Effects of Grafting Azacrown Ether on Thermal and Swelling Properties of Chitosan Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
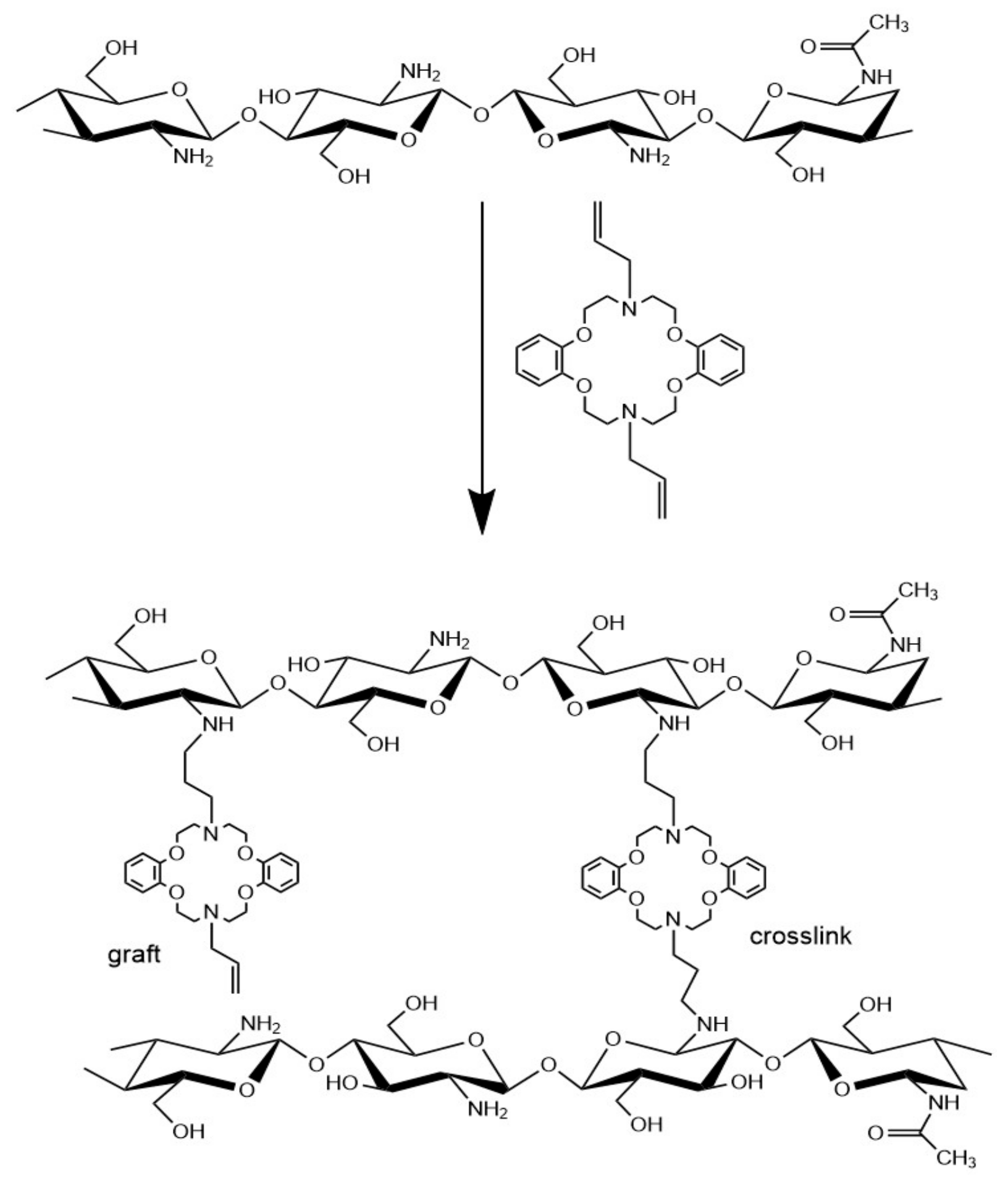
2.2. Preparation of Azacrown Ether/Chitosan Hydrogel Films
2.3. Specific Surface Area, Pore Size, and Density of the Films
2.4. Thermal Analysis
2.4.1. Thermogravimetric Analysis (TGA)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Dynamic Mechanical Analysis (DMA)
2.5. Swelling Behavior
3. Results
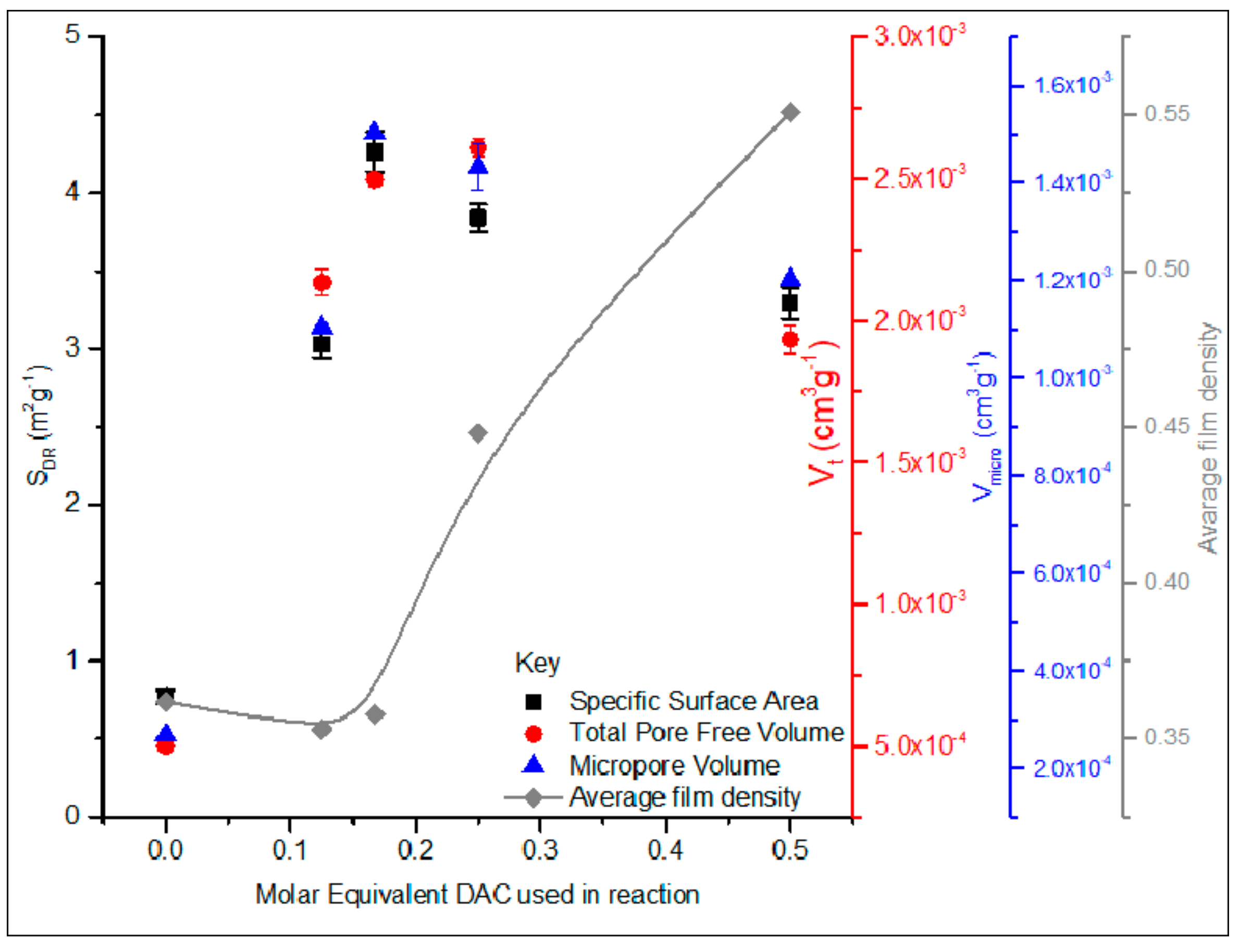
3.1. Films Characterization: Film Porosity, Specific Surface Area, and Density
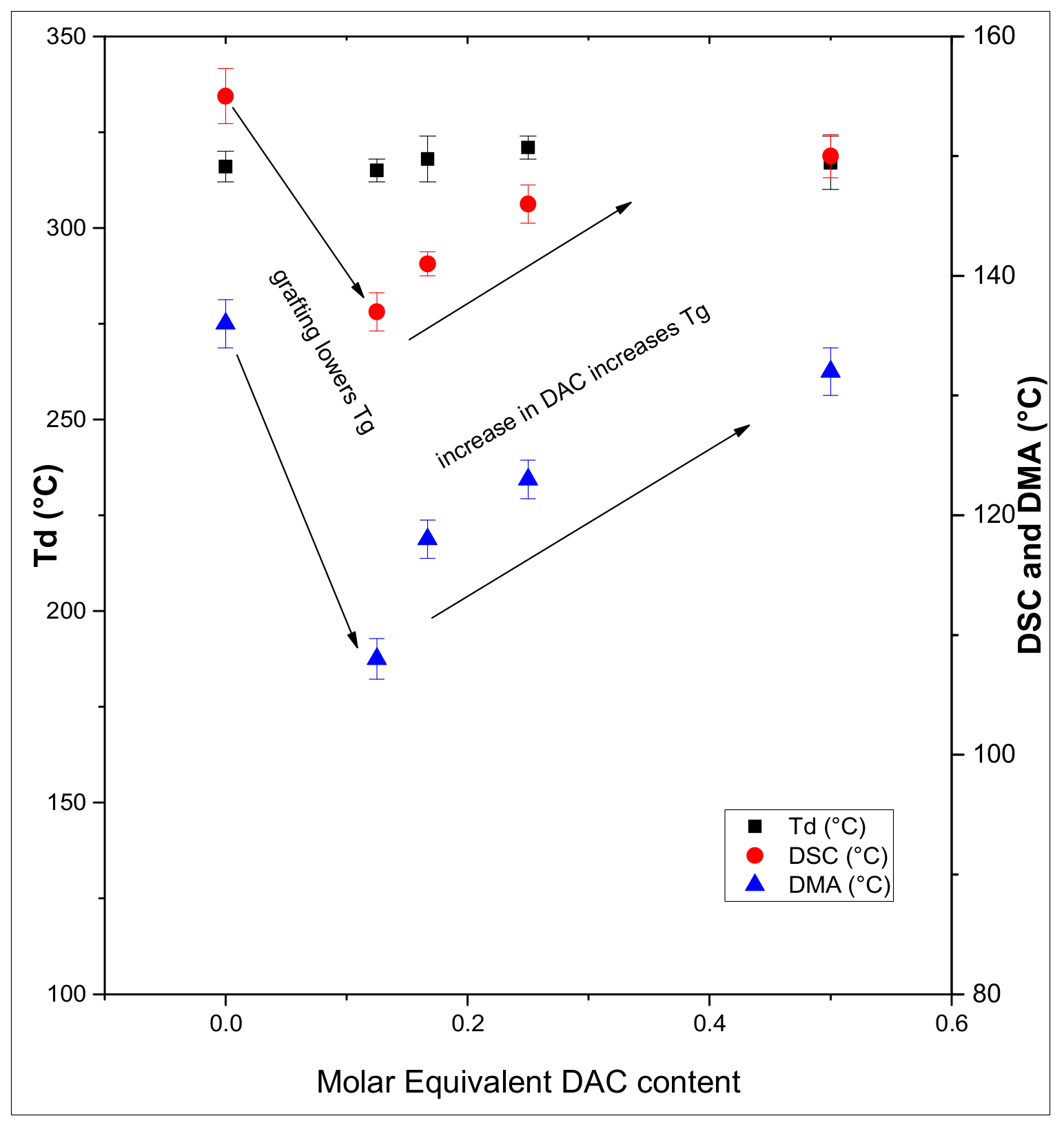
3.2. Thermal Properties of the Synthesized Ch-DAC Films
3.2.1. TGA Analysis
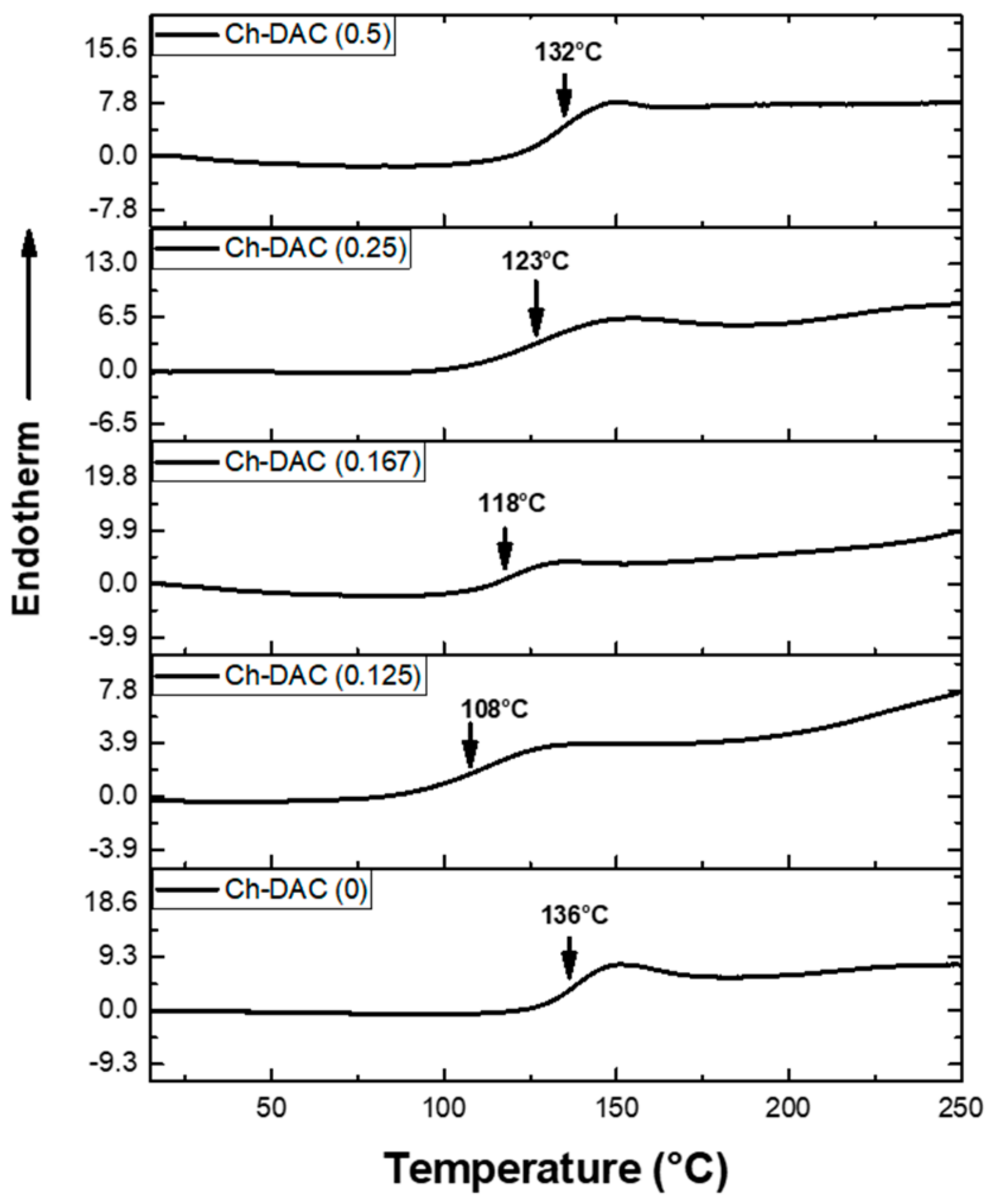
3.2.2. DSC Analysis
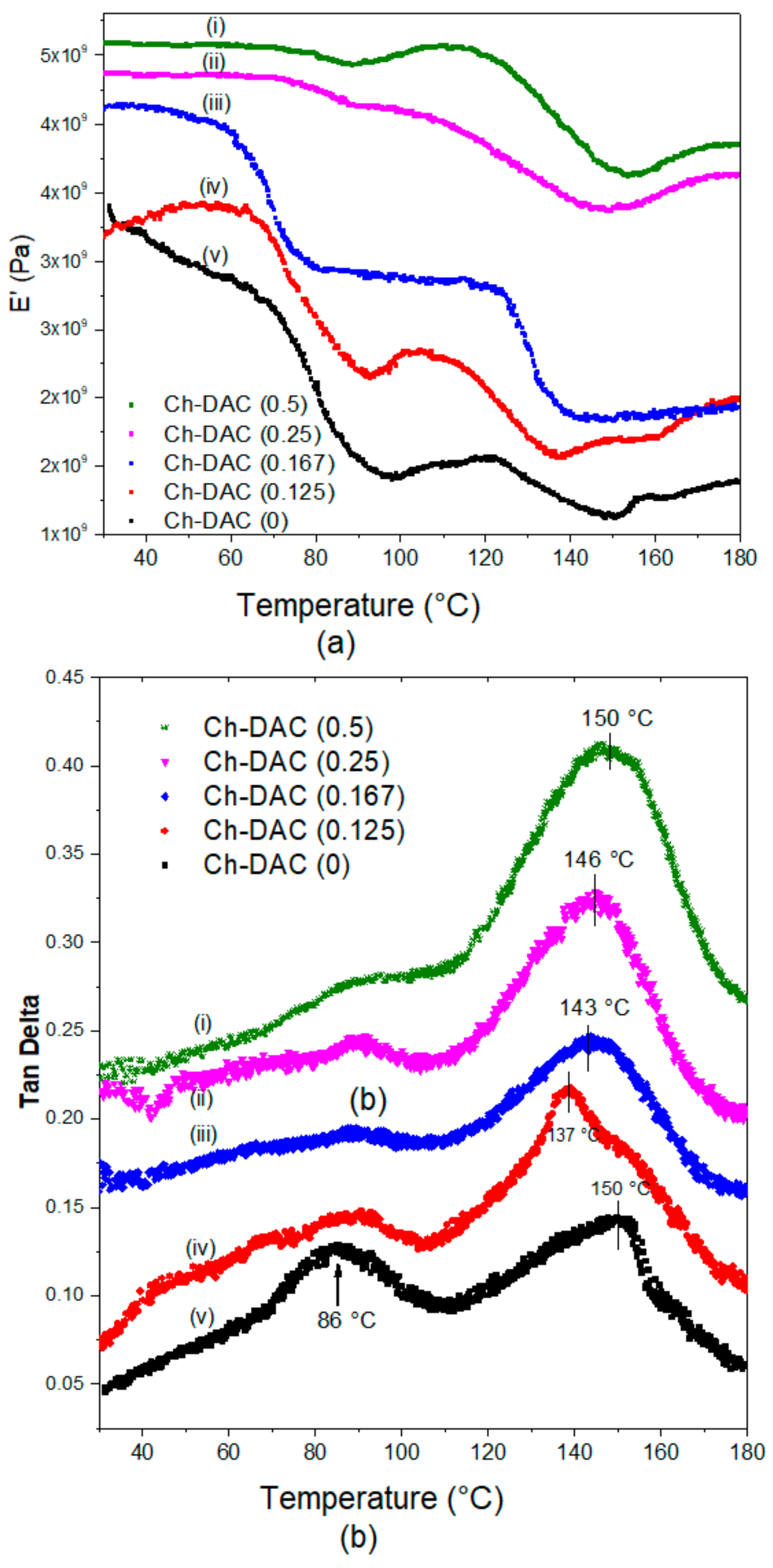
3.2.3. DMA Analysis
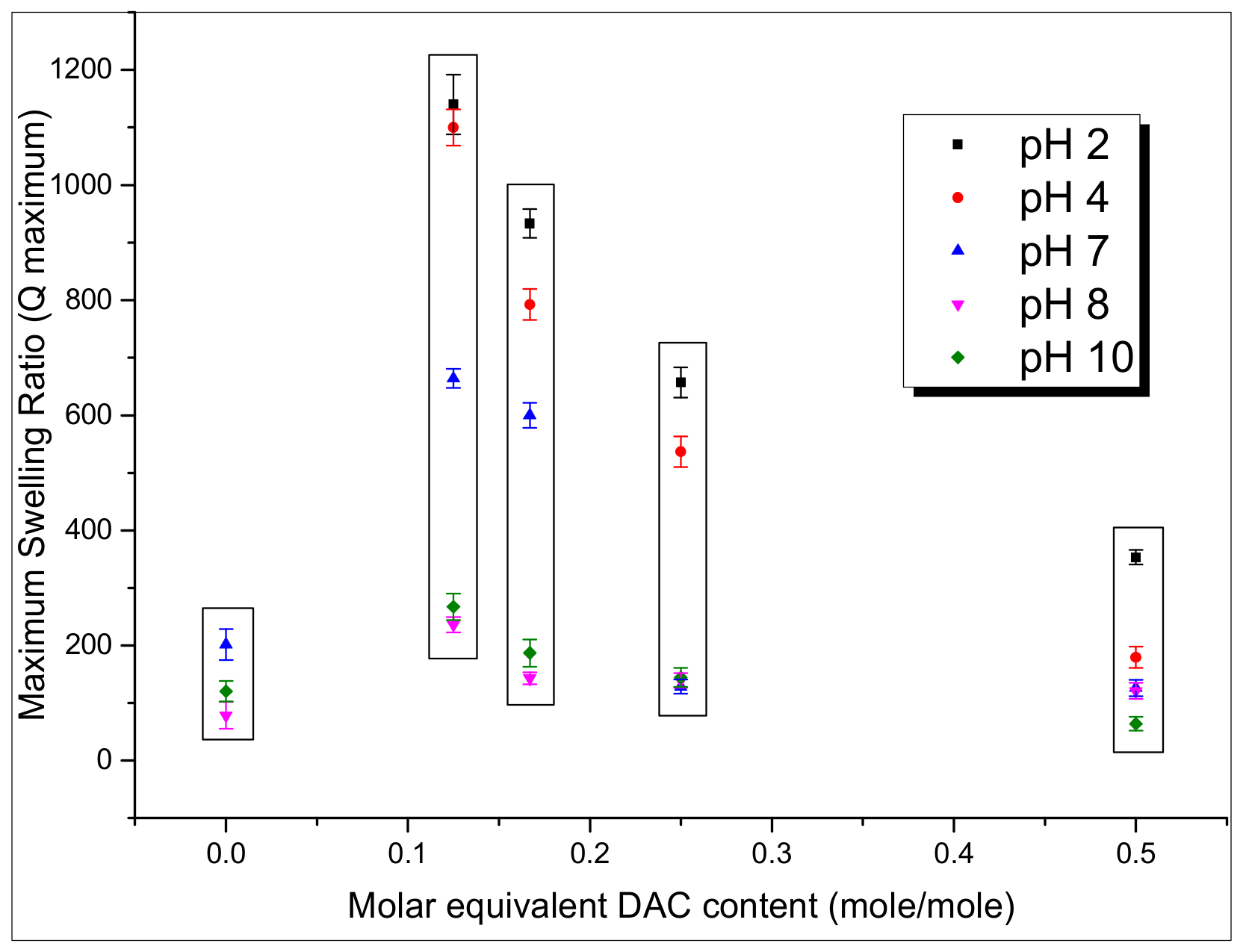
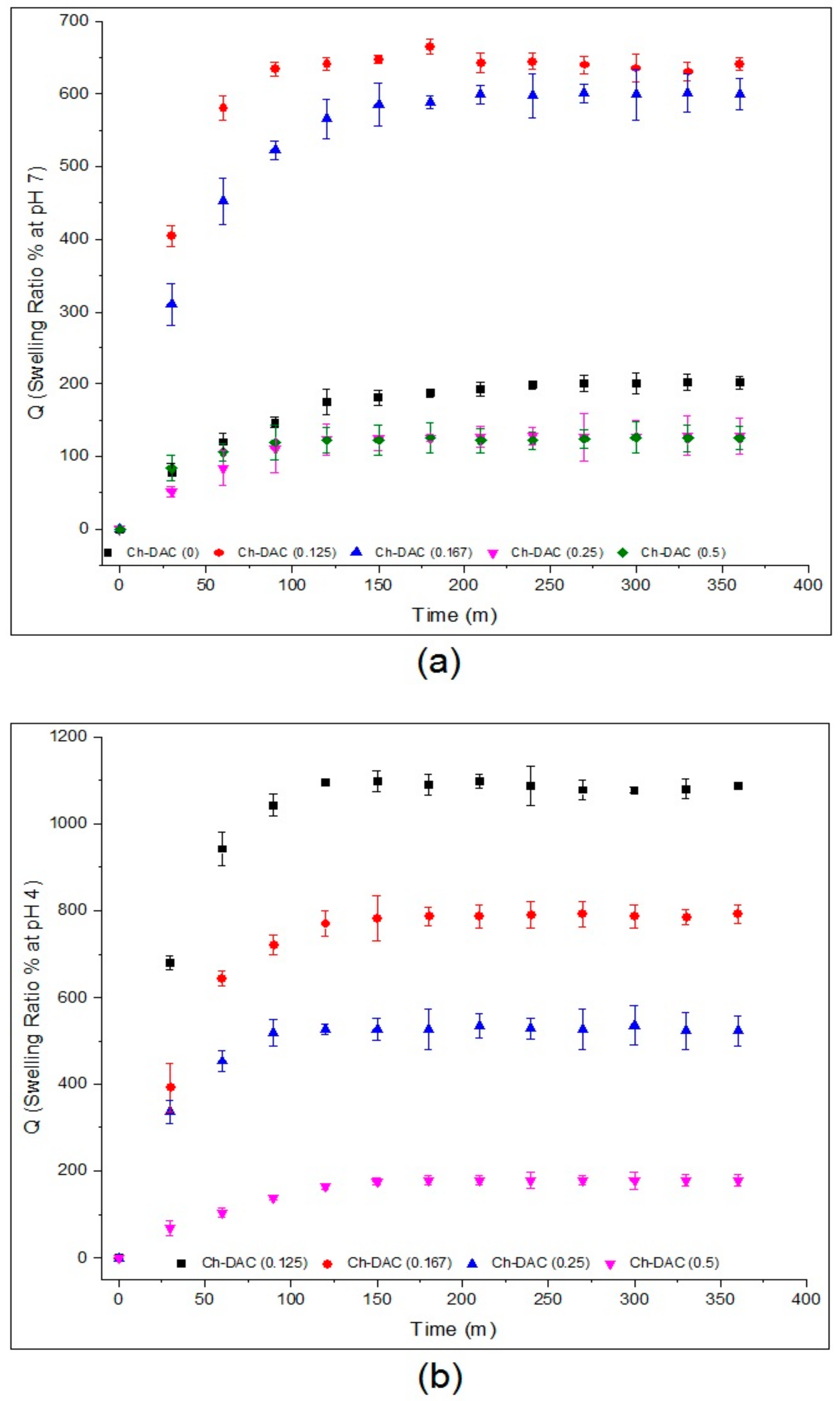
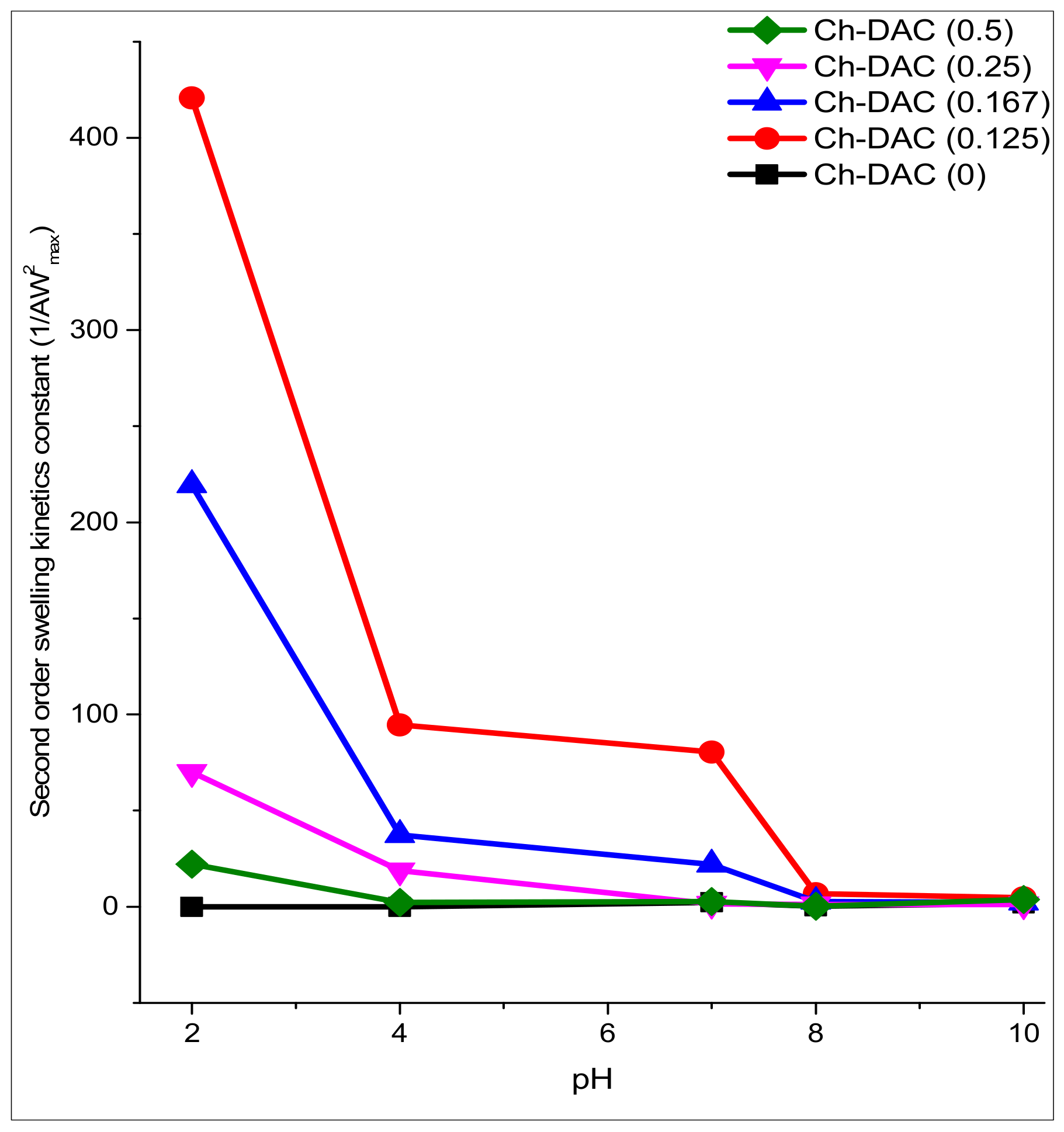
3.3. Swelling Behavior
3.3.1. Water Adsorption Behavior of Films in Various pH Conditions
3.3.2. Swelling Kinetics
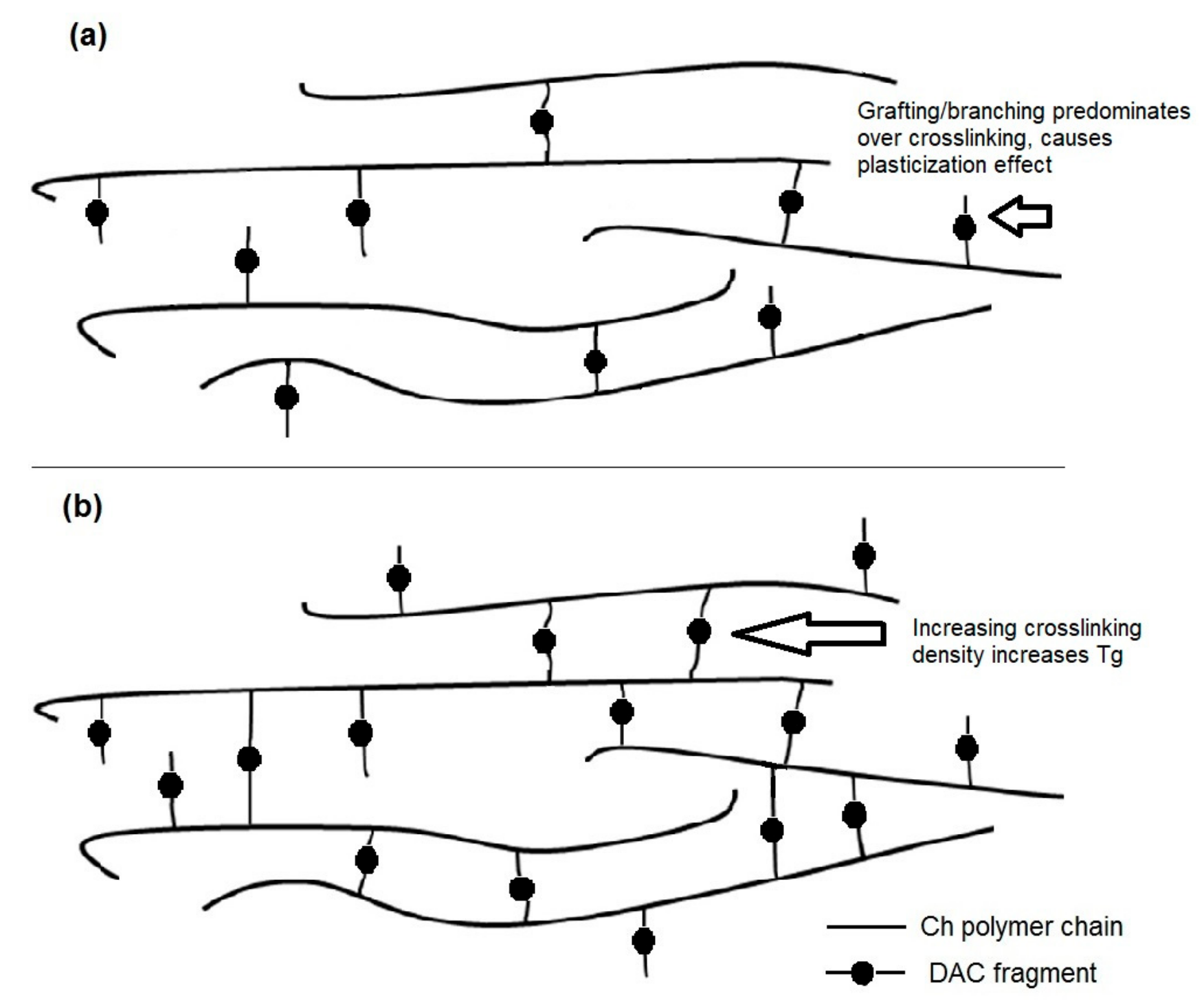
