Interlayer Properties of In-Situ Oxidized Porous Stainless Steel for Preparation of Composite Pd Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
2.2. Membrane Characterization
3. Results and Discussion
3.1. Support Characterization
3.2. Intermediate Layer Formation by In-Situ Oxidation
3.3. Palladium Composite Membrane Preparation
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Hanley; E.S.; Deane; J.P.; Gallachóir; B.P.Ó. The role of hydrogen in low carbon energy futures—A review of existing perspectives. Renew. Sustain. Energy Rev. 2017. [Google Scholar] [CrossRef]
- Vivas, F.J.; de las Heras, A.; Segura, F.; Andújar, J.M. A review of energy management strategies for renewable hybrid energy systems with hydrogen backup. Renew. Sustain. Energy Rev. 2018, 82, 126–155. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. "Renewable” hydrogen: Prospects and challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Uyar, T.S.; Beşikci, D. Integration of hydrogen energy systems into renewable energy systems for better design of 100% renewable energy communities. Int. J. Hydrog. Energy 2017, 42, 2453–2456. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- García-García, F.R.; Soria, M.A.; Mateos-Pedrero, C.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Li, K. Dry reforming of methane using Pd-based membrane reactors fabricated from different substrates. J. Memb. Sci. 2013, 435, 218–225. [Google Scholar] [CrossRef]
- Kim, C.-H.; Han, J.-Y.; Lim, H.; Lee, K.-Y.; Ryi, S.-K. Methane steam reforming using a membrane reactor equipped with a Pd-based composite membrane for effective hydrogen production. Int. J. Hydrog. Energy. 2017. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, S.; Kumar, S. Thermodynamic and energy analysis of renewable butanol–ethanol fuel reforming for the production of hydrogen. J. Environ. Chem. Eng. 2017, 5, 5876–5890. [Google Scholar] [CrossRef]
