Novel Method Based on Spin-Coating for the Preparation of 2D and 3D Si-Based Anodes for Lithium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D Substrate Materials
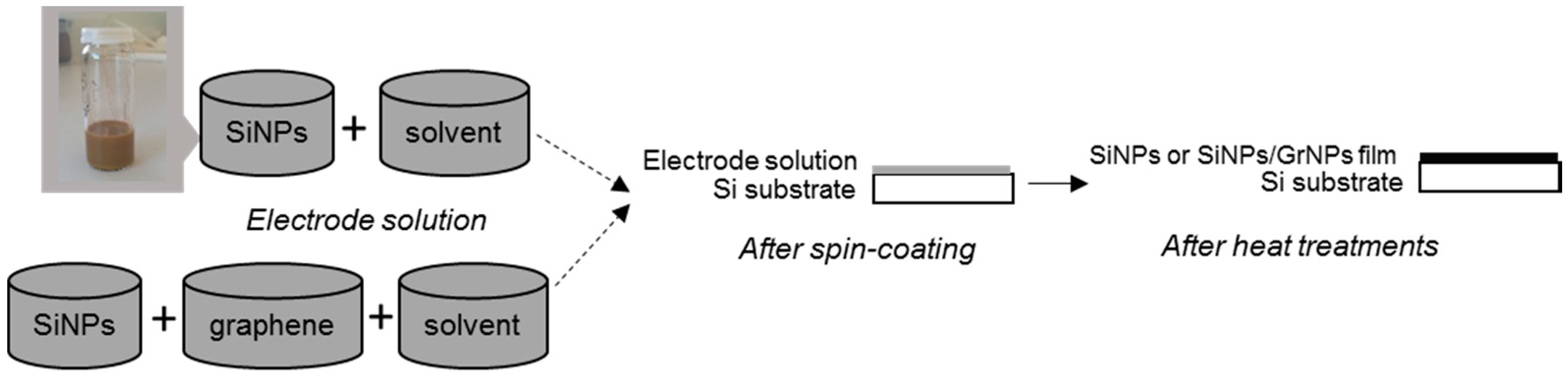
2.2. Electrodes Preparation and Coating Deposition
2.3. Characterization
3. Results and Discussion
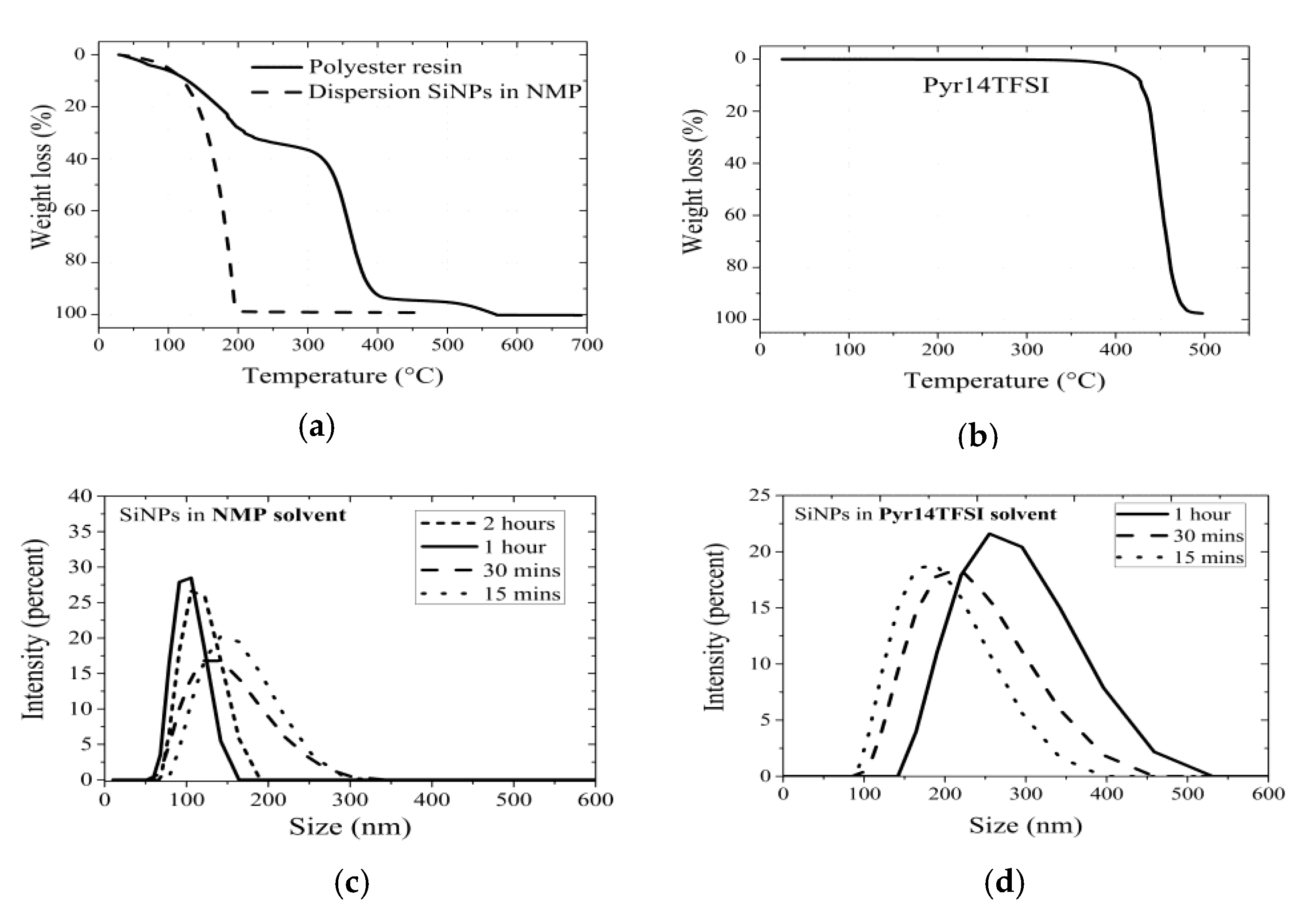
3.1. Thermal Characterization of the Solutions
3.2. Analysis of the Solutions by DLS
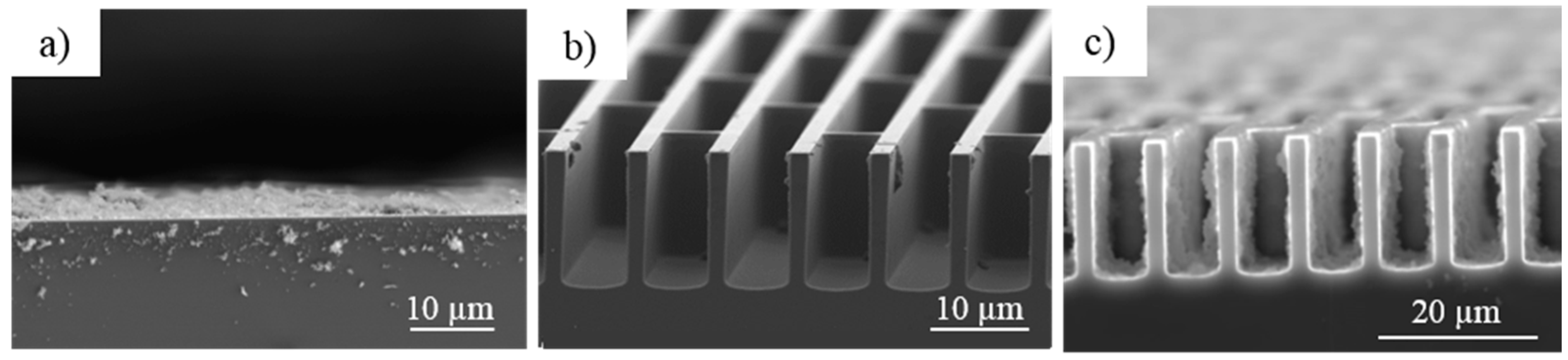
3.3. Morphological Analysis of the 2D and 3D Electrodes
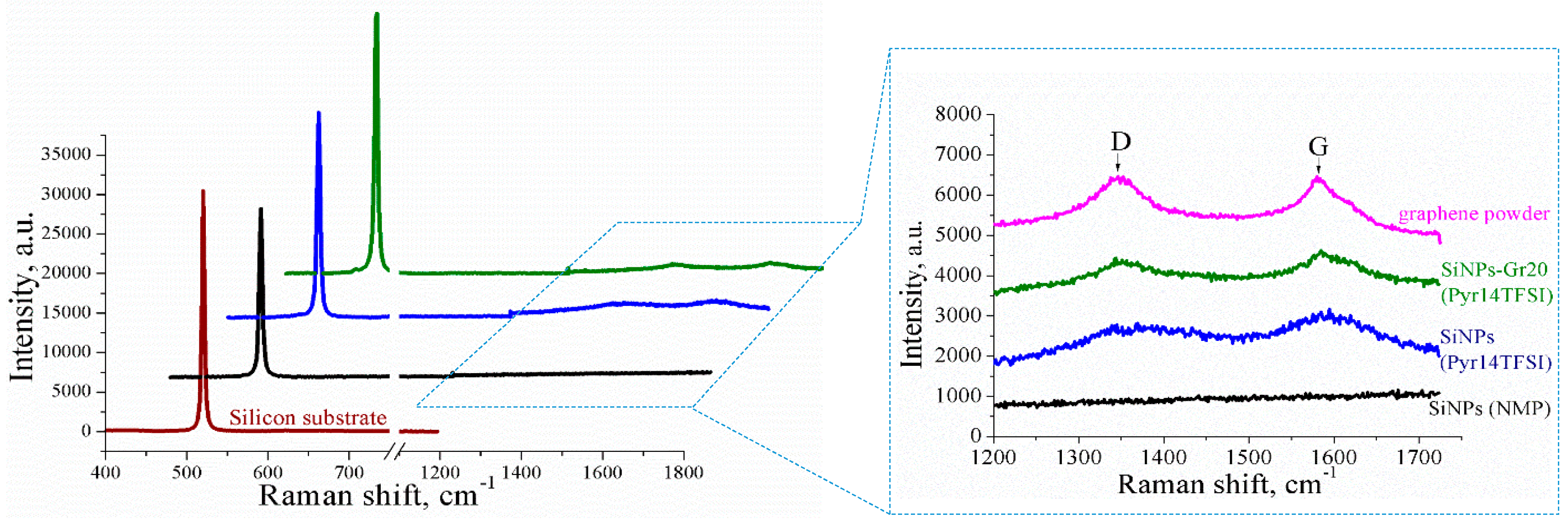
3.4. Raman Characterizations of the Layers
3.5. Electrochemical Properties of the 2D and 3D Electrodes
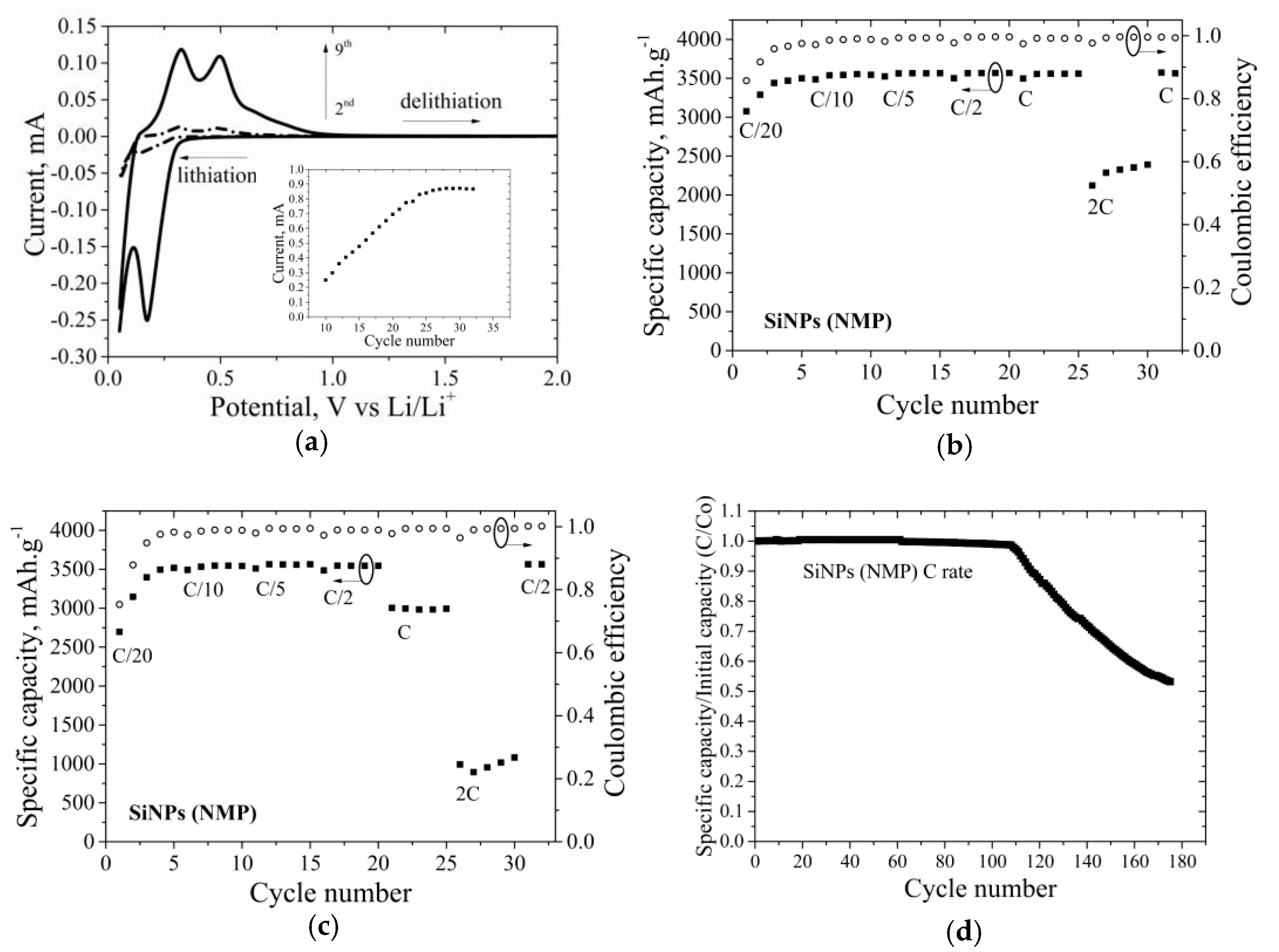
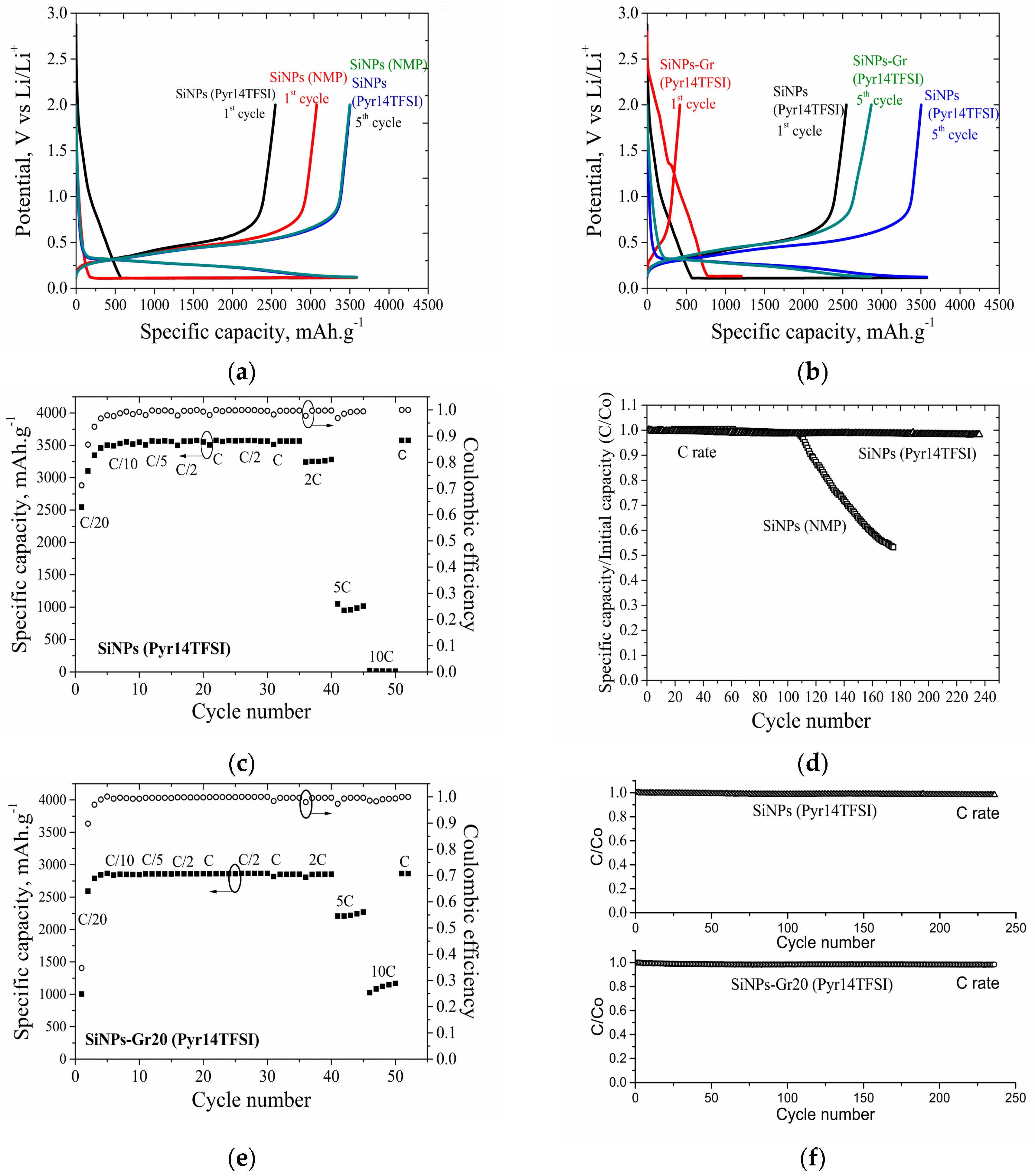
3.5.1. 2D Electrodes
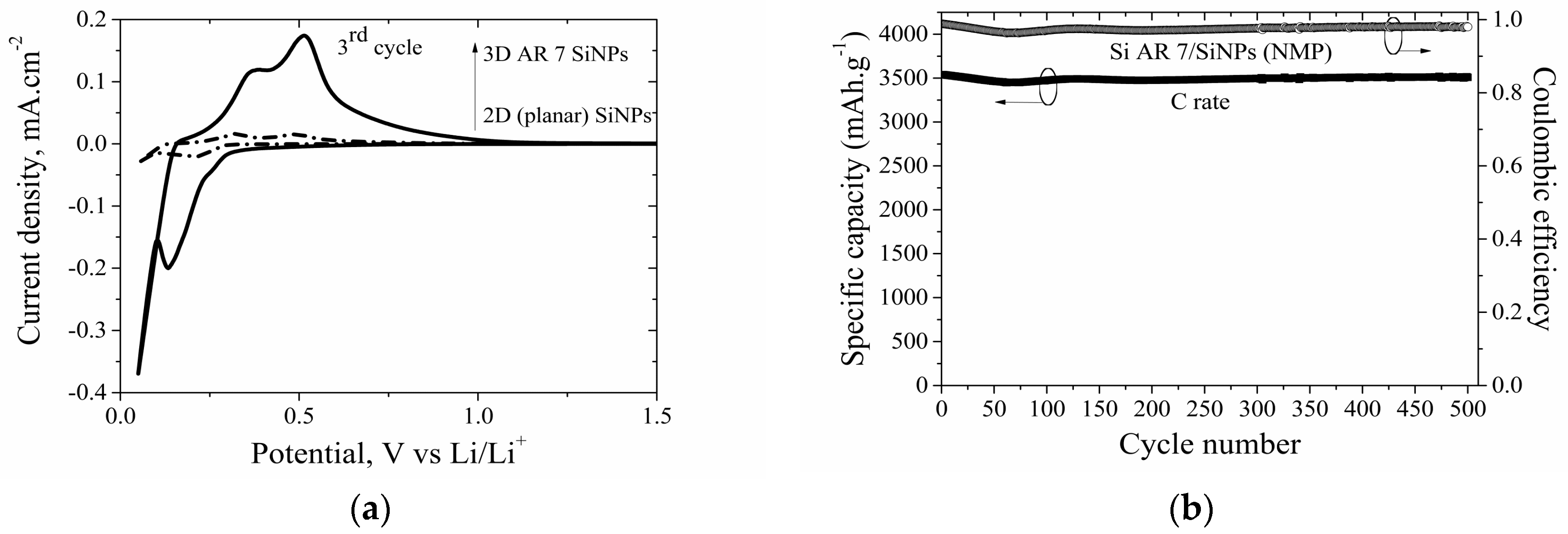
3.5.2. 3D Electrodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1–23. [Google Scholar] [CrossRef]
- Teki, R.; Datta, M.K.; Krishnan, R.; Parker, T.C.; Lu, T.M.; Kumta, P.N.; Koratkar, N. Nanostructured silicon anodes for lithium ion rechargeable batteries. Small 2009, 5, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Luais, E.; Sakai, J.; Desplobain, G.; Gautier, G.; Tran-Van, F.; Ghamouss, F. Thin