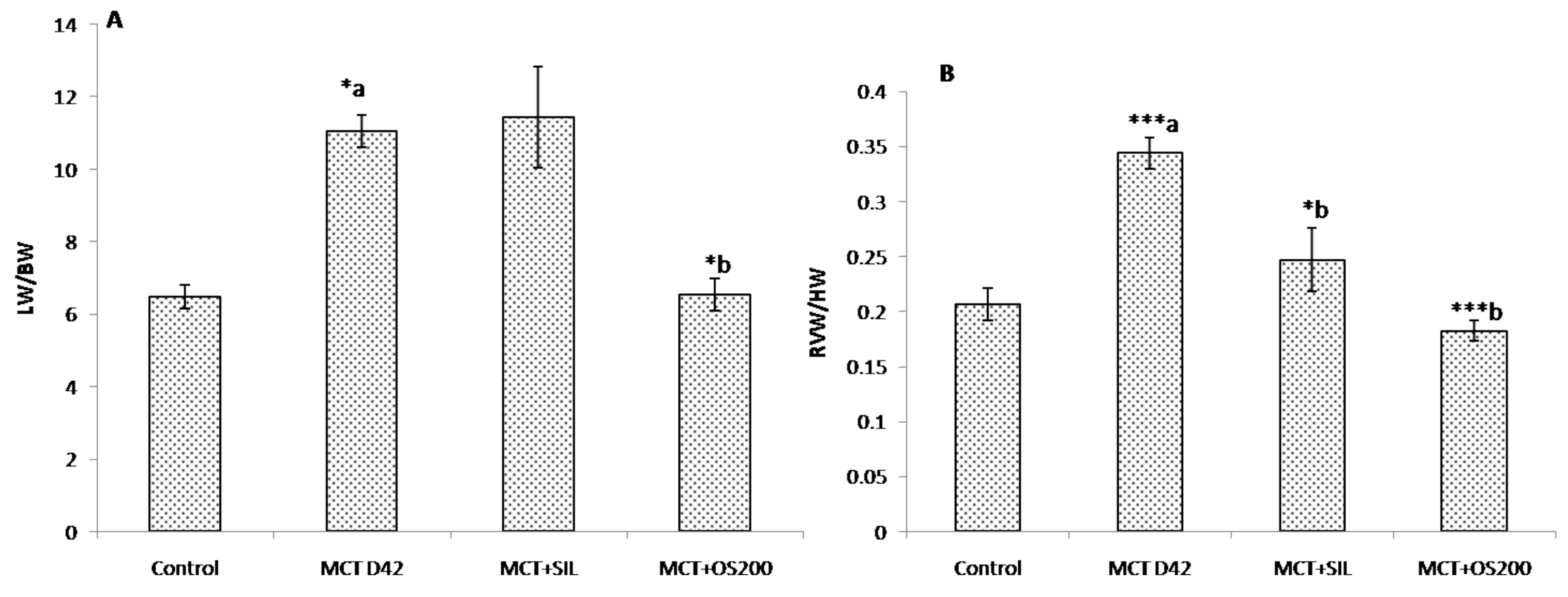
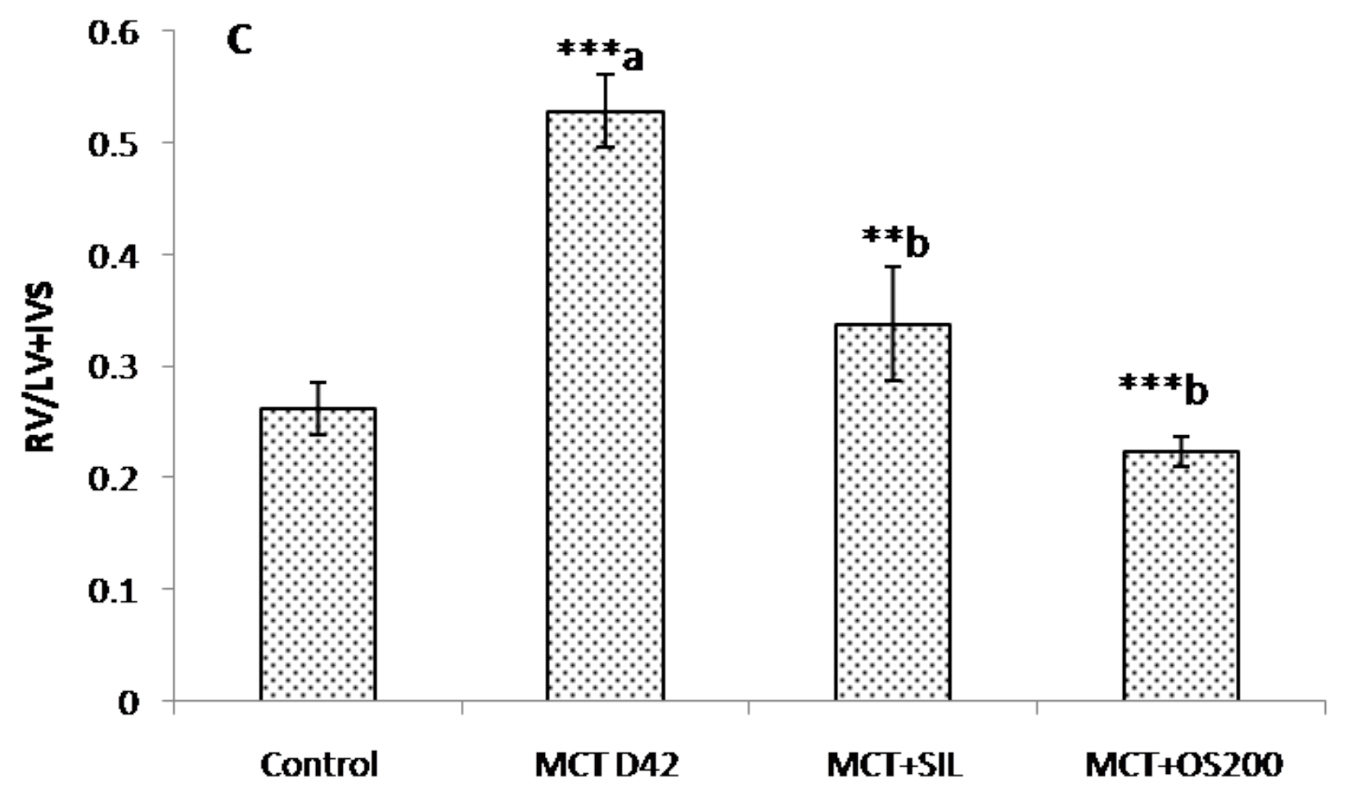
2.5.4. Right Ventricular Hypertrophy Index (RVH Index)
2.5.5. Lung Tissue
2.5.6. Histopathology
2.5.7. Oxidative Stress Markers in Lung Tissue
Thiobarbituric Acid Reactive Substances (TBARS) in Lung Homogenate
Reduced Glutathione (GSH) Levels in Lung Homogenate
Catalase Levels in Lung Homogenate
Superoxide Dismutase (SOD) Enzyme Levels in Lung Homogenate
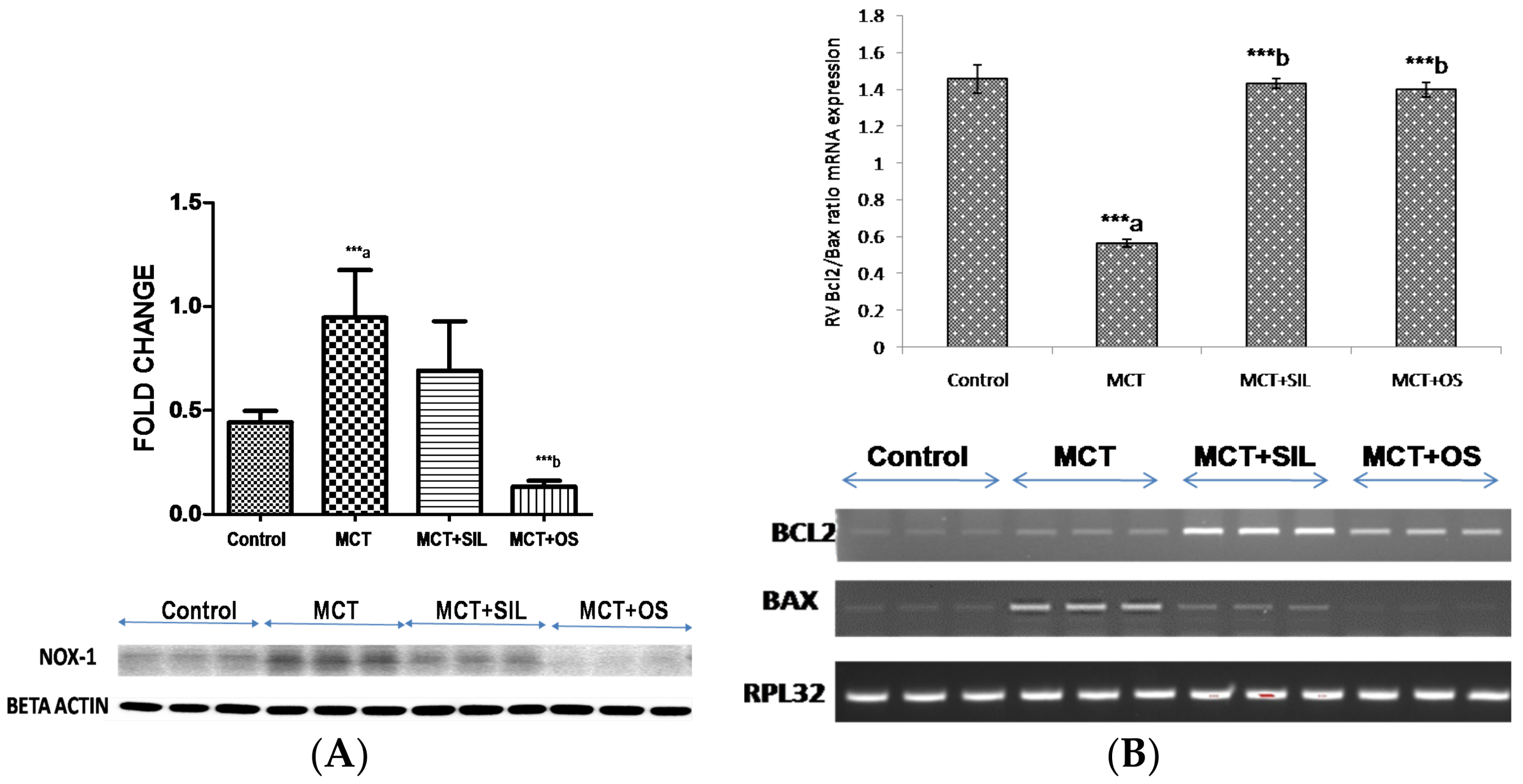
2.5.8. Western Blot Analysis of Lung NADPH Oxidase (Nox-1)
2.5.9. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Morphometric Parameters and Right Ventricular Hypertrophy Index (RVH Index)
3.2. Hemodynamic Parameters
3.3. Echocardiography
3.4. Histopathology
3.5. Study of Oxidative Stress Parameters in Lung Tissue
3.5.1. Thiobarbituric Acid Reactive Substances (TBARS)
3.5.2. Reduced Glutathione (GSH)
3.5.3. Catalase
3.5.4. Superoxide Dismutase (SOD)
3.6. Western Blot Analysis of Lung NADPH Oxidase (Nox-1)
3.7. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, V.; Kahol, A.; Singh, I.P.; Saraf, I.; Shri, R. Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in-vitro and in-vivo models. J. Ethnopharmacol. 2016, 193, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Godhwani, S.; Godhwani, J.L.; Vyas, D.S. Ocimum sanctum—A preliminary study evaluating its immunoregulatory profile in albino rats. J. Ethnopharmacol. 1988, 24, 193–198. [Google Scholar] [CrossRef]
- Singh, S.; Majumdar, D.K.; Rehan, H.M. Evaluation of anti-inflammatory potential of fixed oil of Ocimum sanctum (Holybasil) and its possible mechanism of action. J. Ethnopharmacol. 1996, 54, 19–26. [Google Scholar] [CrossRef]
- Singh, S.; Taneja, M.; Majumdar, D.K. Biological activities of Ocimum sanctum L. fixed oil—An overview. Indian J. Exp. Biol. 2007, 45, 403–412. [Google Scholar] [PubMed]
- Gupta, S.K.; Prakash, J.; Srivastava, S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J. Exp. Biol. 2002, 40, 765–773. [Google Scholar] [PubMed]