- Tian, T.; Li, Q.; He, R.; Tan, Z.; Zhang, Y. Effects of biochemical composition on hydrogen production by biomass gasification. Int. J. Hydrog. Energy 2017, 42, 19723–19732. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Gimžauskaitė, D.; Valinčius, V.; Jeguirim, M. Glycerol steam reforming for hydrogen and synthesis gas production. Int. J. Hydrog. Energy 2017, 42, 12896–12904. [Google Scholar] [CrossRef]
- Devasahayam, S.; Strezov, V. Thermal Decomposition of Magnesium Carbonate with Biomass and Plastic Wastes for Simultaneous Production of Hydrogen and Carbon Avoidance. J. Clean. Prod. 2017, 174, 1089–1095. [Google Scholar] [CrossRef]
- Ayas, N.; Esen, T. Hydrogen production from tea waste. Int. J. Hydrog. Energy. 2016, 41, 8067–8072. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; García-Morales, J.L.; Sales, D. Effect of the addition of glycerol on hydrogen production from industrial municipal solid waste. Fuel 2016, 180, 343–347. [Google Scholar] [CrossRef]
- Tosti, S.; Cavezza, C.; Fabbricino, M.; Pontoni, L.; Palma, V.; Ruocco, C. Production of hydrogen in a Pd-membrane reactor via catalytic reforming of olive mill wastewater. Chem. Eng. J. 2015, 275, 366–373. [Google Scholar] [CrossRef]
- Li, H.; Caravella, A.; Xu, H.Y. Recent progress in Pd-based composite membranes. J. Mater. Chem. A. 2016, 4, 14069–14094. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Arratibel Plazaola, A.; Pacheco Tanaka, D.; van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Alique, D.; Imperatore, M.; Sanz, R.; Calles, J.A.; Giacinti Baschetti, M. Hydrogen permeation in composite Pd-membranes prepared by conventional electroless plating and electroless pore-plating alternatives over ceramic and metallic supports. Int. J. Hydrog. Energy 2016, 41, 19430–19438. [Google Scholar] [CrossRef]