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alexandratos, S.D.; Stine, C.L. Synthesis of ion-selective polymer-supported crown ethers: A review. React. Funct. Polym. 2004, 60, 3–16. [Google Scholar] [CrossRef]
- Wang, M.; Yan, F.; Fang, Z.; Zhang, Y. Preparation of chitosan-graft-benzo-15-crown-5 ether film for heavy metal ions separation. Desalin. Water Treat. 2017, 81, 143–151. [Google Scholar] [CrossRef]
- Mututuvari, T.M.; Tran, C.D. Synergistic adsorption of heavy metal ions and organic pollutants by supramolecular polysaccharide composite materials from cellulose, chitosan and crown ether. J. Hazard. Mater. 2014, 264, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarra, I.A.; Alanazi, F.K.; Radwan, A.A. Chitosan Derivative, A Method for Its Preparation and Its Use as An Adsorption Agent. WIPO Patent WO2013000567, 4 January 2013. [Google Scholar]
- Malkondu, S.; Kocak, A.; Yilmaz, M. Immobilization of Two Azacrown Ethers on Chitosan: Evaluation of Selective Extraction Ability Toward Cu(II) and Ni(II). J. Macromol. Sci. Part A 2009, 46, 745–750. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Zhang, L.; Wang, Y. Preparation and adsorption behavior of macrocyclic tetra-amine derivatives of chitosan. J. Appl. Polym. Sci. 2005, 98, 407–412. