Hydrogen Utilization in Green Fuel Synthesis via CO2 Conversion to Methanol over New Cu-Based Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Catalysts
2.2. Characterization of the Catalysts
2.3. Catalytic Measurements
3. Results
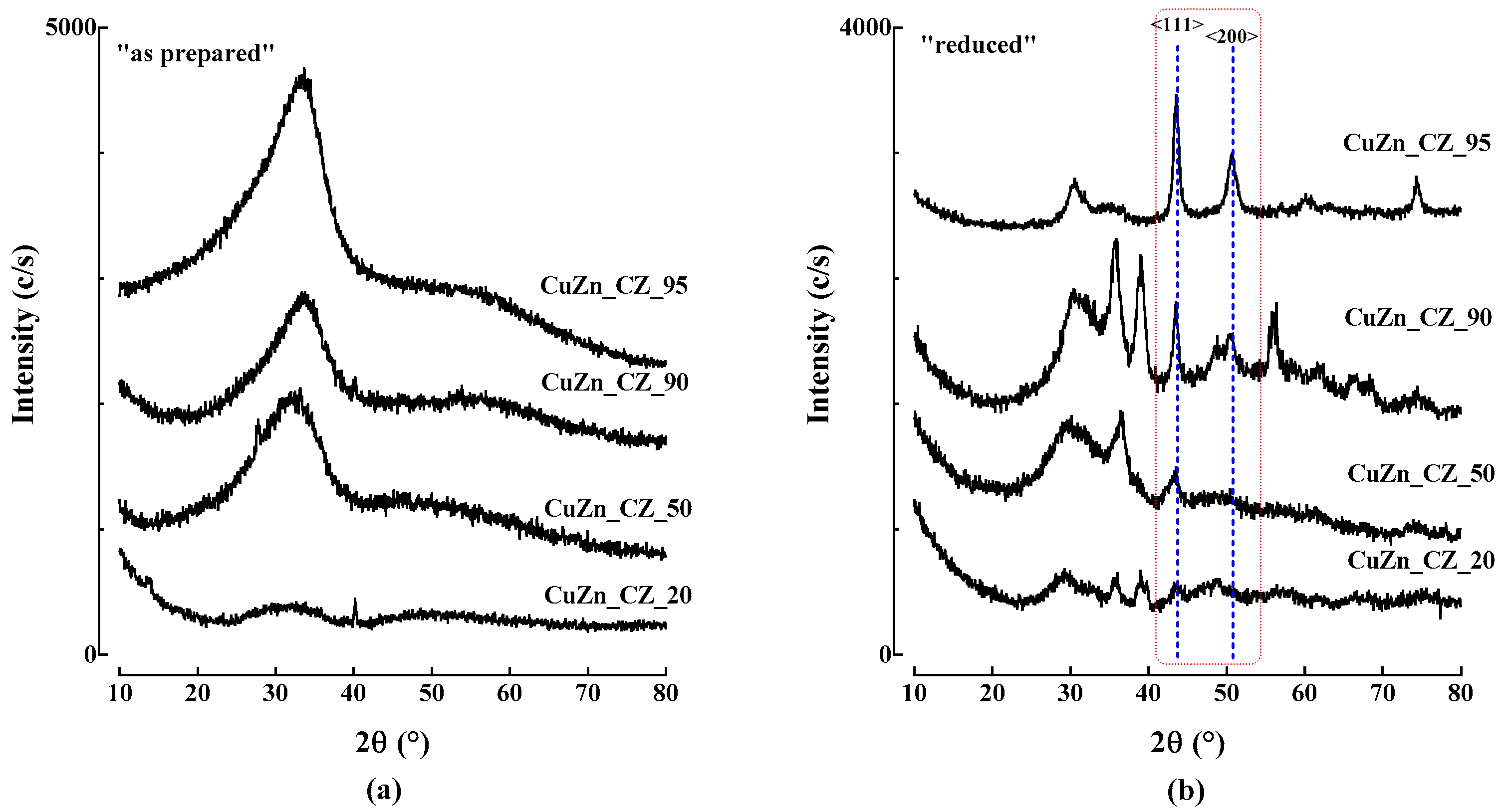
3.1. Chemical-Physical Characterization
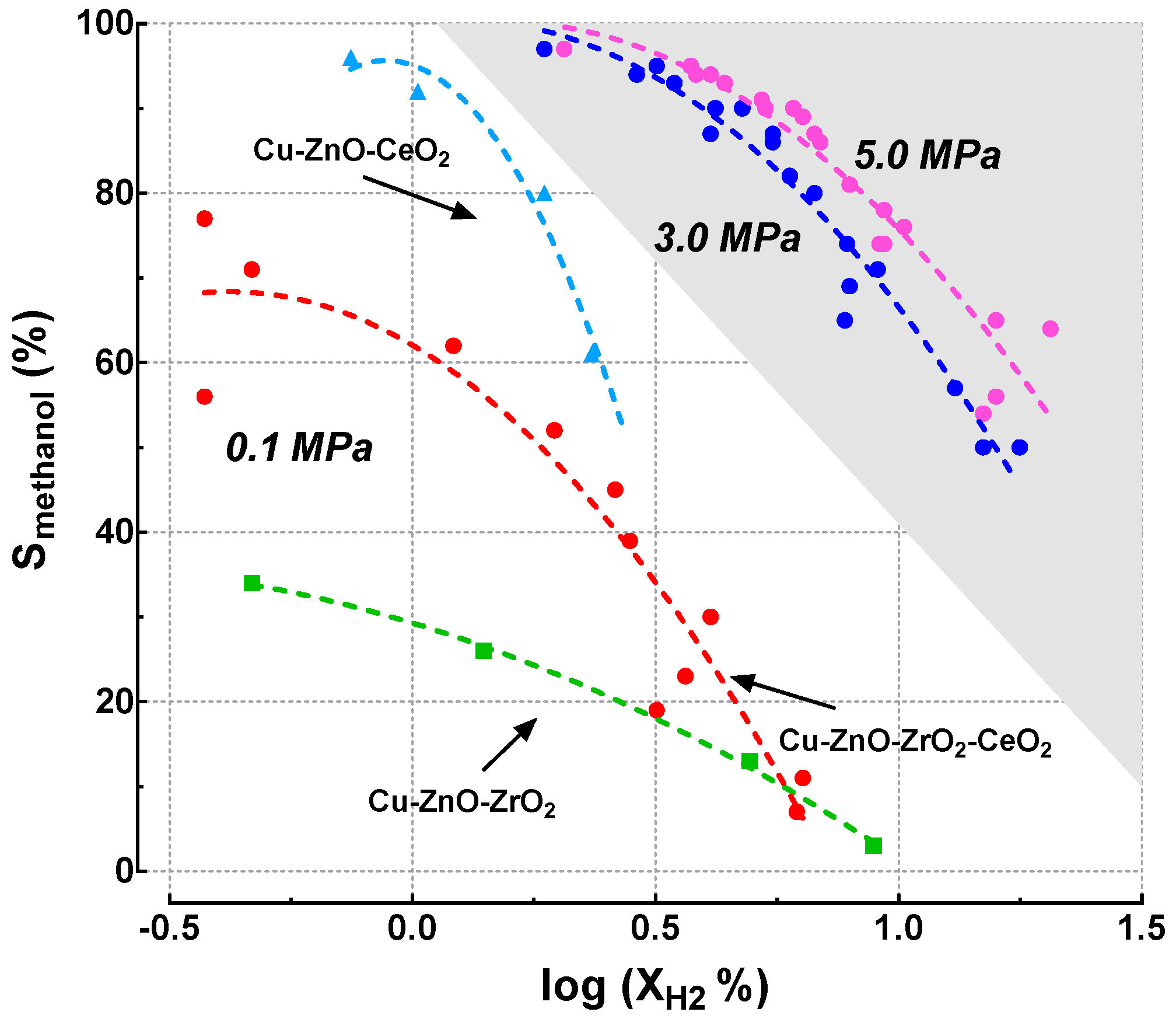
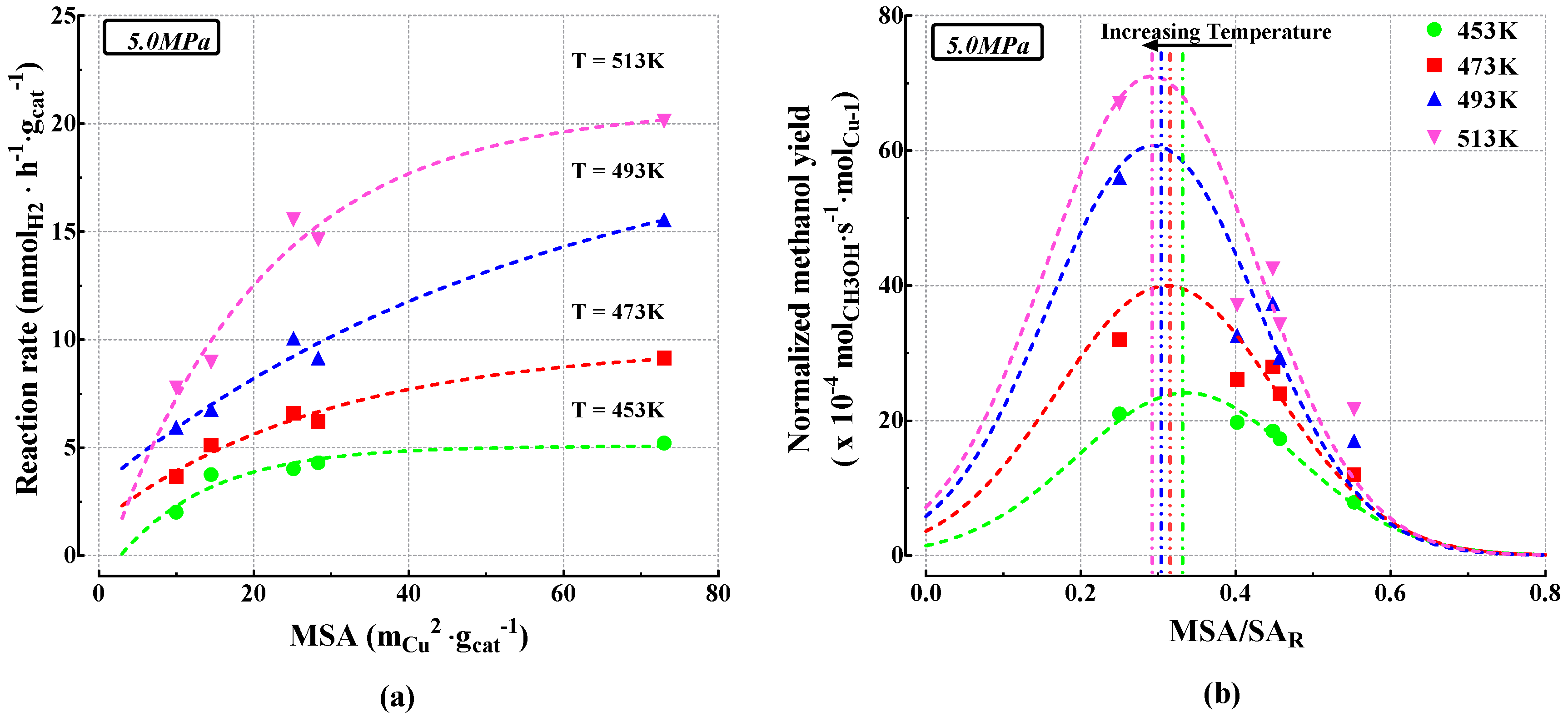
3.2. Catalytic Activity
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Larson, E.D.; Tingjin, R. Synthetic fuel production by indirect coal liquefaction. Energy Sustain. Dev. 2003, 7, 79–102. [Google Scholar] [CrossRef]
- Liu, X.-M.; Lu, G.Q.; Yan, Z.-F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Chen, C.-Y.; Yu, J.C.-C.; Nguyen, V.-H.; Wu, J.C.-S.; Wang, W.-H.; Kočí, K. Reactor Design for CO2 Photo-Hydrogenation toward Solar Fuels under Ambient Temperature and Pressure. Catalysts 2017, 7, 63. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME—Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250. [Google Scholar] [CrossRef]
- Hartadi, Y.; Widmann, D.; Behm, R.J. Methanol formation by CO2 hydrogenation on Au/ZnO catalysts—Effect of total pressure and influence of CO on the reaction characteristics. J. Catal. 2016, 333, 238–250. [Google Scholar] [CrossRef]
- Schlögl, R. Chemistry’s Role in Regenerative Energy. Angew. Chem.-Int. Ed. 2011, 50, 6424–6426. [Google Scholar] [CrossRef] [PubMed]
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd-Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2015, 170–171, 173–185. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Vourros, A.; Garagounis, I.; Kyriakou, V.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Marnellos, G.E.; Konsolakis, M. Carbon dioxide hydrogenation over supported Au nanoparticles: Effect of the support. J. CO2 Util. 2017, 19, 247–256. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Geng, W.; Li, H.; Xiao, L.; Wu, W. Cu-Mo2C/MCM-41: An Efficient Catalyst for the Selective Synthesis of Methanol from CO2. Catalysts 2016, 6, 75. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Spadaro, L.; Trunfio, G. Latest advances in the catalytic hydrogenation of CO2 to methanol/dimethylether. In Transformation and Utilization of Carbon Dioxide, Green Chemistry and Sustainable Technology; Bhanage, B.M., Arai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Deng, K.; Hu, B.; Lu, Q.; Hong, X. Cu/g-C3N4 modified ZnO/Al2O3 catalyst: Methanol yield improvement of CO2 hydrogenation. Catal. Commun. 2017, 100, 81–84. [Google Scholar] [CrossRef]
- Li, S.M.M.-J.; Zeng, Z.; Liao, F.; Hong, H.; Tsang, S.C.E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 2016, 343, 157–163. [Google Scholar] [CrossRef]
- Umegaki, T.; Kojima, Y.; Omata, K. Effect of oxide coating on performance of copper-zinc oxide-based catalyst for methanol synthesis via hydrogenation of carbon dioxide. Materials 2015, 8, 7738–7744. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C.J.A. Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over Cu/ZnO supported on Al2O3 and Nb2O5. J. CO2 Util. 2016, 15, 83–88. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, D.; Guo, X.; Yu, J. Effect of TiO2, ZrO2, and TiO2-ZrO2 on the performance of CuO-ZnO catalyst for CO2 hydrogenation to methanol. Appl. Surf. Sci. 2015, 338, 146–153. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.; Chen, J.C. Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl. Catal. A Gen. 2017, 206, 704–711. [Google Scholar] [CrossRef]