and flexible silicon anode based on integrated macroporous silicon film onto electrodeposited copper current collector. J. Power Sources 2013, 242, 166–170. [Google Scholar] [CrossRef]
- Luais, E.; Ghamouss, F.; Wolfman, J.; Desplobain, G.; Gautier, G.; Tran-Van, F.; Sakai, J. Mesoporous silicon negative electrode for thin film lithium-ion microbatteries. J. Power Sources 2015, 274, 693–700. [Google Scholar] [CrossRef]
- Lee, G.; Schweizer, S.L.; Wehrspohn, R.B. Electrochemical characteristics of palsma-etched black silicon as anodes for Li-ion batteries. J. Vac. Sci. Technol. A 2014, 32, 061202. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Hong, S.S.; Cui, L.; McDonough, J.R.; Bohy, S.; Cui, Y. Si nanoparticle-decorated Si nanowire networks for Li-ion battery anodes. Chem. Commun. 2010, 47, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.D.; Lee, G.H.; Lim, A.H.; Min, K.M.; Kim, J.C.; Shim, H.W.; Park, K.S.; Kim, D.W. Direct assembly of tin-MWCNT 3D-networked anode for rechargeable lithium ion batteries. RSC Adv. 2012, 2, 3315–3320. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, K.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, A.M.; Wan, L.J.; Guo, Y.G. Spin-coated silicon nanoparticle/graphene electrode as a binder-free anode for high-performance lithium-ion batteries. Nano Res. 2012, 5, 845–853. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Langrock, A.; Manivannan, A.; Ehrman, S.H.; Wang, C. Graphene-bonded and encapsulated Si nanoparticles for lithium ion battery anodes. Small 2013, 9, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, X.; Wu, J.; Jang, H.D.; Kung, H.H.; Huang, J. Crumpled graphene-encapsulated Si nanoparticles for lithium ion battery anodes. J. Phys. Chem. Lett. 2012, 3, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Facile synthesis of silicon nanoparticles inserted into graphene sheets as improved anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Dussart, R.; Tillocher, T.; Lefaucheux, P.; Boufnichel, M.; Ranson, P. Plasma cryogenic etching of silicon: From the early days to today’s advanced technologies. J. Phys. Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Tillocher, T.; Dussart, R.; Mellhaoui, X.; Lefaucheux, P.; Boufnichel, M.; Ranson, P. Silicon cryo-etching of deep holes. Microelectron. Eng. 2007, 84, 1120–1123. [Google Scholar] [CrossRef]