- Mohanty, I.; Arya, D.S.; Gupta, S.K. Effect of Curcuma longa and Ocimum sanctum on myocardial apoptosis in experimentally induced myocardial ischemic-reperfusion injury. BMC Complement. Altern. Med. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rehan, H.M.; Majumdar, D.K. Effect of Ocimum sanctum fixed oil on blood pressure, blood clotting time and pentobarbitone-induced sleeping time. J. Ethnopharmacol. 2001, 78, 139–143. [Google Scholar] [CrossRef]
- Sood, S.; Narang, D.; Dinda, A.K.; Maulik, S.K. Chronic oral administration of Ocimum sanctum Linn. augments cardiac endogenous antioxidants and prevents isoproterenol-induced myocardial necrosis in rats. J. Pharm. Pharmacol. 2005, 57, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Uma Devi, P.; Ganasoundari, A.; Vrinda, B.; Srinivasan, K.K.; Unnikrishnan, M.K. Radiation protection by the Ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat. Res. 2000, 154, 455–460. [Google Scholar] [CrossRef]
- Mediratta, P.K.; Dewan, V.; Bhattacharya, S.K.; Gupta, V.S.; Maiti, P.C.; Sen, P. Effect of Ocimum sanctum Linn. on humoral immune responses. Indian J. Med. Res. 1988, 87, 384–386. [Google Scholar] [PubMed]
- Suanarunsawat, T.; Ayutthaya, W.D.N.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Lipid-lowering and antioxidative activities of aqueous extracts of Ocimum sanctum L. leaves in rats fed with a high-cholesterol diet. Oxid. Med. Cell. Longev. 2011, 2011, 962025. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Narang, D.; Thomas, M.K.; Gupta, Y.K.; Maulik, S.K. Effect of Ocimum sanctum Linn. on cardiac changes in rats subjected to chronic restraint stress. J. Ethnopharmacol. 2006, 108, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; VidjayaLetchoumy, P.; Prathiba, D.; Nagini, S. Proliferation, angiogenesis and apoptosis-associated proteins are molecular targets for chemoprevention of MNNG-induced gastric carcinogenesis by ethanolic Ocimum sanctum leaf extract. Singap. Med. J. 2007, 48, 645–651. [Google Scholar]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Andersen, A.; Kirkfeldt, R.E.; Hansen, K.N.; Mellemkjær, S.; Nielsen-Kudsk, J.E. Survival in an incident cohort of patients with pulmonary arterial hypertension in Denmark. Pulm. Circ. 2015, 5, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Veit, F.; Pak, O.; Egemnazarov, B.; Roth, M.; Kosanovic, D.; Seimetz, M.; Sommer, N.; Ghofrani, H.A.; Seeger, W.; Grimminger, F.; et al. Function of NADPH Oxidase 1 in Pulmonary Arterial Smooth Muscle Cells after Monocrotaline-Induced Pulmonary Vascular Remodeling. Antioxid. Redox Signal. 