- Ouyang, B.; Tan, W.; Liu, B. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation. Catal. Commun. 2017, 95, 36–39. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Wang, H.; Sun, Y. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Fluorine-modified Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Commun. 2014, 50, 78–82. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Fei, X.; Liu, D.; Zhang, Y. Catalytic Hydrogenation of CO2 to Methanol: Study of Synergistic Effect on Adsorption Properties of CO2 and H2 in CuO/ZnO/ZrO2 System. Catalysts 2015, 5, 1846–1861. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu-ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bonura, G.; Spadaro, L.; Frusteri, F. Basic evidences for methanol-synthesis catalyst design. Catal. Today 2009, 143, 80–85. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. How oxide carriers control the catalytic functionality of the Cu-ZnO system in the hydrogenation of CO2 to methanol. Catal. Today 2013, 210, 39–46. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. Effects of oxide carriers on surface functionality and process performance of the Cu-ZnO system in the synthesis of methanol via CO2 hydrogenation. J. Catal. 2013, 300, 141–151. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M.; Melián-Cabrera, I.; Navarro, R.M.; Fierro, J.L.G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Evans, J.W.; Wainwright, M.S.; Bridgewater, A.J.; Young, D.J. On the determination of copper surface area by reaction with nitrous oxide. Appl. Catal. 1983, 7, 75–83. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Chen, S. Effect of promoter SiO2, TiO2 or SiO2-TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for methanol synthesis from CO2 hydrogenation. Appl. Catal. A Gen. 2012, 415–416, 118–123. [Google Scholar] [CrossRef]
