3.1 Reaction Stoichiometries and the Effect of m-β-Cyclodextrin
The H-transfer reactions from polyphenols to DPPH can be very effectively assessed by monitoring the decay of
A515, using as molar absorptivity
ε = 11,240 M
−1 cm
−1 and considering the purity of the reagent. The decay in
A515 is initiated following addition of the antioxidant(s) to the DPPH solution [
10] and potent antioxidants may provoke a rapid decay over 1–2 min, as a result of the transfer of H-atoms of the antioxidant that possess low C-H bond dissociation enthalpies (fast step). This step is followed by a much slower decline in
A515, which corresponds to the donation by the antioxidant(s) of the residual H-atoms (slow step) [
11,
12].
A simple hypothesis considers that an antioxidant AH bears
n independent antioxidant subunits, which may all transfer a single H atom to DPPH with the same second-order rate constant
k [
4]. Such a background can be described as follows:
As mentioned above, the initial (fast) step of the reaction actually represents the donation of the most readily abstracted H-atoms from the antioxidant. Hence the initial reaction rate
R0 could be given as:
where
c is the initial antioxidant concentration,
c0 is the initial DPPH concentration and
k1 the reaction rate constant of the first abstracted H-atom. Therefore
k would be
. Based on Beer-Lambert’s law, the [DPPH] that reacts with the first H-atom may be represented as
A0–
Af, where
A0 and
Af correspond to the initial and final
A515. Thus by replacing [DPPH] with
A0–
Af, the Equation (3) can be transformed after integration, as follows:
The slope of the straight line obtained after plotting ) as a function of time t, equals k1.
On such a theoretical basis, OLL extracts obtained with or without m-
β-CD were assayed with the aim to clarifying the role of m-
β-CD on the antiradical effects exerted by OLL polyphenols. To this purpose, the extracts generated were adjusted at a final
CTP of 0.1 g L
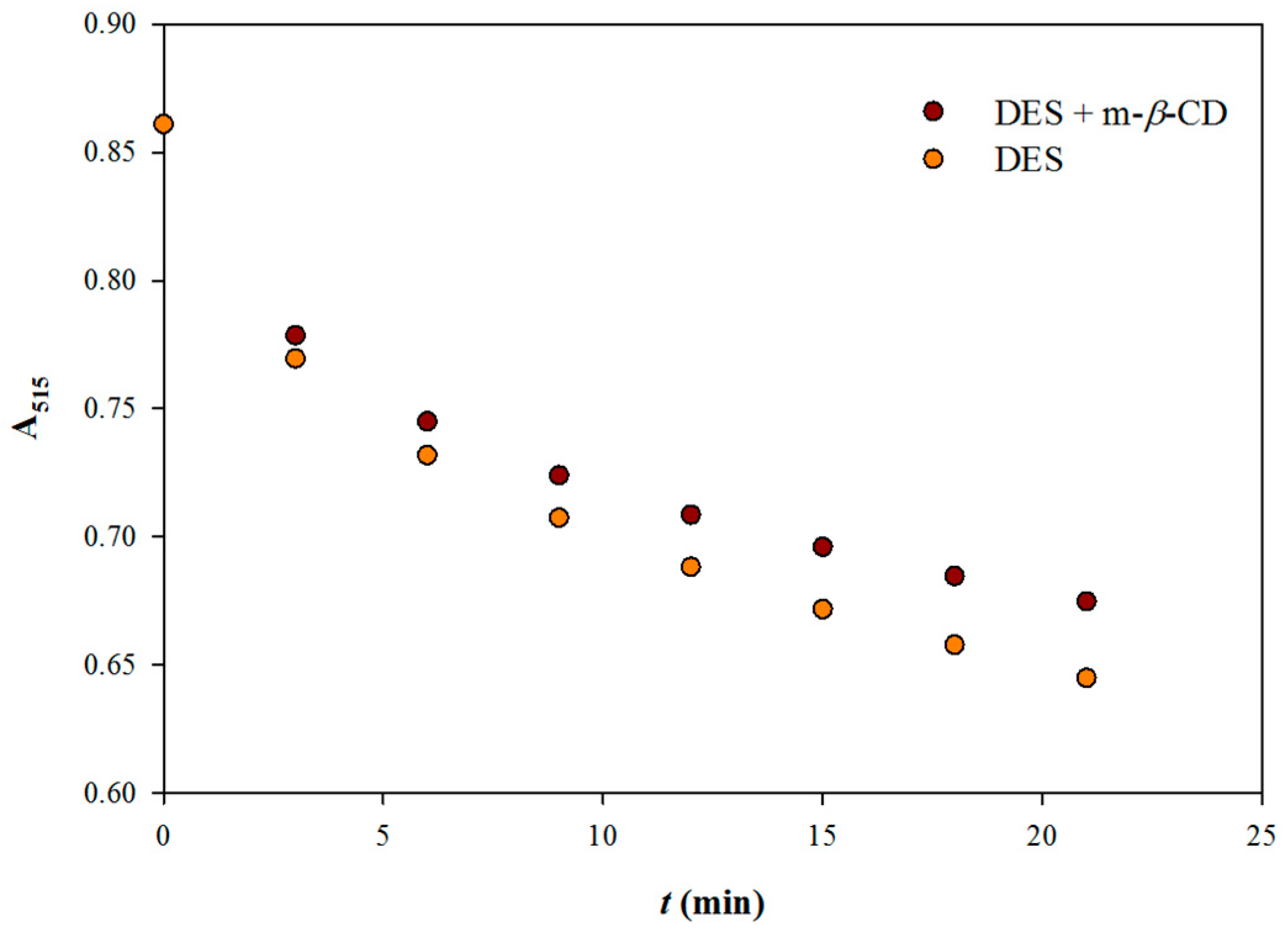
−1 and reaction with DPPH was monitored up to 2 minutes (
