Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production
Abstract
:1. Introduction
2. Thermodynamic Modeling
2.1. Material and Methods
2.2. Reactions Involved in the Autothermal Reforming of Crude Glycerol
- Steam Reforming
ΔH298K = 127.67 kJ/mol ΔH298K = 49.5 kJ/mol ΔH298K = 283.8 kJ/mol - Partial Oxidation
ΔH298K = −603.5 kJ/mol ΔH298K = −102.5.5 kJ/mol ΔH298K = −926.2 kJ/mol - Water Gas Shift
ΔH298K = −41.8 kJ/mol - Methanation Reactions
ΔH298K = −206.8 kJ/mol ΔH298K = −165 kJ/mol - Methane Dry Reforming
ΔH298K = 246 kJ/mol - Glycerol Reforming
ΔH298K = −78.3 kJ/mol - Methanol Reforming
ΔH298K = 9.6 kJ/mol - Propanol Reforming
ΔH298K = −159 kJ/mol
3. Results and Discussions
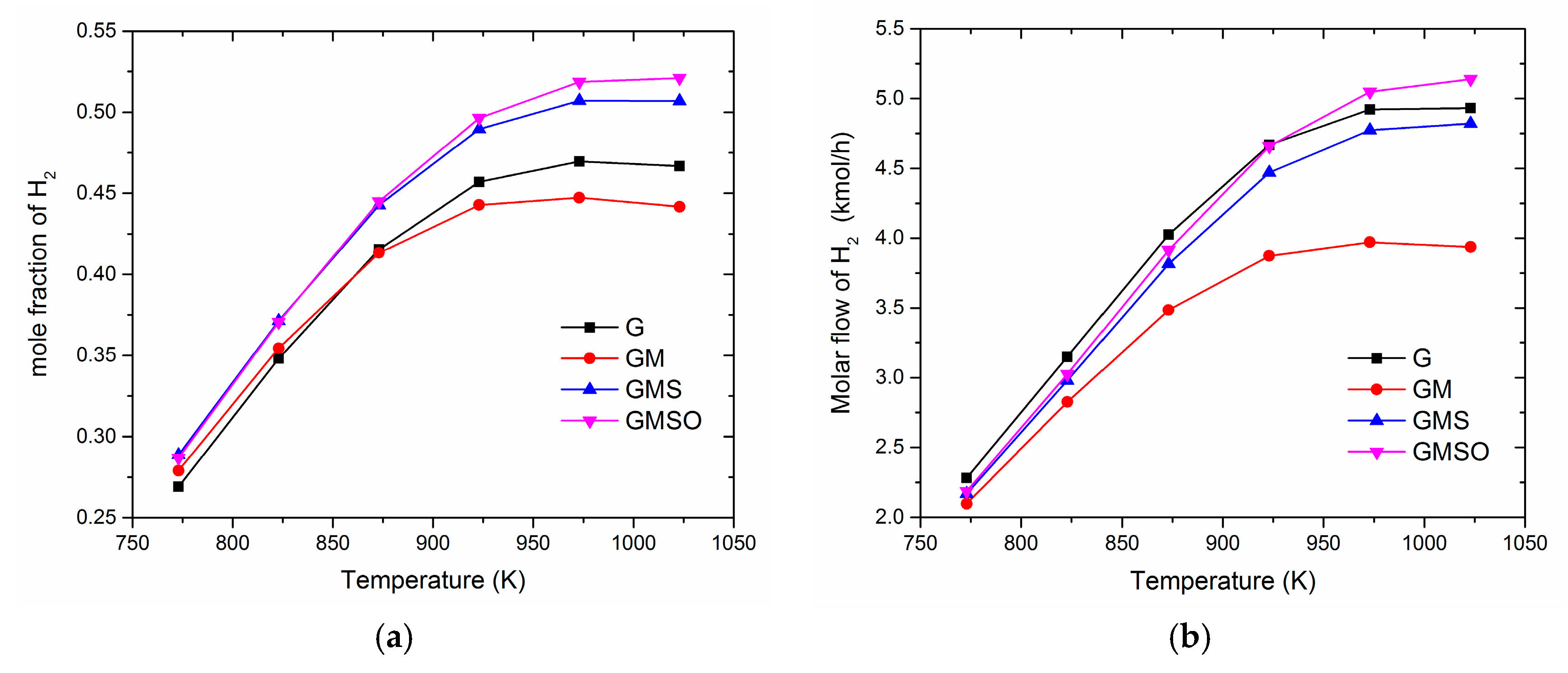
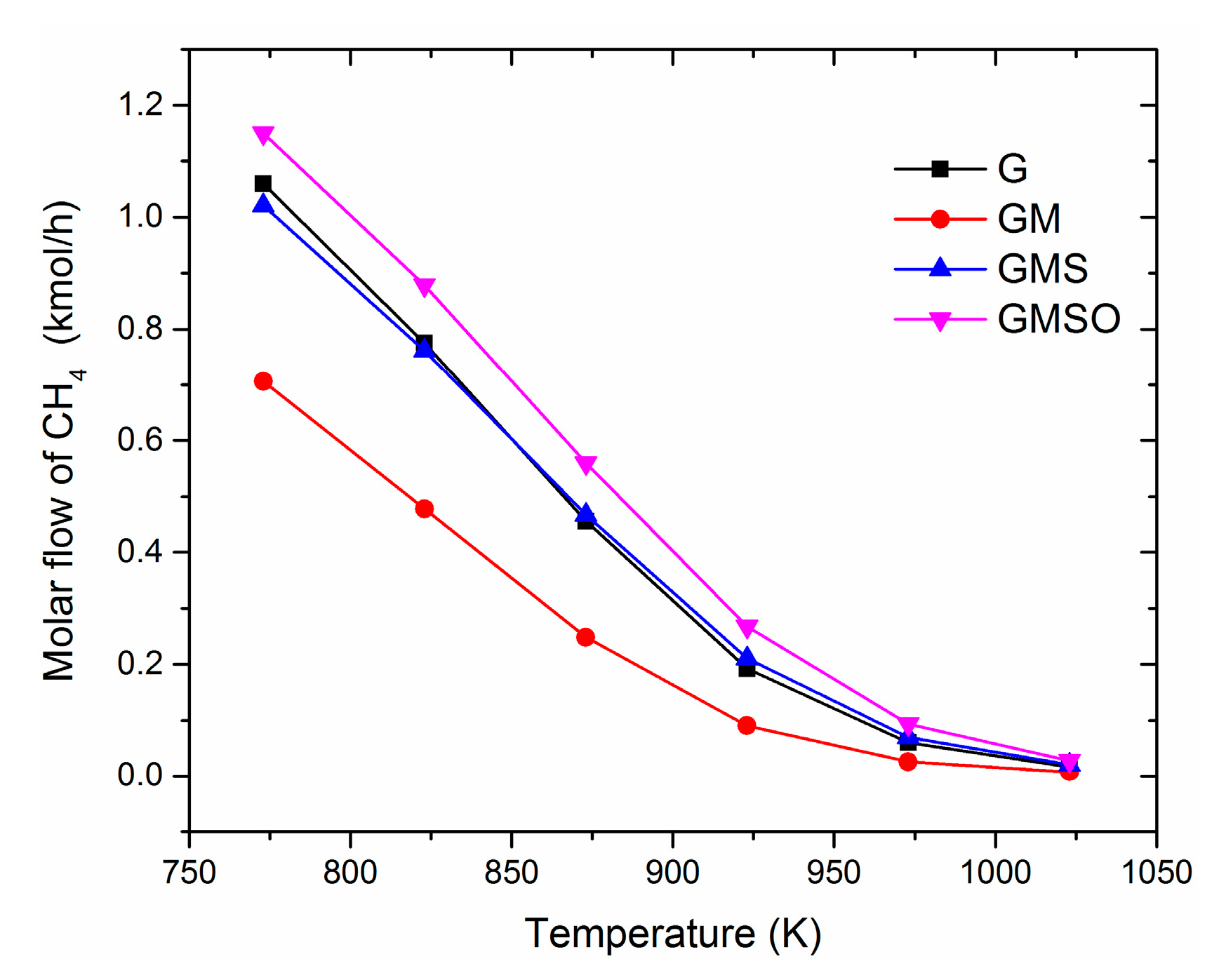
3.1. Effect of Operating Temperature
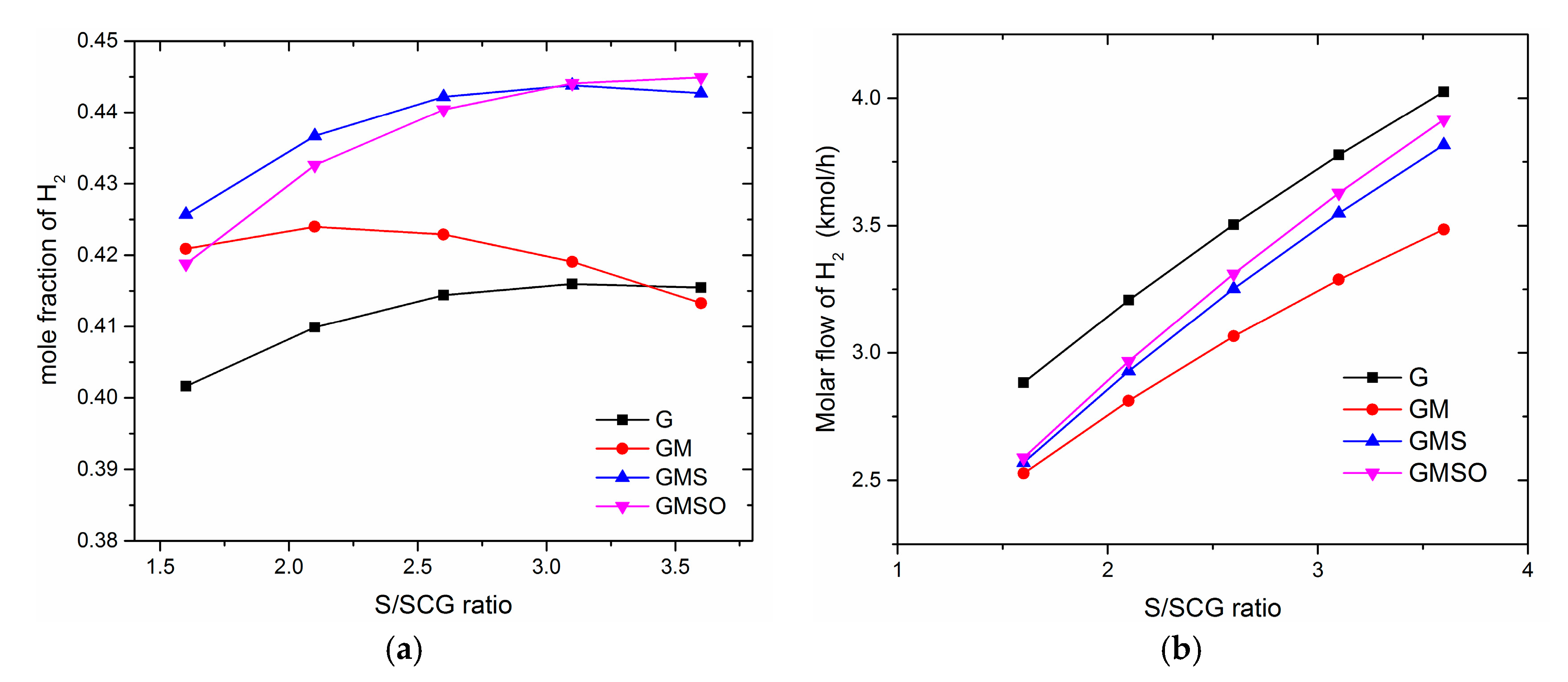
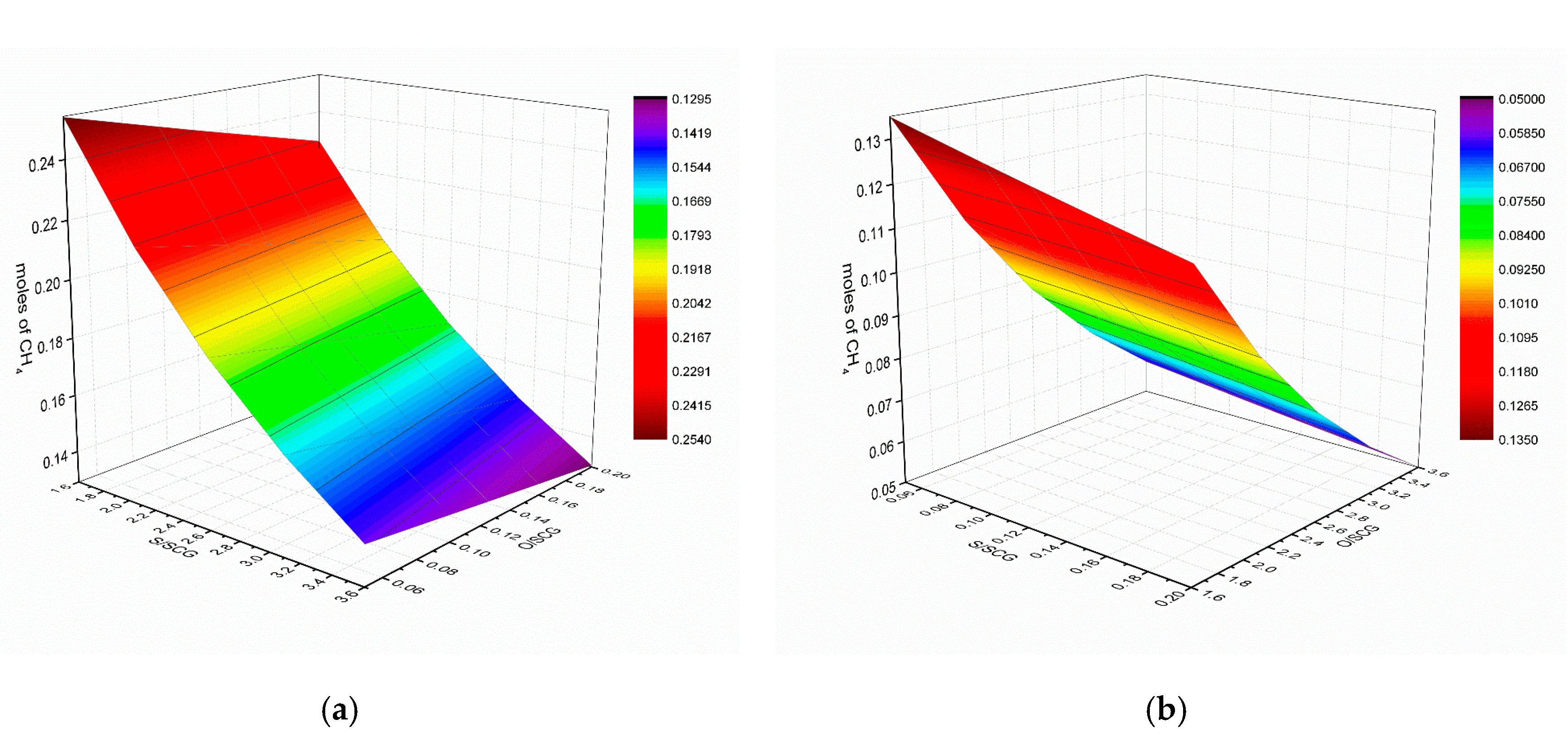
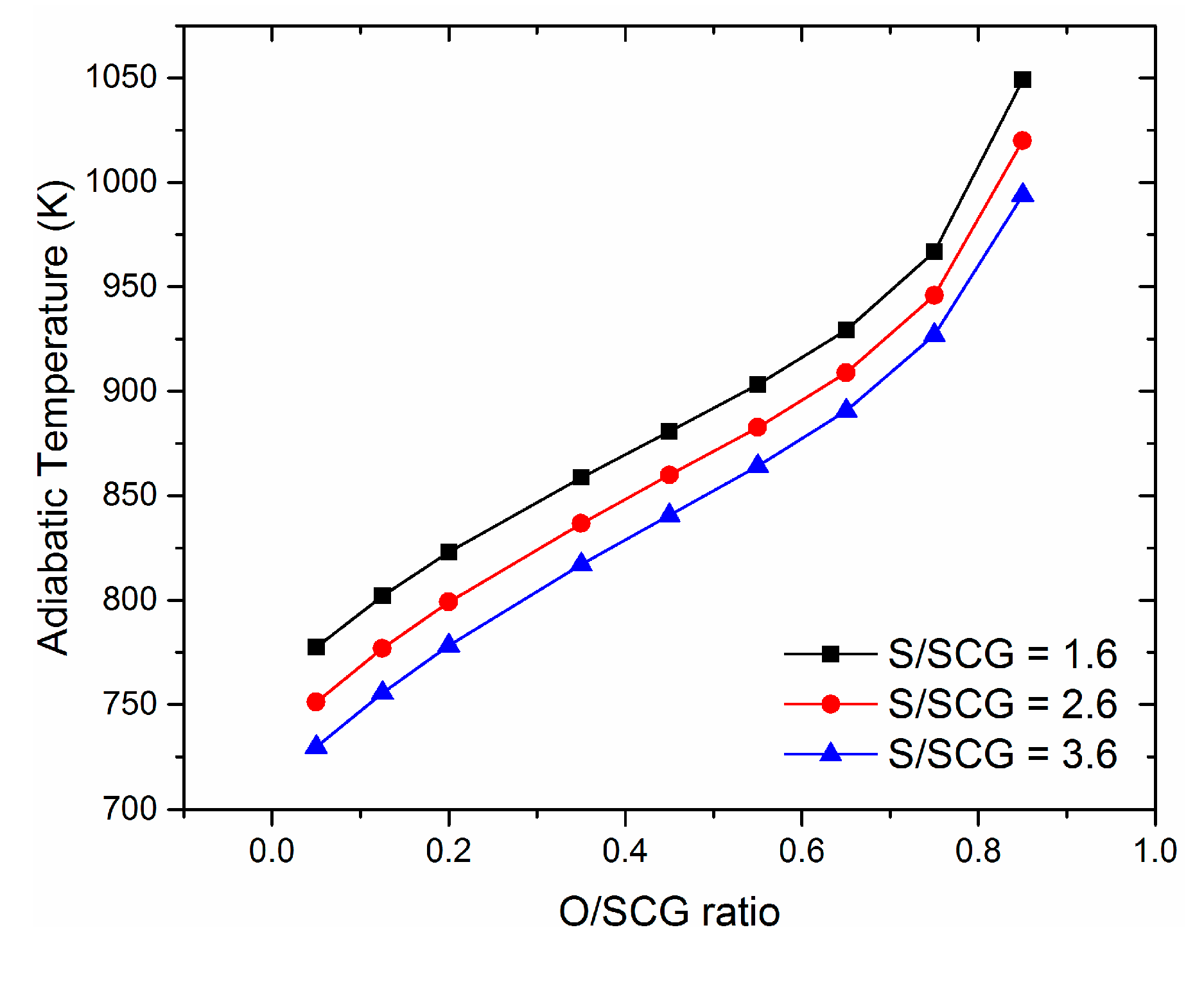