2013, 19, 2213–2231. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.Y.; Montezano, A.C.; Harvey, A.P.; Nilsen, M.; MacLean, M.R.; Touyz, R.M. Nicotinamide Adenine Dinucleotide Phosphate Oxidase-Mediated Redox Signaling and Vascular Remodeling by 16α-Hydroxyestrone in Human Pulmonary Artery Cells: Implications in Pulmonary Arterial Hypertension. Hypertension 2016, 68, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mao, M.; Qiu, Y.; Liu, G.; Sheng, T.; Yu, X.; Wang, S.; Zhu, D. Key Role of ROS in the Process of 15-Lipoxygenase/15-Hydroxyeicosatetraenoiccid-Induced Pulmonary Vascular Remodeling in Hypoxia Pulmonary Hypertension. PLoS ONE 2016, 11, e0149164. [Google Scholar] [CrossRef] [PubMed]
- Paffett, M.L.; Lucas, S.N.; Campen, M.J. Resveratrol Reverses Monocrotaline-Induced Pulmonary Vascular and Cardiac Dysfunction: A Potential Role for Atrogin-1 in Smooth Muscle. Vascul. Pharmacol. 2012, 56, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zuckerbraun, B.S.; Chin, B.Y.; Wegiel, B.; Billiar, T.R.; Czsimadia, E.; Rao, J.; Shimoda, L.; Ifedigbo, E.; Kanno, S.; Otterbein, L.E. Carbon monoxide reverses established pulmonary hypertension. J. Exp. Med. 2006, 203, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Harada, T.; Kikuzuki, R.; Yamawaki, H.; Hara, Y. Effects of telmisartan on right ventricular remodeling induced by monocrotaline in rats. J. Pharmacol. Sci. 2009, 111, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwase, M.; Kanazawa, H.; Kawata, N.; Yoshimori, Y.; Hashimoto, K.; Yokoi, T.; Noda, A.; Takagi, K.; Koike, Y.; et al. Progressive Development of Pulmonary Hypertension Leading to Right Ventricular Hypertrophy Assessed by Echocardiography in Rats. Exp. Anim. 2003, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Slama, M.; Susic, D.; Varagic, J.; Ahn, J.; Frohlich, E.D. Echocardiographic measurement of cardiac output in rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H691–H697. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.B.; Kurtz, B.; Raher, M.J.; Shaik, R.S.; Waxman, A.; Derumeaux, G.; Halpern, E.F.; Bloch, K.D.; Scherrer-Crosbie, M. Noninvasive assessment of murine pulmonary arterial pressure: Validation and application to models of pulmonary hypertension. Circ. Cardiovasc. Imaging 2010, 3, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kosanovic, D.; Kojonazarov, B.; Luitel, H.; Dahal, B.K.; Sydykov, A.; Cornitescu, T.; Janssen, W.; Brandes, R.P.; Davie, N.; Ghofrani, H.A.; et al. Therapeutic efficacy of TBC3711 in monocrotaline-induced pulmonary hypertension. Respir. Res. 2011, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Mendes, L.; Rudd, M.A.; Russo, G.; Loscalzo, J.; Zhang, Y.-Y. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H364–H371. [Google Scholar] [CrossRef] [PubMed]
- Werchan, P.M.; Summer, W.R.; Gerdes, A.M.; McDonough, K.H. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am. J. Physiol. 1989, 256, H1328–H1336. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.E.; Li, D.; Zhan, X.; Garcia, J.G.N.; Tuder, R.M.; Hassoun, P.M.; Johns, R.A. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H938–H945. [Google Scholar] [CrossRef] [PubMed]
- Silverton, N.; Meineri, M.; Djaiani, G. The controversy of right ventricular systolic pressure: Is it time to abandon the pulmonary artery catheter? Anaesthesia 2015, 70, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Wölkart, G.; Haleen, S. Defective intracellular calcium handling in monocrotaline-induced right ventricular hypertrophy: Protective effect of long-term endothelin-A receptor blockade with 2-benzo[1,3]dioxol-5-yl-3-benzyl-4-(4-methoxy-phenyl-)-4-oxobut-2-enoate-sodium (PD 155080). J. Pharmacol. Exp. Ther. 2002, 300, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Falcao-Pires, I.; Gonçalves, N.; Henriques-Coelho, T.; Moreira-Gonçalves, D.; Roncon-Albuquerque, R., Jr.; Leite-Moreira, A.F. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H2007–H2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Mao, L.; Rajagopal, S. Hemodynamic Characterization of Rodent Models of Pulmonary Arterial Hypertension. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cilley, R.E.; Wang, J.Y.; Coran, A.G. Lung injury produced by moderate lung overinflation in rats. J. Pediatr. Surg. 1993, 28, 488–493. [Google Scholar] [CrossRef]