Figure 1, upper plot). Determination of
k1 was performed by tracing the second order kinetics (
Figure 1, lower plot) and gave values of 1.925 and 2.221 M
−1 s
−1, for the extract obtained with DES/m-
β-CD and DES, respectively. The slower reaction rate of the extract obtained with DES/m-
β-CD compared with that obtained only with DES could not be interpreted as weaker antiradical activity, but only as a measure of the radical scavenging rate. This is because several polyphenolic antioxidants were shown to respond differently in kinetic and stoichiometric assays based on reaction with DPPH [
13].
Thus in order to have a more integrated picture, the total stoichiometries (
nt) were also determined by extending the reaction of each extract with DPPH, up to 21 min (
Figure 2), using the following equation:
where
CTP is the total polyphenol concentration of the extracts, which as mentioned above was adjusted to 0.1 g GAE L
−1. Determination of
nt for the DES/m-
β-CD and DES extracts gave corresponding values of 1.05 × 10
−4 and 1.92 × 10
−4 mol g
−1, indicating higher stoichiometry for the extract in the absence of m-
β-CD.
Considering both
k1 and
nt, it could be argued that the extract obtained only with DES displayed superior radical scavenging potency. This finding contrasted previous ones, which demonstrated that polyphenol-containing extracts obtained with the aid of various cyclodextrins, such as Melissa officinalis leaf extract [
14] and pomegranate fruit extract [
15] exhibited increased antiradical activity. Likewise, simple phenolics such as rosmarinic acid [
16], chlorogenic acid [
17] and
trans-resveratrol [
18], and quercetin and glycosides therof [
19], showed improved antioxidant properties when they were encapsulated in cyclodextrins. However, a detailed study on the inclusion complexes of tea catechins suggested that the nature of the polyphenol, as well as the orientation of the encapsulated molecule inside the cyclodextrin cavity, may affect antioxidant potency either negatively or positively [
20].