3.2. Effect of Steam to Synthetic Crude Glycerol (S/SCG)
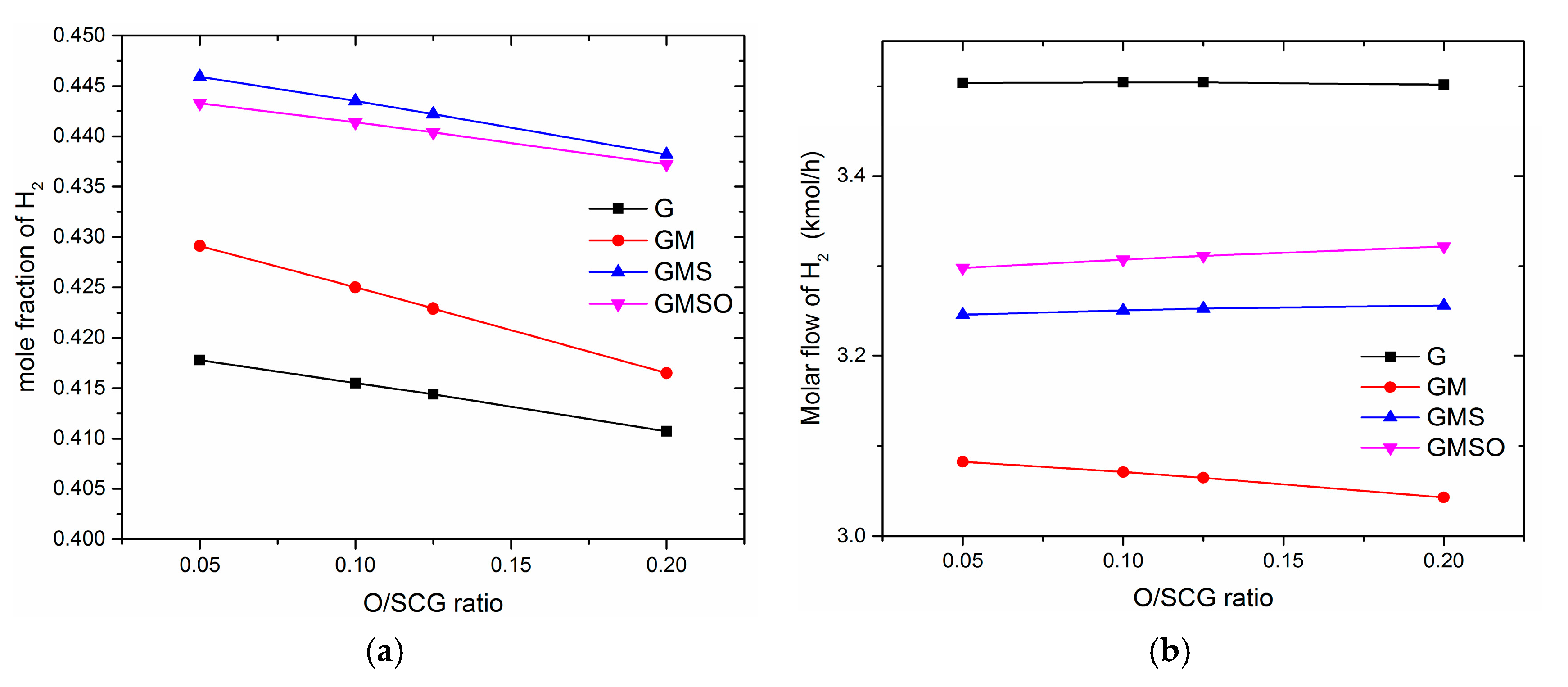
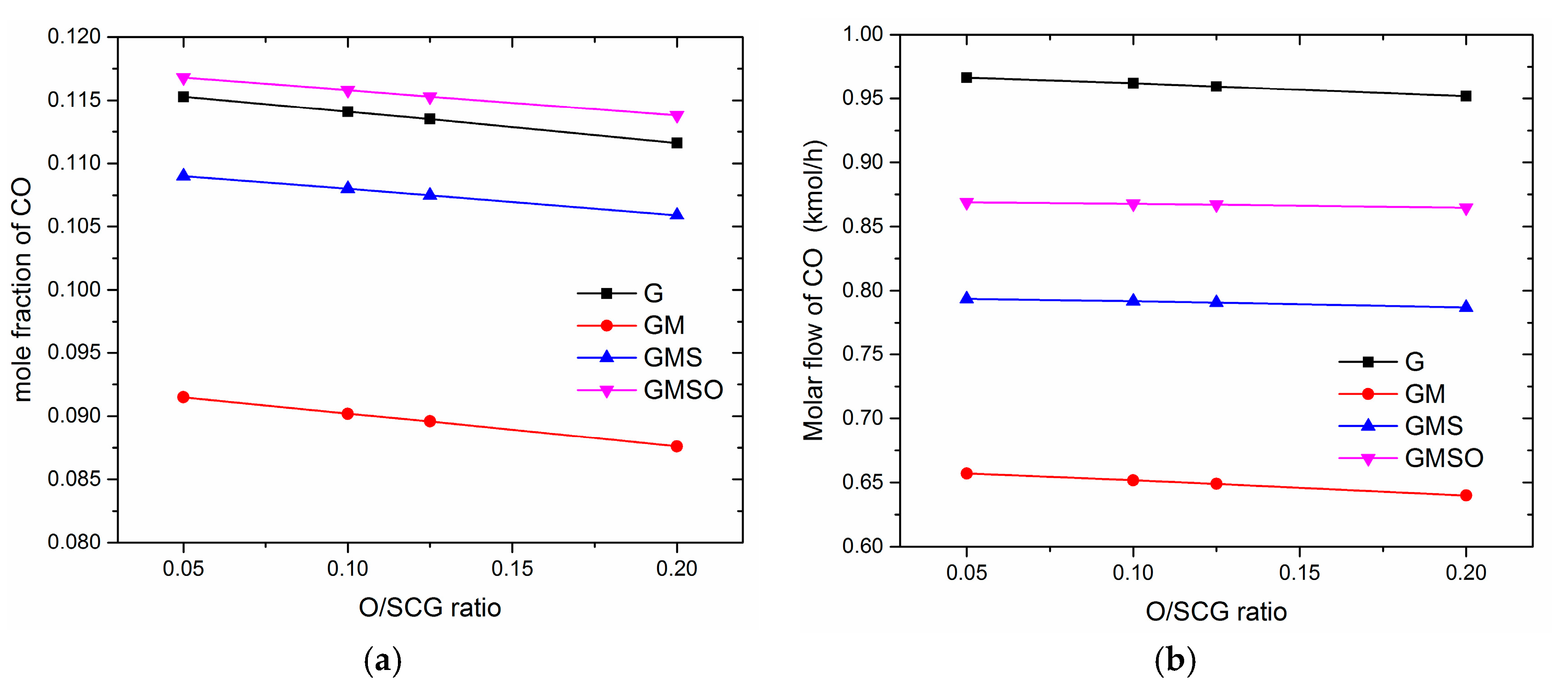
3.3. Effect of Oxygen to Synthetic Crude Glycerol (O/SCG)
3.4. Thermoneutral Conditions
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- EIA. International Energy Outlook 2014 (IEO2014). Available online: https://www.iea.org/publications/freepublications/publication/WEO2014.pdf (accessed on 4 April 2017).
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield–A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Preservatives; Synapse Info Resources: Endicott, NY, USA, 2004. [Google Scholar]
- Ooi, T.; Leong, W.; Jamaludin, R. Potential source of sterols from a palm kernel oil methyl ester residue of an oleochemical plant. PORIM Bull. 1993, 27, 25–37. [Google Scholar]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Sabourin-Provost, G.; Hallenbeck, P.C. High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresour. Technol. 2009, 100, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Abdul Ghani, A.M. Hydrogen Production by the Catalytic Autothermal Reforming of Synthetic Crude Glycerol in a Packed Bed Tubular Reactor. Available online: http://ourspace.uregina.ca/handle/10294/5756 (accessed on 10 January 2017).