- Arena, F.; Di Chio, R.; Fazio, B.; Espro, C.; Spiccia, L.; Palella, A.; Spadaro, L. Probing the functionality of nanostructured MnCeOx catalysts in the carbon monoxide oxidation. Part I. Influence of cerium addition on structure and CO oxidation activity. Appl. Catal. B Environ. 2017, 210, 14–22. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdiet, J.W. Concepts of Modern Catalysis and Kinetics; Wiley-VCH Verlag GmgH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Bartholomew, C.H.; Ferrauto, R.J. Foundamentals of Industrial Catalytic Processes; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Li, L.; Mao, D.; Yu, J.; Guo, X. Highly selective hydrogenation of CO2 to methanol over CuO-ZnO-ZrO2 catalysts prepared by a surfactant-assisted co-precipitation method. J. Power Sources 2015, 279, 394–404. [Google Scholar] [CrossRef]
- Angelo, L.; Kobl, K.; Tejada, L.M.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.-C. Study of CuZnMOx oxides (M = Al, Zr, Ce, CeZr) for the catalytic hydrogenation of CO2 into methanol. C. R. Chim. 2015, 18, 250–260. [Google Scholar] [CrossRef]
- Arena, F.; Famulari, P.; Trunfio, G.; Bonura, G.; Frusteri, F.; Spadaro, L. Probing the factors affecting structure and activity of the Au/CeO2 system in total and preferential oxidation of CO. Appl. Catal. B Environ. 2006, 66, 81–91. [Google Scholar] [CrossRef]
- Arena, F.; Famulari, P.; Interdonato, N.; Bonura, G.; Frusteri, F.; Spadaro, L. Physico-chemical properties and reactivity of Au/CeO2 catalysts in total and selective oxidation of CO. Catal. Today 2006, 116, 384–390. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2002. [Google Scholar]