- Li, X.; Li, A.; Lim, C.J.; Grace, J.R. Hydrogen permeation through Pd-based composite membranes: Effects of porous substrate, diffusion barrier and sweep gas. J. Memb. Sci. 2016, 499, 143–155. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L. H2 production via water gas shift in a composite Pd membrane reactor prepared by the pore-plating method. Int. J. Hydrog. Energy 2014, 39, 4739–4748. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. On the high pressure performance of thin supported Pd–23%Ag membranes—Evidence of ultrahigh hydrogen flux after air treatment. J. Memb. Sci. 2011, 378, 28–34. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; van Sint Annaland, M.; Pacheco Tanaka, D.A. Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Calles, J.A.; Sanz, R.; Alique, D.; Furones, L. Thermal stability and effect of typical water gas shift reactant composition on H2 permeability through a Pd-YSZ-PSS composite membrane. Int. J. Hydrogen Energy. 2014, 39, 1398–1409. [Google Scholar] [CrossRef]
- Santucci, A.; Borgognoni, F.; Vadrucci, M.; Tosti, S. Testing of dense Pd–Ag tubes: Effect of pressure and membrane thickness on the hydrogen permeability. J. Memb. Sci. 2013, 444, 378–383. [Google Scholar] [CrossRef]
- Tosti, S. Supported and laminated Pd-based metallic membranes. Int. J. Hydrog. Energy 2003, 28, 1445–1454. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Fletcher, S.; Speight, J.D.; Book, D. Hydrogen permeation through porous stainless steel for palladium-based composite porous membranes. J. Memb. Sci. 2016, 515, 22–28. [Google Scholar] [CrossRef]