- Luo, N.; Zhao, X.; Cao, F.; Xiao, T.; Fang, D. Thermodynamic study on hydrogen generation from different glycerol reforming processes. Energy Fuels 2007, 21, 3505–3512. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, M.; Li, S.; Wang, S.; Ma, X. Thermodynamic analysis of hydrogen production from glycerol autothermal reforming. Int. J. Hydrogen Energy 2009, 34, 5683–5690. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.; Haryanto, A. A comparative thermodynamic and experimental analysis on hydrogen production by steam reforming of glycerin. Energy Fuels 2007, 21, 2306–2310. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Ollero, P.; Serrera, A. Thermodynamic analysis of the autothermal reforming of glycerol using supercritical water. Int. J. Hydrogen Energy 2011, 36, 12186–12199. [Google Scholar] [CrossRef]
- Swami, S.M.; Abraham, M.A. Integrated catalytic process for conversion of biomass to hydrogen. Energy Fuels 2006, 20, 2616–2622. [Google Scholar] [CrossRef]
- Authayanun, S.; Arpornwichanop, A.; Paengjuntuek, W.; Assabumrungrat, S. Thermodynamic study of hydrogen production from crude glycerol autothermal reforming for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 6617–6623. [Google Scholar] [CrossRef]
- Hyprotech, a Subsidiary of Aspen Technology, Inc. HYSYS Plant 3.1 Documentation. Available online: http://home.aspentech.com/ (accessed on 12 January 2017).
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Shirley, A.; Kolaczkowski, S. Evaluation of thermodynamically favourable operating conditions for production of hydrogen in three different reforming technologies. J. Power Sources 2002, 108, 213–225. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K.; Fang, L.; Li, Y. Thermodynamic Analysis of Hydrogen Production from Oxidative Steam Reforming of Ethanol. Energy Fuels 2008, 22, 1365–1370. [Google Scholar] [CrossRef]
- Narischat, N.; Takeguchi, T.; Mori, T.; Iwamura, S.; Ogino, I.; Mukai, S.R.; Ueda, W. Effect of the mesopores of carbon supports on the CO tolerance of Pt 2 Ru 3 polymer electrolyte fuel cell anode catalyst. Int. J. Hydrogen Energy 2016, 41, 13697–13704. [Google Scholar] [CrossRef]
- Gandia, L.M.; Arzamedi, G.; Diéguez, P.M. Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Newnes: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Thermodynamic analysis of glycerol steam reforming for hydrogen production with in situ hydrogen and carbon dioxide separation. J. Power Sources 2015, 273, 423–430. [Google Scholar] [CrossRef]
- Remón, J.; Giménez, J.R.; Valiente, A.; García, L.; Arauzo, J. Production of gaseous and liquid chemicals by aqueous phase reforming of crude glycerol: Influence of operating conditions on the process. Energy Convers. Manag. 2016, 110, 90–112. [Google Scholar] [CrossRef]
- Silva, J.M.; Soria, M.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef]





| Parameters | Standard Condition | Operational Array | ||
|---|---|---|---|---|
| Reformer temperature (K) | 873 | 773–923 | ||
| Pressure (atm) | 1 | - | ||
| S/SCG | 2.6 | 1.6–3.6 | ||
| O/SCG | 0.125 | 0.05–0.2 | ||
| Feed Composition in Weight % | ||||
| Components | Glycerol | Methanol | Soap | Oleic Acid |
| pure glycerol (G) | 100 | 0 | 0 | 0 |
| glycerol + methanol (GM) | 80 | 20 | 0 | 0 |
| glycerol + methanol + soap (GMS) | 53 | 13 | 34 | 0 |
| synthetic crude glycerol (GMSO) | 51 | 12 | 32 | 4 |
| Component | S/G Ratio | O/G Ratio | Adiabatic Temperature (K) | Molar Flow of H2 (kmol/h) |
|---|---|---|---|---|
| Synthetic crude glycerol | 1.6 | 0.75 | 966.6 | 3.7025 |
| 2.6 | 0.75 | 945.9 | 3.9986 | |
| 3.6 | 0.75 | 927 | 4.2269 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimmy, U.; Mohamedali, M.; Ibrahim, H. Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production. ChemEngineering 2017, 1, 4. https://doi.org/10.3390/chemengineering1010004
Jimmy U, Mohamedali M, Ibrahim H. Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production. ChemEngineering. 2017; 1(1):4. https://doi.org/10.3390/chemengineering1010004
Chicago/Turabian StyleJimmy, Uwem, Mohanned Mohamedali, and Hussameldin Ibrahim. 2017. "Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production" ChemEngineering 1, no. 1: 4. https://doi.org/10.3390/chemengineering1010004