- Kunkes, E.L.; Studt, F.; Abild-Pedersen, F.; Schlögl, R.; Behrens, M. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: Is there a common intermediate or not? J. Catal. 2015, 328, 43–48. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In-Situ Infrared Study of Methanol Synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1997, 172, 222–237. [Google Scholar] [CrossRef]
- Spadaro, L.; Arena, F.; Granados, M.L.; Ojeda, M.; Fierro, J.L.G.; Frusteri, F. Metal-support interactions and reactivity of Co/CeO2 catalysts in the Fischer-Tropsch synthesis reaction. J. Catal. 2005, 234, 451–462. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In-Situ Infrared Study of Methanol Synthesis from H2/CO over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1998, 178, 153–173. [Google Scholar] [CrossRef]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 Adsorption on Tetragonal and Monoclinic Zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Tada, S.; Watanabe, F.; Kiyota, K.; Shimoda, N.; Hayashi, R.; Takahashi, M.; Nariyuki, A.; Igarashi, A.; Satokawa, S. Ag addition to CuO-ZrO2 catalysts promotes methanol synthesis via CO2 hydrogenation. J. Catal. 2017, 351, 107–118. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. J. Catal. 2016, 343, 133–146. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Z.; Zhao, N.; Wei, W.; Hu, T.; Sun, Y. Methanol synthesis from CO2-rich syngas over a ZrO2 doped CuZnO catalyst. Catal. Today 2006, 115, 222–227. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Wu, D.; Fan, W.-H.; Zhang, Y.-L.; Deng, J.-F. A practical approach for the preparation of high activity Cu/ZnO/ZrO2 catalyst for methanol synthesis from CO2 hydrogenation. Appl. Catal. A Gen. 1998, 171, 45–55. [Google Scholar] [CrossRef]
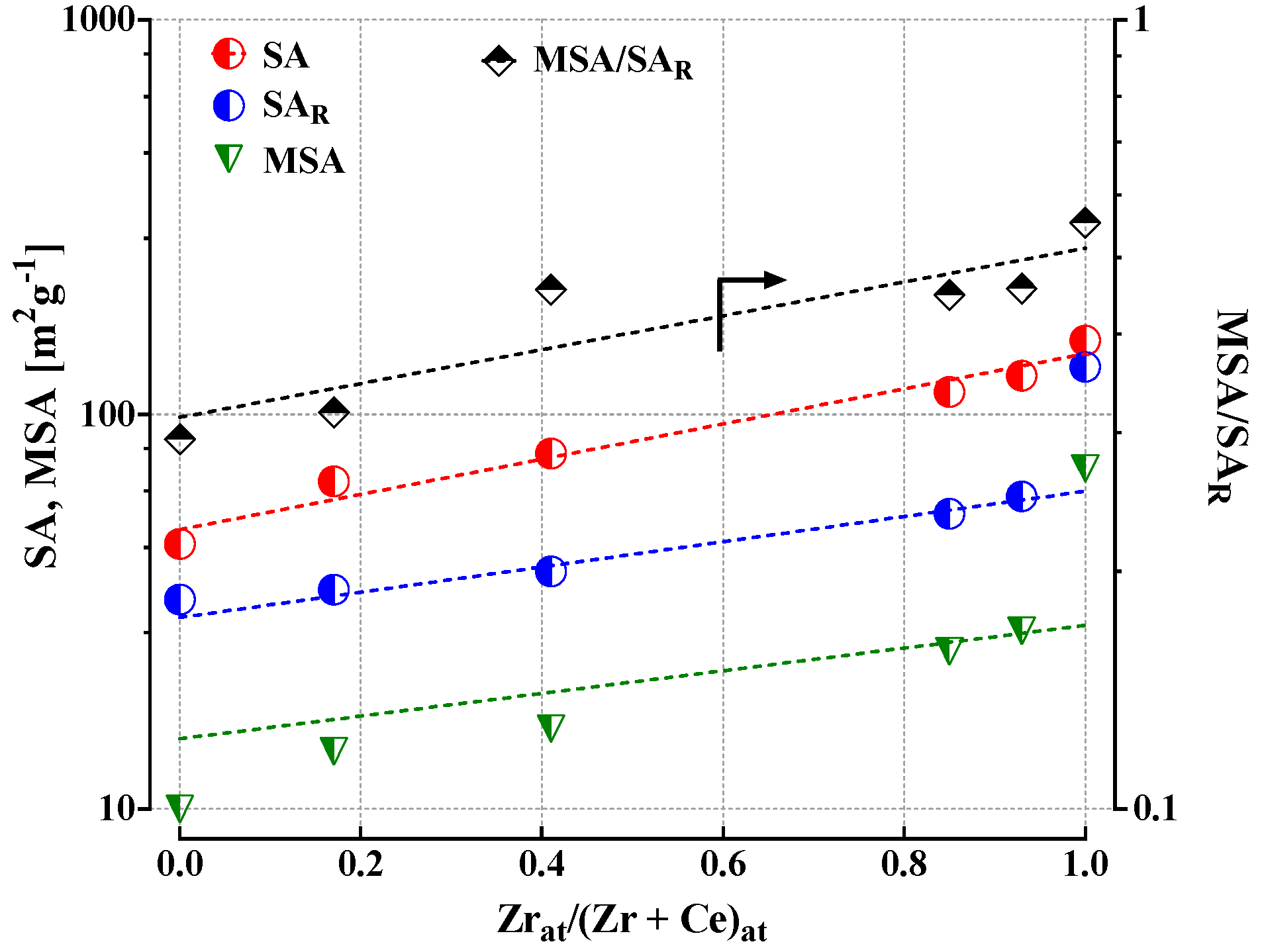