- Nakajima, T.; Kume, T.; Ikeda, Y.; Shiraki, M.; Kurokawa, H.; Iseki, T.; Kajitani, M.; Tanaka, H.; Hikosaka, H.; Takagi, Y.; et al. Effect of concentration polarization on hydrogen production performance of ceramic-supported Pd membrane module. Int. J. Hydrog. Energy 2015, 40, 11451–11456. [Google Scholar] [CrossRef]
- Abate, S.; Díaz, U.; Prieto, A.; Gentiluomo, S.; Palomino, M.; Perathoner, S.; Corma, A.; Centi, G. Influence of Zeolite Protective Overlayer on the Performances of Pd Thin Film Membrane on Tubular Asymmetric Alumina Supports. Ind. Eng. Chem. Res. 2016, 55, 4948–4959. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Sint Annaland, M.; Arias, P.L.; Tanaka, D.A.P. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Memb. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Richter, H. 4—Large-scale ceramic support fabrication for palladium membranes. In Palladium Membrane Technology for Hydrogen Production, Carbon Capture and Other Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 69–82. [Google Scholar] [CrossRef]
- Huang, Y.; Dittmeyer, R. Preparation of thin palladium membranes on a porous support with rough surface. J. Memb. Sci. 2007, 302, 160–170. [Google Scholar] [CrossRef]
- Lee, J.-H.; Han, J.-Y.; Kim, K.-M.; Ryi, S.-K.; Kim, D.-W. Development of homogeneous Pd–Ag alloy membrane formed on porous stainless steel by multi-layered films and Ag-upfilling heat treatment. J. Memb. Sci. 2015, 492, 242–248. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L.; Ordóñez, S.; Marín, P.; Corengia, P.; Fernandez, E. Preparation, testing and modelling of a hydrogen selective Pd/YSZ/SS composite membrane. Int. J. Hydrog. Energy 2011, 36, 15783–15793. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.; Rui, Z.; Liu, P.; Li, Y.; Lin, Y.S. High-Temperature Stability of Palladium Membranes on Porous Metal Supports with Different Intermediate Layers. Ind. Eng. Chem. Res. 2009, 48, 1880–1886. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Xu, H. “Defect-free” interlayer with a smooth surface and controlled pore-mouth size for thin and thermally stable Pd composite membranes. Int. J. Hydrog. Energy 2016, 41, 1002–1009. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Soria, M.A.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Modifications of porous stainless steel previous to the synthesis of Pd membranes. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2010; pp. 779–783. [Google Scholar]
- Yepes, D.; Cornaglia, L.M.; Irusta, S.; Lombardo, E.A. Different oxides used as diffusion barriers in composite hydrogen permeable membranes. J. Memb. Sci. 2006, 274, 92–101. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, K.-H. Hydrogen separation by Pd alloy composite membranes: Introduction of diffusion barrier. J. Memb. Sci. 2001, 192, 177–185. [Google Scholar] [CrossRef]
- Calles, J.A.; Sanz, R.; Alique, D. Influence of the type of siliceous material used as intermediate layer in the preparation of hydrogen selective palladium composite membranes over a porous stainless steel support. Int. J. Hydrog. Energy 2012, 37, 6030–6042. [Google Scholar] [CrossRef]
- Qiao, A.; Zhang, K.; Tian, Y.; Xie, L.; Luo, H.; Lin, Y.S.; Li, Y. Hydrogen separation through palladium–copper membranes on porous stainless steel with sol–gel derived ceria as diffusion barrier. Fuel 2010, 89, 1274–1279. [Google Scholar] [CrossRef]
- Tong, J.; Matsumura, Y.; Suda, H.; Haraya, K. Thin and dense Pd/CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane. Sep. Purif. Technol. 2005, 46, 1–10. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Ahn, H.-S.; Park, J.-S.; Kim, D.-W. Pd-Cu alloy membrane deposited on CeO2 modified porous nickel support for hydrogen separation. Int. J. Hydrog. Energy 2014, 39, 4698–4703. [Google Scholar] [CrossRef]