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Wang, Y.-T.; Tang, Y.-R. Studies of some ultratrace elements in antarctic water via crown ether crosslinked chitosan. J. Appl. Polym. Sci. 2003, 90, 806–809. [Google Scholar] [CrossRef]
- Changhong, P.; Weijun, Y.; Motang, T. Chemical modification of chitosan: Synthesis and characterization of chitosan-crown ethers. J. Appl. Polym. Sci. 2003, 87, 2221–2225. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Tang, Y. Synthesis of crosslinked chitosan-crown ethers and evaluation of these products as adsorbents for metal ions. J. Appl. Polym. Sci. 1998, 70, 501–506. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Tan, S.; Cheng, G. Preparation of Chitosan Derivatives. Synthesis of N-Schiff Base Type and N-Secondary Amino Type Chitosan-Crown Ethers. Polym. J. 1998, 30, 843–845. [Google Scholar] [CrossRef]
- Tang, X.-H.; Tan, S.-Y.; Wang, Y.-T. Study of the synthesis of chitosan derivatives containing benzo-21-crown-7 and their adsorption properties for metal ions. J. Appl. Polym. Sci. 2002, 83, 1886–1891. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, S. Synthesis and characterization of macrocyclic polyamine derivative of chitosan. J. Appl. Polym. Sci. 2003, 89, 924–929. [Google Scholar] [CrossRef]