| Sample | Chemical Composition (wt%) | Physical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CuO | ZnO | ZrO2 | CeO2 | SA (m2·g−1) | PV (cm3·g−1) | APD (a) (nm) | MSA (c) (m2·g−1) | MSA/SAR | ||
| CuZn_CZ_100 | 44.2 | 13.4 | 42.1 | 0.0 | 154 | 0.34 | 9.0 | - | - | |
| R (b) | - | - | - | - | 132 | 0.28 | 8.0 | 73 | 0.55 | |
| CuZn_CZ_95 | 42.8 | 13.2 | 38.9 | 4.2 | 125 | 0.40 | 11.2 | - | - | |
| R (b) | - | - | - | - | 62 | 0.20 | 12.9 | 28 | 0.45 | |
| CuZn_CZ_90 | 42.6 | 12.8 | 34.0 | 8.3 | 114 | 0.60 | 18.7 | - | - | |
| R (b) | - | - | - | - | 56 | 0.21 | 10.8 | 25 | 0.44 | |
| CuZn_CZ_50 | 39.0 | 12.3 | 15.6 | 31.8 | 80 | 0.89 | 48.6 | - | - | |
| R (b) | - | - | - | - | 40 | 0.20 | 15.7 | 16 | 0.40 | |
| CuZn_CZ_20 | 40.1 | 13.0 | 5.8 | 40.3 | 68 | 0.32 | 20.8 | - | - | |
| R (b) | - | - | - | - | 36 | 0.20 | 21.0 | 14 | 0.38 | |
| CuZn_CZ_0 | 38.7 | 12.2 | 0.0 | 48.8 | 47 | 0.24 | 20.0 | - | - | |
| R (b) | - | - | - | - | 34 | 0.17 | 20.0 | 10 | 0.29 | |
| 0.1 MPa | (%) | (%) | Reaction Rate (mmolH2·h−1·gcat−1) | |||||||||
| 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | |
| CUZN_CZ_100 | 0.5 | 1.4 | 4.9 | 8.9 | 34 | 26 | 13 | 3 | 0.46 | 1.37 | 4.85 | 8.69 |
| CUZN_CZ_95 | 0.4 | 2.6 | 3.6 | 6.2 | 56 | 45 | 23 | 7 | 0.37 | 2.56 | 3.57 | 6.04 |
| CUZN_CZ_90 | 0.5 | 2.0 | 4.1 | 6.3 | 71 | 52 | 30 | 11 | 0.46 | 1.92 | 4.02 | 6.22 |
| CUZN_CZ_20 | 0.4 | 1.2 | 2.8 | 3.2 | 77 | 62 | 39 | 19 | 0.37 | 1.19 | 2.74 | 3.11 |
| CUZN_CZ_0 | 0.7 | 1.0 | 1.9 | 2.3 | 96 | 92 | 80 | 61 | 0.73 | 1.01 | 1.83 | 2.29 |
| 3.0 MPa | (%) | (%) | Reaction Rate (mmolH2·h−1·gcat−1) | |||||||||
| 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | |
| CUZN_CZ_100 | 4.1 | 7.8 | 13.1 | 17.7 | 87 | 74 | 57 | 50 | 4.02 | 7.68 | 12.81 | 17.38 |