- Boufnichel, M.; Aachboun, S.; Grangeon, F.; Lefaucheux, P.; Ranson, P. Profile control of high aspect ratio trenches of silicon. I. Effect of process parameters on local bowing. J. Vac. Sci. Technol. B 2002, 20, 1508–1513. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, H.W.; Ki, J.K.; Lim, Y.J.; Kim, Y.; Ahn, J.H. Nanocomposite quasi-solid-state electrolyte for highsafety lithium batteries. Nano Res. 2017, 1–11. [Google Scholar] [CrossRef]
- Lewandowski, A.; Swiderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Vincent, A.; Poirot, N. Procédé de Dépôt d’un Matériau Inorganique sur un Substrat Notamment Texturé à L’échelle du Micron ou Submicronique. French Patent FR3010919, 27 March 2015. (In French). [Google Scholar]
- Baggetto, L.; Niessen, R.A.H.; Roozeboom, F.; Notten, P.H.L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Funct. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- He, D.; Bai, F.; Li, L.; Shen, L.; Kung, H.H.; Bao, N. Fabrication of sandwich—Structured Si nanoparticles-graphene nanocomposites for high-performance lithium-ion batteries. Electrochimica Acta 2015, 169, 409–415. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, J.; Yang, J.; Xie, J.; Ma, L. Silicon@carbon hollow core-shell heterostructures novel anode materials for lithium ion batteries. Electrochim. Acta 2013, 87, 663–668. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Pu, X.; Jiang, Y.; Cheng, L.; Jiao, Z. One-step synthesis of high conductivity silver nanoparticle-reduced graphene oxide composite films by electron beam irradiation. Appl. Surf. Sci. 2015, 349, 570–575. [Google Scholar] [CrossRef]
- Chung, C.K.; Wu, B.H. Effect of amorphous Si layer on the reaction of carbon and silicon in the C/Si multilayer by high vacuum annealing. Thin Solid Films 2006, 515, 1985–1991. [Google Scholar] [CrossRef]
- Green, M.; Fielder, E.; Scrosati, B.; Wachtler, M.; Moreno, J.S. Structured silicon anodes for lithium battery applications. Electrochem. Solid-State Lett. 2003, 6, A75–A79. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, H.; Chu, K.L.; Zhuang, Y. Anodized macroporous silicon anode for integration of lithium-ion batteries on chips. J. Electron. Mater. 2012, 41, 2369–2375. [Google Scholar] [CrossRef]
- Kang, D.K.; Corno, J.A.; Gole, J.L.; Shin, H.C. Microstructured nanopore-walled porous silicon as an anode material for rechargeable lithium batteries. J. Electrochem. Soc. 2008, 15, A276–A281. [Google Scholar] [CrossRef]