- Tarditi, A.; Gerboni, C.; Cornaglia, L. PdAu membranes supported on top of vacuum-assisted ZrO2-modified porous stainless steel substrates. J. Memb. Sci. 2013, 428, 1–10. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Preparation of “pore-fill” type Pd–YSZ–γ-Al2O3 composite membrane supported on α-Al2O3 tube for hydrogen separation. J. Memb. Sci. 2008, 320, 436–441. [Google Scholar] [CrossRef]
- Hwang, K.-R.; Oh, D.-K.; Lee, S.-W.; Park, J.-S.; Song, M.-H.; Rhee, W.-H. Porous stainless steel support for hydrogen separation Pd membrane; fabrication by metal injection molding and simple surface modification. Int. J. Hydrog. Energy 2017, 42, 14583–14592. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Meng, B.; Zhang, X.; Liang, Q.; Pan, H.; Liu, S. TS-1 zeolite as an effective diffusion barrier for highly stable Pd membrane supported on macroporous [small alpha]-Al2O3 tube. RSC Adv. 2013, 3, 4821–4834. [Google Scholar] [CrossRef]
- Bosko, M.L.; Ojeda, F.; Lombardo, E.A.; Cornaglia, L.M. NaA zeolite as an effective diffusion barrier in composite Pd/PSS membranes. J. Memb. Sci. 2009, 331, 57–65. [Google Scholar] [CrossRef]
- Nozaki, T.; Hatano, Y.; Yamakawa, E.; Hachikawa, A.; Ichinose, K. Improvement of high temperature stability of Pd coating on Ta by HfN intermediate layer. Int. J. Hydrog. Energy 2010, 35, 12454–12460. [Google Scholar] [CrossRef]
- Samingprai, S.; Tantayanon, S.; Ma, Y.H. Chromium oxide intermetallic diffusion barrier for palladium membrane supported on porous stainless steel. J. Memb. Sci. 2010, 347, 8–16. [Google Scholar] [CrossRef]
- Guazzone, F.; Engwall, E.E.; Ma, Y.H. Effects of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes. Catal. Today 2006, 118, 24–31. [Google Scholar] [CrossRef]