- Yang, Z.; Shu, J.; Zhang, L.; Wang, Y. Preparation and adsorption behavior for metal ions of cyclic polyamine derivative of chitosan. J. Appl. Polym. Sci. 2006, 100, 3018–3023. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Tang, Y. Preparation and adsorption properties of metal ions of crosslinked chitosan azacrown ethers. J. Appl. Polym. Sci. 1999, 74, 3053–3058. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Tang, Y. Synthesis and adsorption properties for metal ions of mesocyclic diamine-grafted chitosan-crown ether. J. Appl. Polym. Sci. 2000, 75, 1255–1260. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Wang, Y. Synthesis and evaluation of chitosan aryl azacrown ethers as adsorbents for metal ions. J. Appl. Polym. Sci. 2000, 77, 3093–3098. [Google Scholar] [CrossRef]
- Yang, Z.; Zhuang, L.; Tan, G. Preparation and adsorption behavior for metal of chitosan crosslinked by dihydroxy azacrown ether. J. Appl. Polym. Sci. 2002, 85, 530–535. [Google Scholar] [CrossRef]
- Dhakal, R.P.; Oshima, T.; Baba, Y. Synthesis of Unconventional Materials Using Chitosan and Crown Ether for Selective Removal of Precious Metal Ions. World Acad. Sci. Eng. Technol. 2009, 56, 204–208. [Google Scholar]