- Ito, T.; Okada, T.; Miyashita, H.; Nomoto, T.; Nonaka-Sarukawa, M.; Uchibori, R.; Maeda, Y.; Urabe, M.; Mizukami, H.; Kume, A.; et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ. Res. 2007, 101, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Prié, S.; Leung, T.K.; Cernacek, P.; Ryan, J.W.; Dupuis, J. The orally active ET(A) receptor antagonist (+)-(S)-2-(4,6-dimethoxy-pyrimidin-2-yloxy)-3-methoxy-3,3-diphe nyl-propionic acid (LU 135252) prevents the development of pulmonary hypertension and endothelial metabolic dysfunction in monocrotaline-treated rats. J. Pharmacol. Exp. Ther. 1997, 282, 1312–1318. [Google Scholar] [PubMed]
- Kay, J.M.; Keane, P.M.; Suyama, K.L.; Gauthier, D. Angiotensin converting enzyme activity and evolution of pulmonary vascular disease in rats with monocrotaline pulmonary hypertension. Thorax 1982, 37, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Banerjee, S.K.; Wang, D.W.; Alzamora, R.; Huang, X.N.; Pastor-Soler, N.M.; Hallows, K.R.; McGaffin, K.R.; Ahmad, F. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J. Mol. Cell. Cardiol. 2010, 49, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Meghwani, H.; Prabhakar, P.; Mohammed, S.A.; Seth, S.; Hote, M.P.; Banerjee, S.K.; Arava, S.; Ray, R.; Maulik, S.K. Beneficial effects of aqueous extract of stem bark of Terminalia arjuna (Roxb.), An ayurvedic drug in experimental pulmonary hypertension. J. Ethnopharmacol. 2017, 197, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Boissiere, J.; Gautier, M.; Machet, M.-C.; Hanton, G.; Bonnet, P.; Eder, V. Doppler tissue imaging in assessment of pulmonary hypertension-induced right ventricle dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2450–H2455. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Gong, Q.H.; Wei, L.W.; Wu, Q.; Huang, X.-N. Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol. Pharm. Bull. 2008, 31, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Ilgin, S.; Atli, O.; Ergun, B.; Sirmagul, B. The effects of gender difference on monocrotaline-induced pulmonary hypertension in rats. Hum. Exp. Toxicol. 