- Chen, X.; Gerasopoulos, K.; Guo, J.; Brown, A.; Wang, C.; Ghodssi, R.; Culver, J.N. A patterned 3D silicon anode fabricated by electrodeposition on a virus-structured current collector. Adv. Funct. Mater. 2011, 21, 380–387. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chao, S.C.; Wu, H.C.; Wu, N.L. Study on solid-electrolyte-interphase of Si and C-coated Si electrodes in lithium cells. J. Electrochem. Soc. 2009, 156, A95–A102. [Google Scholar] [CrossRef]
- Shu, J.; Li, H.; Yang, R.; Shi, Y.; Huang, X. Cage-like carbon nanotubes/Si composite as anode material for lithium ion batteries. Electrochem. Commun. 2006, 8, 51–54. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.X.; Cao, A.M.; Wan, L.J.; Guo, Y.G. Efficient 3D conducting networks built by graphene sheets and carbon nanoparticles for high-performance silicon anode. ACS Appl. Mater. Interfaces 2012, 4, 2824–2828. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.C.; Jun, S.H.; Lee, K.Y.; Kim, M.H. Effect of surface structure on the irreversible capacity of various graphitic carbon electrodes. J. Electrochem. Soc. 1999, 146, 1664–1671. [Google Scholar] [CrossRef]