| CUZN_CZ_95 | 3.5 | 5.5 | 7.7 | 13.1 | 93 | 86 | 65 | 57 | 3.38 | 5.40 | 7.59 | 12.81 |
| CUZN_CZ_90 | 3.5 | 5.5 | 9.1 | 14.9 | 93 | 87 | 71 | 50 | 3.38 | 5.40 | 8.87 | 14.63 |
| CUZN_CZ_20 | 2.9 | 4.2 | 6.0 | 7.9 | 94 | 90 | 82 | 69 | 2.84 | 4.12 | 5.85 | 7.77 |
| CUZN_CZ_0 | 1.9 | 3.2 | 4.8 | 6.7 | 97 | 95 | 90 | 80 | 1.83 | 3.11 | 4.66 | 6.59 |
| 5.0 MPa | (%) | (%) | Reaction Rate (mmolH2·h−1·gcat−1) | |||||||||
| 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | 453 K | 473 K | 493 K | 513 K | |
| CUZN_CZ_100 | 5.3 | 9.3 | 15.9 | 20.5 | 90 | 78 | 65 | 64 | 5.21 | 9.15 | 15.55 | 20.12 |
| CUZN_CZ_95 | 4.4 | 6.3 | 9.3 | 14.9 | 93 | 89 | 74 | 54 | 4.30 | 6.22 | 9.15 | 14.63 |
| CUZN_CZ_90 | 4.1 | 6.7 | 10.3 | 15.9 | 94 | 87 | 76 | 56 | 4.02 | 6.59 | 10.06 | 15.55 |
| CUZN_CZ_20 | 3.8 | 5.2 | 6.9 | 9.1 | 94 | 91 | 86 | 74 | 3.75 | 5.12 | 6.77 | 8.96 |
| CUZN_CZ_0 | 2.1 | 3.7 | 6.1 | 7.9 | 97 | 95 | 90 | 81 | 2.01 | 3.66 | 5.95 | 7.77 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaro, L.; Santoro, M.; Palella, A.; Arena, F. Hydrogen Utilization in Green Fuel Synthesis via CO2 Conversion to Methanol over New Cu-Based Catalysts. ChemEngineering 2017, 1, 19. https://doi.org/10.3390/chemengineering1020019
Spadaro L, Santoro M, Palella A, Arena F. Hydrogen Utilization in Green Fuel Synthesis via CO2 Conversion to Methanol over New Cu-Based Catalysts. ChemEngineering. 2017; 1(2):19. https://doi.org/10.3390/chemengineering1020019
Chicago/Turabian StyleSpadaro, Lorenzo, Mariarita Santoro, Alessandra Palella, and Francesco Arena. 2017. "Hydrogen Utilization in Green Fuel Synthesis via CO2 Conversion to Methanol over New Cu-Based Catalysts" ChemEngineering 1, no. 2: 19. https://doi.org/10.3390/chemengineering1020019