2013, 32, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Nasu, T.; Sonoda, H.; Ito, K.M.; Ikeda, M.; Ito, K. Evaluation of olmesartan medoxomil in the rat monocrotaline model of pulmonary hypertension. J. Cardiovasc. Pharmacol. 2008, 51, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, T.K.; Wanstall, J.C. Pulmonary vascular remodeling: A target for therapeutic intervention in pulmonary hypertension. Pharmacol. Ther. 2001, 92, 1–20. [Google Scholar] [CrossRef]
- Bogdan, S.; Seferian, A.; Totoescu, A.; Dumitrache-Rujinski, S.; Ceausu, M.; Coman, C.; Ardelean, C.M.; Dorobantu, M.; Bogdan, M. Sildenafil reduces inflammation and prevents pulmonary arterial remodeling of the monocrotaline-induced disease in the wistar rats. Maedica 2012, 7, 109–116. [Google Scholar] [PubMed]
- Burch, G.H.; Jensen, L.R.; Pappas, J.; Hammond, E.H.; Banner, W., Jr.; Shaddy, R.E. Growth factor expression and effects of amrinone in monocrotaline-induced pulmonary hypertension in rats. Biochem. Mol. Med. 1996, 58, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Koskenvuo, J.W.; Mirsky, R.; Zhang, Y.; Angeli, F.S.; Jahn, S.; Alastalo, T.P.; Schiller, N.B.; Boyle, A.J.; Chatterjee, K.; De Marco, T.; et al. A comparison of echocardiography to invasive measurement in the evaluation of pulmonary arterial hypertension in a rat model. Int. J. Cardiovasc. Imaging 2010, 26, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Demarco, V.G.; Whaley-Connell, A.T.; Sowers, J.R.; Habibi, J.; Dellsperger, K.C. Contribution of oxidative stress to pulmonary arterial hypertension. World J. Cardiol. 2010, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ghouleh, I.A.; Sahoo, S.; Meijles, D.N.; Amaral, J.H.; de Jesus, D.S.; Sembrat, J.; Rojas, M.; Goncharov, D.A.; Goncharova, E.A.; Pagano, P.J. Endothelial Nox1 oxidase assembly inhuman pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin. Sci. 2017, 131, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Olson, S.; Pinto, J.T.; Gupte, S.; Wu, J.M.; Hu, F.; Ballabh, P.; Podlutsky, A.; Losonczy, G.; et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 2009, 54, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Manea, S.A.; Constantin, A.; Manda, G.; Sasson, S.; Manea, A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015, 5, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Davis, M.; Cornwell, W. Pulmonary arterial hypertension. Curr. Probl. Cardiol. 