Hydrophobicity would be an issue in this regard, because cyclodextrin/polyphenol inclusion complexes may be better stabilized with molecules having higher hydrophobicity [
21]. On the other hand, hydrogen bonding could also greatly affect the antioxidant behaviour of the complexed polyphenols, because if there is extended intermolecular hydrogen bond development between the encapsulated and the host molecule, then radical scavenging is abrogated [
22]. Such a claim was made for the apparent null effect of hydroxypropyl
β-CD on caffeic acid antioxidant potency [
23], where intramolecular hydrogen bond between the hydroxyl groups of the
o-diphenol moiety would not allow for intermolecular interactions. On the basis of the above concepts, it could be supported that there might be a slower reaction for the OLL extract with DPPH in the presence of m-
β-CD. This phenomenon might be ascribed to the inclusion of OLL polyphenols inside the m-
β-CD cavity, which could slow down H-atom transfer to DPPH due to steric effects. Such a hypothesis would be concurred by the fact that complexation of oleuropein, the most abundant polyphenolic antioxidant in OLL, most probably involves deep insertion of the dihydroxyphenethyl moiety inside the cavity from its secondary side, as demonstrated for OLL interactions with
β-cyclodextrin (
β-CD) [
21]. The formation of similar inclusion complexes with of
β-CD has also been shown for chlorogenic acid [
24].
3.2 Interactions with Ascorbic Acid
In an earlier study, interactions of polyphenol-containing extract with ascorbic acid (AA) were very effectively examined using response surface methodology [