- Vargas, C.O.A.; Caballero, A.; Morales, J. Can the performance of graphene nanosheets for lithium storage in Li-ion batteries be predicted? Nanoscale 2012, 4, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.; Knoops, H.C.M.; Niessen, R.A.H.; Kessels, W.M.M.; Notten, P.H.L. 3D negative electrode stacks for integrated all-solid-state lithium-ion microbatteries. J. Mater. Chem. 2010, 20, 3703–3708. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Graetz, J.; Ahn, C.C.; Yazami, R.; Fultz, B. Highly reversible lithium storage in nanostructured silicon. Electrochem. Solid-State Lett. 2003, 6, A194–A197. [Google Scholar] [CrossRef]
- Pereira-Nabais, C.; Swiatowska, J.; Chagnes, A.; Ozanam, F.; Gohier, A.; Tran-Van, P.; Cojocaru, C.S.; Cassir, M.; Marcus, P. Interphase chemistry of Si electrodes used as anodes in Li-ion batteries. Appl. Surf. Sci. 2013, 266, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Aurbach, D.; Talyosef, Y.; Markovsky, B.; Markevich, E.; Zinigrad, E.; Asraf, L.; Gnanaraj, J.S.; Kim, H.J. Design of electrolyte solutions for Li and Li-ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar] [CrossRef]
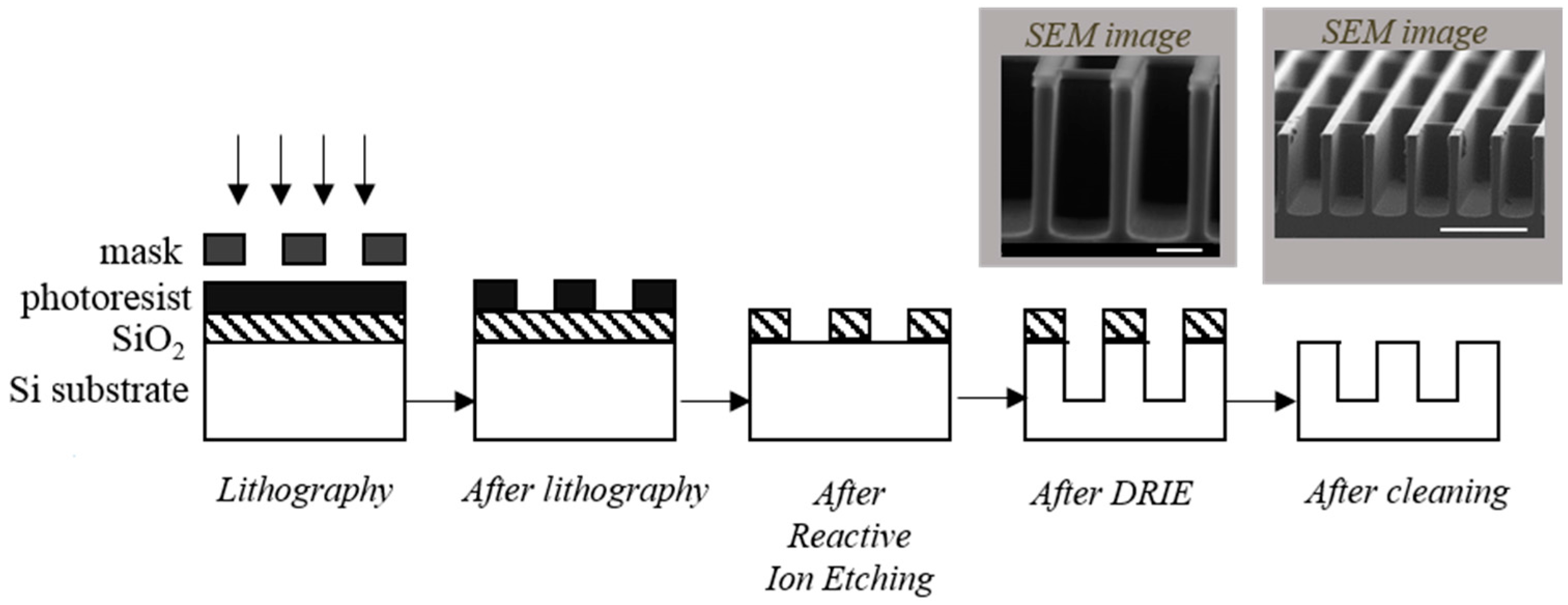