- Ma, Y.H.; Akis, B.C.; Ayturk, M.E.; Guazzone, F.; Engwall, E.E.; Mardilovich, I.P. Characterization of Intermetallic Diffusion Barrier and Alloy Formation for Pd/Cu and Pd/Ag Porous Stainless Steel Composite Membranes. Ind. Eng. Chem. Res. 2004, 43, 2936–2945. [Google Scholar] [CrossRef]
- Iulianelli, A.; Alavi, M.; Bagnato, G.; Liguori, S.; Wilcox, J.; Rahimpour, R.M.; Eslamlouyan, R.; Anzelmo, B.; Basile, A. Supported Pd-Au Membrane Reactor for Hydrogen Production: Membrane Preparation, Characterization and Testing. Molecules 2016, 21, 581. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L. New synthesis method of Pd membranes over tubular {PSS} supports via “pore-plating” for hydrogen separation processes. Int. J. Hydrog. Energy 2012, 37, 18476–18485. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Ordóñez, S.; Marín, P.; Alique, D.; Furones, L. Modelling and simulation of permeation behaviour on Pd/PSS composite membranes prepared by “pore-plating” method. J. Memb. Sci. 2013, 446, 410–421. [Google Scholar] [CrossRef]
- Mardilovich, I.P.; Engwall, E.; Ma, Y.H. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes. Desalination 2002, 144, 85–89. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 301–353. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kim, C.-H.; Lim, H.; Lee, K.-Y.; Ryi, S.-K. Diffusion barrier coating using a newly developed blowing coating method for a thermally stable Pd membrane deposited on porous stainless-steel support. Int. J. Hydrog. Energy 2017, 42, 12310–12319. [Google Scholar] [CrossRef]
- Washington, P. II.C.3 High Performance Palladium-Based Membrane for Hydrogen Separation and Purification; Department of Energy: Washington, DC, USA, 2011; pp. 58–61. [Google Scholar]
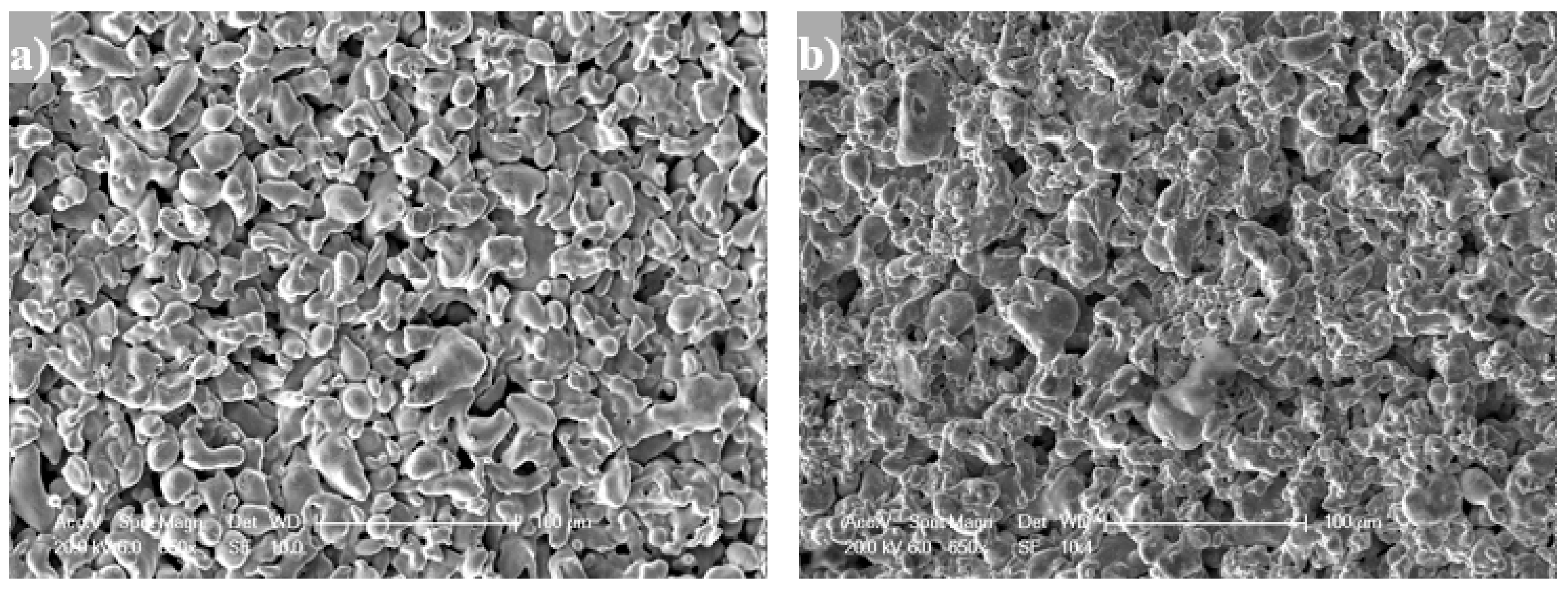