8]. It was proposed that by combining fixed amounts of AA and total polyphenols is a rather unilateral approach, providing limited information, whereas the simultaneous variation of concentrations within predetermined ranges may be more illustrative of the kind of interactions. This is because it has been demonstrated that the relevant amounts of AA and polyphenols in a mixture may significantly affect the overall antioxidant effect [
25].
On these grounds, a response surface design was deployed to evaluate interactions between OLL extract and AA. Evaluation of term contribution by performing ANOVA showed that
CTP and
CAA and their quadratic terms exerted statistically significant effects on the
AAR of the mixtures. However, cross terms were non-significant in this regard (
p > 0.05) and thus they were omitted from the models (mathematical equations), which are presented in their final form in
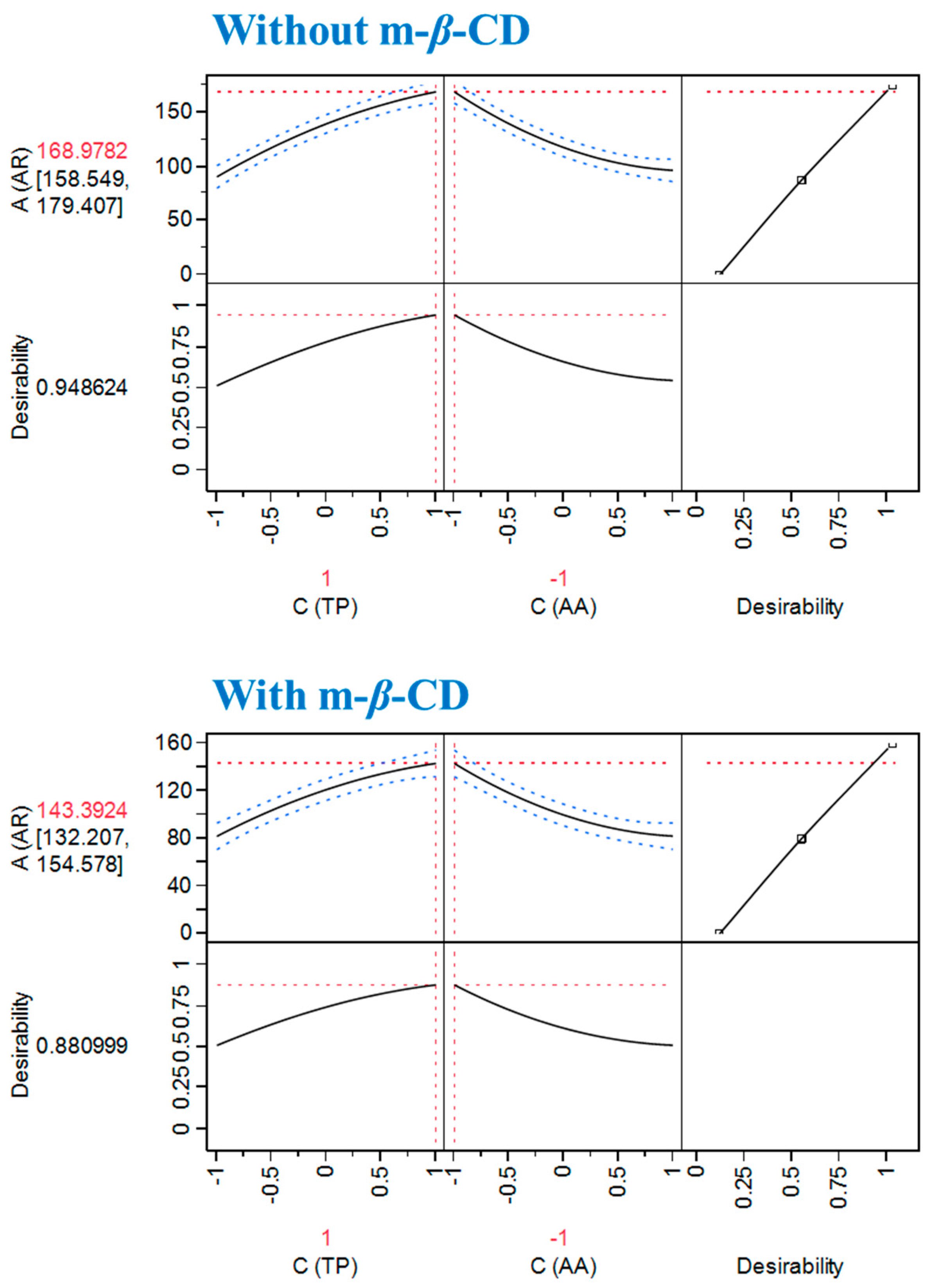
Table 3. The use of the desirability function (
Figure 3) enabled the determination of the settings recommended to achieve
AAR maximisation. Under these
CTP and
CAA combinations, maximum
AAR was estimated to be 168.98 ± 10.43 and 143.39 ± 11.18 μmol DPPH g
−1 dw, for the extracts obtained with DES/m-
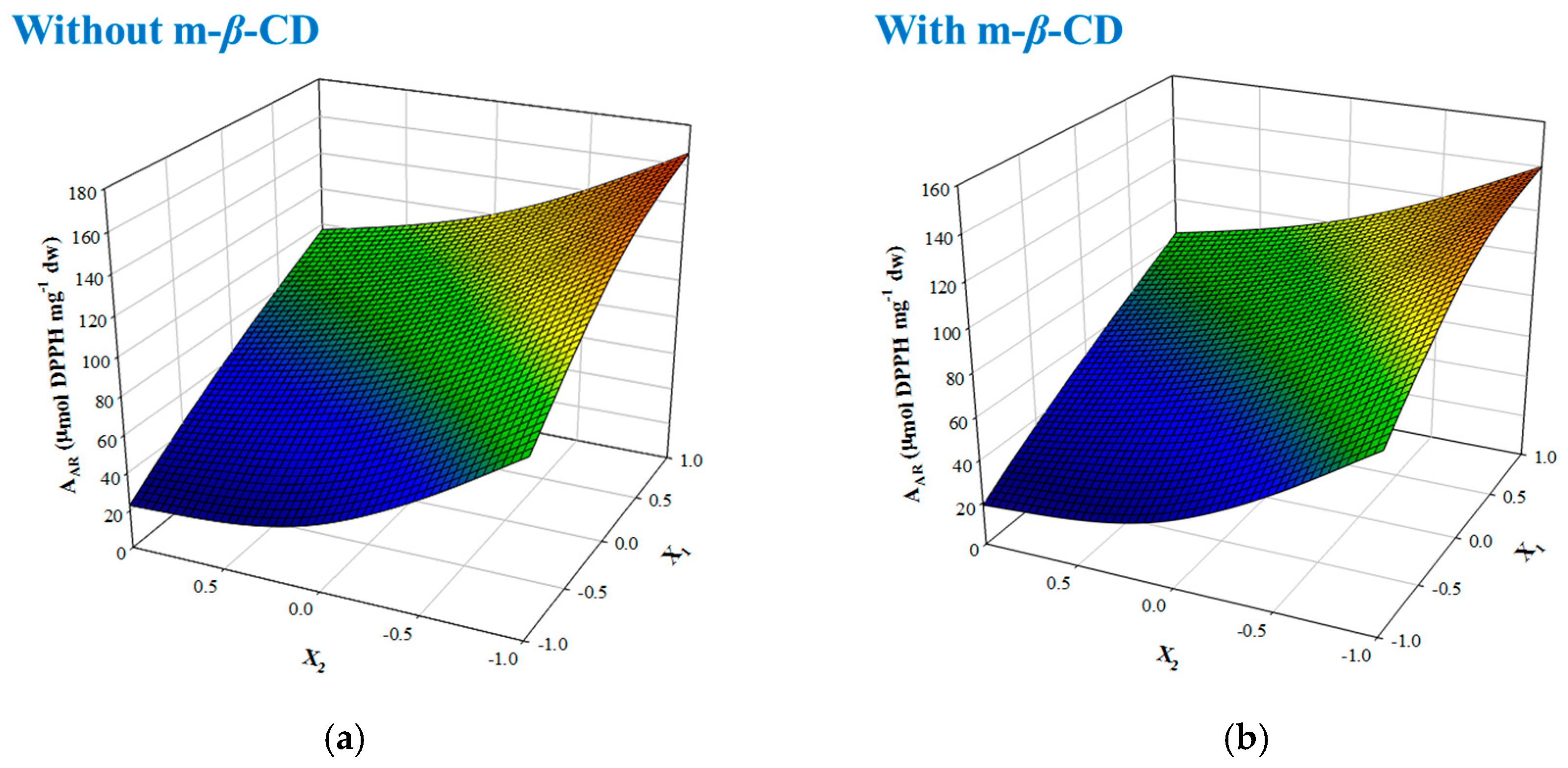
β-CD and DES, respectively. As can be seen in
Figure 4, the interaction pattern with AA was identical, but the difference of 15% in performance was a further confirmation that the OLL extract could act as a better radical scavenger in the absence of m-