| Etching Conditions | E1 | E2 |
|---|---|---|
| SF6 gas flow (sccm) | 200 | 200 |
| O2 gas flow (sccm) | 13 | 13 |
| Temperature (°C) | −104 | −102 |
| Self-bias (V) | −42 | −42 |
| Source power (W) | 653–844 | 682–844 |
| Time (s) | 180 | 450 |
| Pressure (Pa) | 2.8 | 2.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabard, M.; Zaghrioui, M.; Chouteau, D.; Grimal, V.; Tillocher, T.; Ghamouss, F.; Poirot, N. Novel Method Based on Spin-Coating for the Preparation of 2D and 3D Si-Based Anodes for Lithium Ion Batteries. ChemEngineering 2017, 1, 5. https://doi.org/10.3390/chemengineering1010005
Gabard M, Zaghrioui M, Chouteau D, Grimal V, Tillocher T, Ghamouss F, Poirot N. Novel Method Based on Spin-Coating for the Preparation of 2D and 3D Si-Based Anodes for Lithium Ion Batteries. ChemEngineering. 2017; 1(1):5. https://doi.org/10.3390/chemengineering1010005
Chicago/Turabian StyleGabard, Marie, Mustapha Zaghrioui, David Chouteau, Virginie Grimal, Thomas Tillocher, Fouad Ghamouss, and Nathalie Poirot. 2017. "Novel Method Based on Spin-Coating for the Preparation of 2D and 3D Si-Based Anodes for Lithium Ion Batteries" ChemEngineering 1, no. 1: 5. https://doi.org/10.3390/chemengineering1010005