- Zhang, X.; Ding, S.; Wang, Y.; Feng, X.; Peng, Q.; Ma, S. Synthesis and adsorption properties of metal ions of novel azacrown ether crosslinked chitosan. J. Appl. Polym. Sci. 2006, 100, 2705–2709. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, Y.; Liu, H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr. Polym. 2003, 53, 425–430. [Google Scholar] [CrossRef]
- Radwan, A.A.; Alanazi, F.K.; Alsarra, I.A. Microwave Irradiation-Assisted Synthesis of a Novel Crown Ether Crosslinked Chitosan as a Chelating Agent for Heavy Metal Ions (M+n). Molecules 2010, 15, 6257–6268. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, Y.; Peng, C.; Tang, Y. Synthesis and adsorption properties for metal ions of crosslinked chitosan acetate crown ethers. J. Appl. Polym. Sci. 1999, 71, 2069–2074. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Y.; Qian, S. Study on the adsorption properties of novel crown ether crosslinked chitosan for metal ions. J. Appl. Polym. Sci. 2002, 84, 29–34. [Google Scholar] [CrossRef]
- Zhikuan Yang, Y.Y. Synthesis, characterization, and adsorption properties of chitosan azacrown ethers bearing hydroxyl group. J. Appl. Polym. Sci. 2001, 81, 1793–1798. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, X.; Feng, X.; Wang, Y.; Ma, S.; Peng, Q.; Zhang, W. Synthesis of N,N′-diallyl dibenzo 18-crown-6 crown ether crosslinked chitosan and their adsorption properties for metal ions. React. Funct. Polym. 2006, 66, 357–363. [Google Scholar] [CrossRef]
- Bradshaw, J.S.; Krakowiak, K.E.; Izatt, R.M. Aza-Crown Macrocycles; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Krakowiak, K.E.; Bradshaw, J.S.; Zamecka-Krakowiak, D.J. Synthesis of aza-crown ethers. Chem. Rev. 1989, 89, 929–972. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and Kinetic Data for Macrocycle Interaction with Cations, Anions, and Neutral Molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials 2017, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Statistical Mechanics of Swelling of Network Structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. S. Pac. J. Nat. Appl. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Toeri, J.; Laborie, M.-P. Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6. Molecules 2016, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, A.M.M. A study of the porous structure of active carbons using a variety of methods. Q. Rev. Chem. Soc. 1955, 9, 101–114. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Choi, H.-K.; Cho, C.-S. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials 2001, 22, 923–928. [Google Scholar] [CrossRef]
- Dong, Y.; Ruan, Y.; Wang, H.; Zhao, Y.; Bi, D. Studies on glass transition temperature of chitosan with four techniques. J. Appl. Polym. Sci. 2004, 93, 1553–1558. [Google Scholar] [CrossRef]
- Butler, M.F.; Clark, A.H.; Adams, S. Swelling and Mechanical Properties of Biopolymer Hydrogels Containing Chitosan and Bovine Serum Albumin. Biomacromolecules 2006, 7, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Tasselli, F.; Mirmohseni, A.; Seyed Dorraji, M.S.; Figoli, A. Mechanical, swelling and adsorptive properties of dry–wet spun chitosan hollow fibers crosslinked with glutaraldehyde. React. Funct. Polym. 2013, 73, 218–223. [Google Scholar] [CrossRef]
- Somasundaran, P. (Ed.) Encyclopedia of Surface and Colloid Science; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Ofner, C.M.; Schott, H. Swelling Studies of Gelatin I: Gelatin Without Additives. J. Pharm. Sci. 1986, 75, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Ofner, C.M.; Schott, H. Swelling Studies of Gelatin II: Effect of Additives. J. Pharm. Sci. 1987, 76, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Auditore, L.; Loria, D.; Trimarchi, M.; Wanderlingh, U. Radiation synthesis and characterization of poly(ethylene oxide)/chitosan hydrogels. J. Appl. Polym. Sci. 2013, 127, 217–223. [Google Scholar] [CrossRef]
- Katime, I.; Valderruten, N.; Quintana, J.R. Controlled release of aminophylline from poly (N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym. Int. 2001, 50, 869–879. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toeri, J.; Laborie, M.-P. Effects of Grafting Azacrown Ether on Thermal and Swelling Properties of Chitosan Films. ChemEngineering 2017, 1, 16. https://doi.org/10.3390/chemengineering1020016
Toeri J, Laborie M-P. Effects of Grafting Azacrown Ether on Thermal and Swelling Properties of Chitosan Films. ChemEngineering. 2017; 1(2):16. https://doi.org/10.3390/chemengineering1020016
Chicago/Turabian StyleToeri, Julius, and Marie-Pierre Laborie. 2017. "Effects of Grafting Azacrown Ether on Thermal and Swelling Properties of Chitosan Films" ChemEngineering 1, no. 2: 16. https://doi.org/10.3390/chemengineering1020016