β-CD.
The optimal estimated ratio of
CTP/
CAA to attain maximum
AAR was in both cases 7/1 (
Table 4), which clearly indicated that the switching of
CAA to higher levels would not provide higher
AAR. This particular antiradical behaviour of the OLL extract/AA would most probably be ascribed to the nature of the major radical scavengers occurring in OLL extracts. In previous studies pertaining to
AAR of mixtures of polyphenol-containing extracts with AA, it was shown that grape stem extracts displayed the highest performance when combined with AA at a
CTP/
CAA ratio of 1/1 [
8], but for grape seed extracts optimal ratio of 0.82/1 was also determined [
26]. These findings suggested that the nature of principal antioxidant polyphenols in an extract might greatly define the antiradical effects. Investigations with pure polyphenols including quercetin, hesperetin and ferulic acid, revealed that interactions with AA at molar ratio 1:1 yielded antagonism [
27], a behaviour that was confirmed by a following detailed study on reducing power, employing response surface methodology [
28]. In this study, however, it was shown that the maximum response in hesperetin/AA mixtures was achieved at a molar ratio of 5.2/1, which clearly indicated that the nature of polyphenolic antioxidant may define the molar ratio that could yield maximum antioxidant effect, in combination with AA. Furthermore, other authors supported that compounds that are co-extracted with polyphenols from OLL could also interfere [
29], but such an effect remains to be elucidated.