2011, 36, 461–517. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.; Lecour, S.; Butrous, G.; Thienemann, F.; Sliwa, K. A comprehensive review: The evolution of animal models in pulmonary hypertension research; are we there yet? Pulm. Circ. 2013, 3, 739–756. [Google Scholar] [CrossRef] [PubMed]

| Name | Direction | Sequences | Product Size | Temperature |
|---|---|---|---|---|
| BCL-2 | Forward | CCATGACTGAGGGACCAACT | 150 bp | 56 °C |
| Reverse | CTCTTCTTCCTGCCCTTCCT | |||
| BAX | Forward | TGCAGAGGATGATTGCGACT | 200 bp | 60 °C |
| Reverse | GATCAGCTCGGGCACTTTAG | |||
| RPL32 | Forward | AGATTCAAGGGCCAGATCCT | 175 bp | 57 °C |
| Reverse | CGATGGCTTTTCGGTTCTTA |
| Groups | TBARS (nmol/mg protein) | GSH (mg/mg protein) | Catalase (U/mg protein) | SOD (U/mg protein) |
|---|---|---|---|---|
| Control | 19.60 ± 2.5 | 27.36 ± 3.0 | 0.85 ± 0.04 | 110.51 ± 10.8 |
| MCT | 155.46 ± 13.4 ***,a | 13.98 ± 2.0 ***,a | 0.21 ± 0.01 ***,a | 35.4 ± 5.7 ***,a |
| MCT + SIL | 72.36 ± 12.7 **,b | 17.22 ± 3.1 | 0.18 ± 0.02 | 51.98 ± 3.5 |
| MCT + OS | 99.05 ± 20.5 *,b | 12.99 ± 1.9 | 0.51 ± 0.04 ***,b | 41.49 ± 6.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meghwani, H.; Prabhakar, P.; Mohammed, S.A.; Dua, P.; Seth, S.; Hote, M.P.; Banerjee, S.K.; Arava, S.; Ray, R.; Maulik, S.K. Beneficial Effect of Ocimum sanctum (Linn) against Monocrotaline-Induced Pulmonary Hypertension in Rats. Medicines 2018, 5, 34. https://doi.org/10.3390/medicines5020034
Meghwani H, Prabhakar P, Mohammed SA, Dua P, Seth S, Hote MP, Banerjee SK, Arava S, Ray R, Maulik SK. Beneficial Effect of Ocimum sanctum (Linn) against Monocrotaline-Induced Pulmonary Hypertension in Rats. Medicines. 2018; 5(2):34. https://doi.org/10.3390/medicines5020034
Chicago/Turabian StyleMeghwani, Himanshu, Pankaj Prabhakar, Soheb A. Mohammed, Pamila Dua, Sandeep Seth, Milind P. Hote, Sanjay K. Banerjee, Sudheer Arava, Ruma Ray, and Subir Kumar Maulik. 2018. "Beneficial Effect of Ocimum sanctum (Linn) against Monocrotaline-Induced Pulmonary Hypertension in Rats" Medicines 5, no. 2: 34. https://doi.org/10.3390/medicines5020034