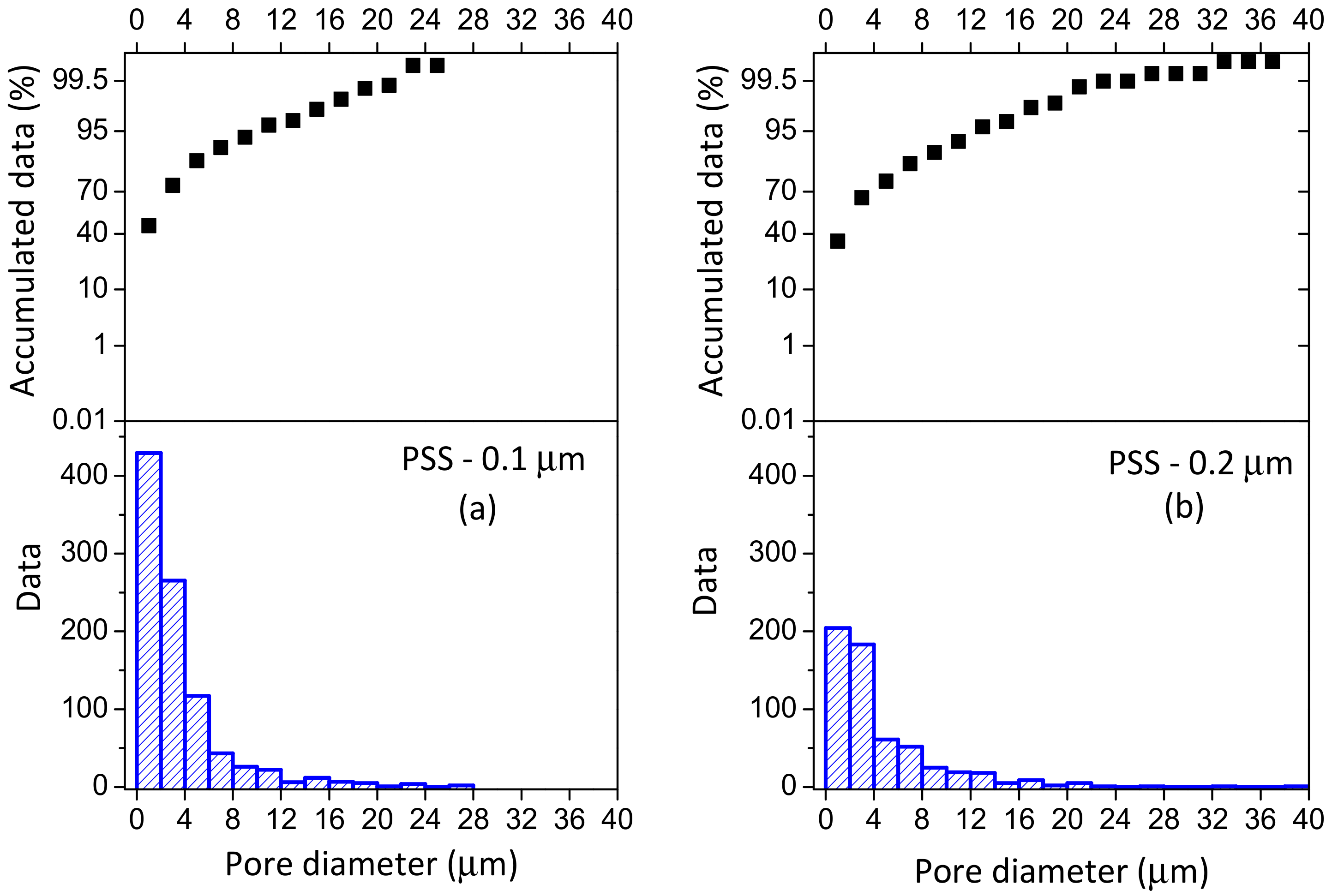
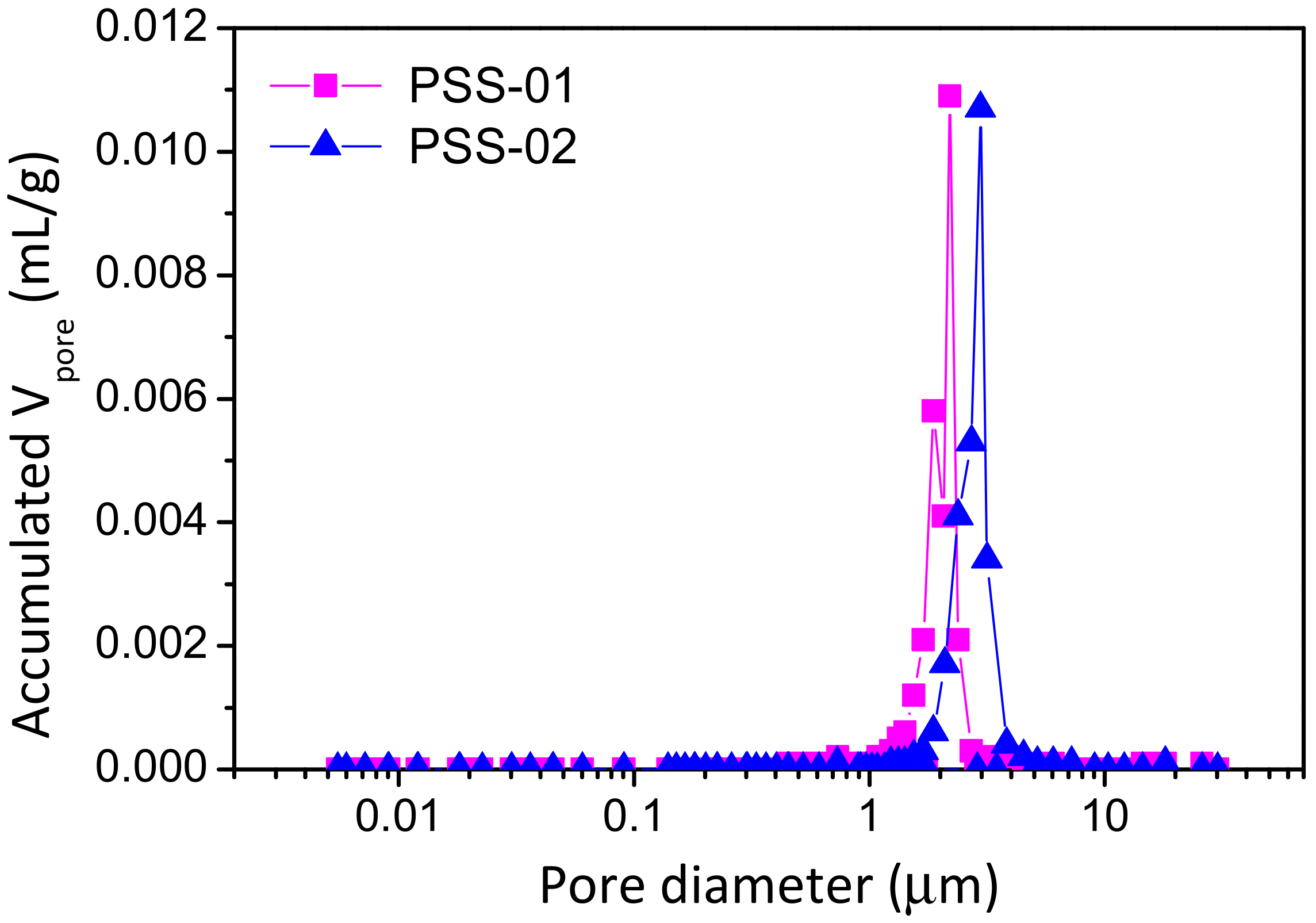


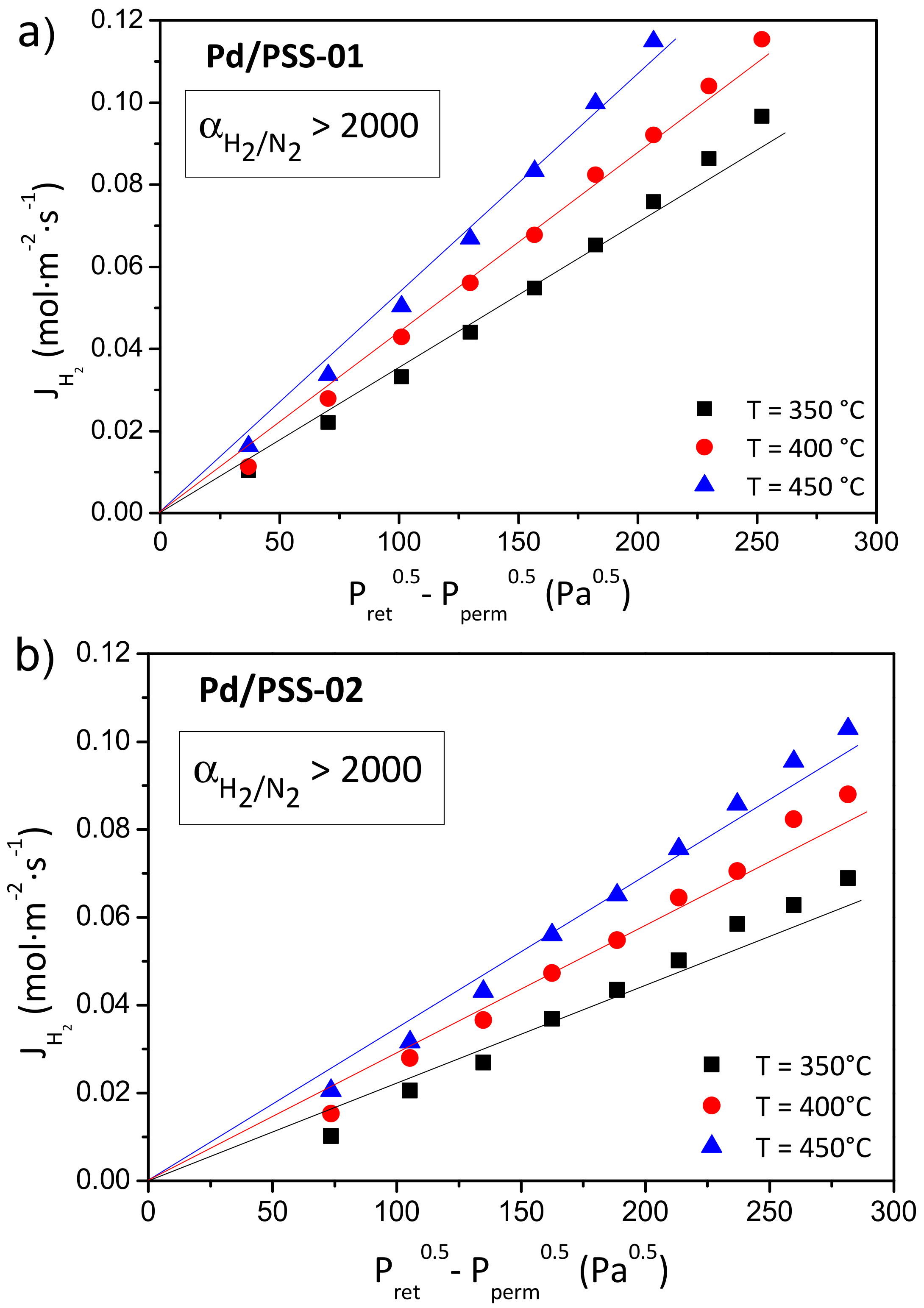
| Sample | Media Grade (μm) | Tox (°C) | ∆m/L (g/m) | ε (%) | dp (μm) | Ra (μm) | Elemental Composition EDAS (wt % ) | ||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | O | |||||||
| PSS-01 | 0.1 | - | - | 21.5 | 3.5 | 11.8 | 63.3 | 17.0 | 0.0 |
| PSS-01-550 | 0.1 | 550 | 1.5 | 15.7 | 3.1 | 9.4 | 61.2 | 16.1 | 7.5 |
| PSS-01-600 | 0.1 | 600 | 5.8 | 7.3 | 2.1 | 9.0 | 61.0 | 13.5 | 11.4 |
| PSS-01-650 | 0.1 | 650 | 7.4 | 5.7 | 1.7 | 8.9 | 59.2 | 10.6 | 19.9 |
| PSS-01-700 | 0.1 | 700 | 19.2 | 1.4 | 0.6 | 6.5 | 75.5 | 1.2 | 23.3 |
| PSS-02 | 0.2 | - | - | 19.6 | 4.5 | 12.7 | 63.9 | 16.9 | 0.0 |
| PSS-02-550 | 0.2 | 550 | 2.0 | 15.4 | 3.4 | 9.9 | 61.9 | 16.3 | 7.4 |
| PSS-02-600 | 0.2 | 600 | 4.4 | 10.6 | 2.3 | 9.2 | 57.6 | 13.3 | 16.1 |
| PSS-02-650 | 0.2 | 650 | 6.6 | 6.3 | 2.1 | 8.7 | 56.3 | 14.2 | 17.8 |
| PSS-02-700 | 0.2 | 700 | 15.5 | 1.6 | 0.8 | 6.2 | 77.2 | 1.5 | 21.3 |
| Sample | Media Grade (μm) | Tox (°C) | ∆P (bar) | (mol m−2 s−1) | (mol m−2 s−1) | |
|---|---|---|---|---|---|---|
| PSS-01 | 0.1 | - | 0.5 | 0.30 | 0.79 | 2.7 |
| 1.0 | 0.72 | 1.78 | 2.5 | |||
| PSS-01-550 | 0.1 | 550 | 0.5 | 0.21 | 0.59 | 2.9 |
| 1.0 | 0.49 | 1.37 | 2.8 | |||
| PSS-01-600 | 0.1 | 600 | 0.5 | 0.12 | 0.31 | 2.7 |
| 1.0 | 0.27 | 0.73 | 2.7 | |||
| PSS-01-650 | 0.1 | 650 | 0.5 | 0.09 | 0.25 | 2.7 |
| 1.0 | 0.21 | 0.57 | 2.7 | |||
| PSS-01-700 | 0.1 | 700 | 0.5 | - | - | - |
| 1.0 | - | - | - | |||
| PSS-02 | 0.2 | - | 0.5 | 0.63 | 1.52 | 2.4 |
| 1.0 | 1.48 | 3.61 | 2.4 | |||
| PSS-02-550 | 0.2 | 550 | 0.5 | 0.48 | 1.16 | 2.4 |
| 1.0 | 1.16 | 2.90 | 2.5 | |||
| PSS-02-600 | 0.2 | 600 | 0.5 | 0.33 | 1.02 | 3.1 |
| 1.0 | 0.82 | 2.36 | 2.9 | |||
| PSS-02-650 | 0.2 | 650 | 0.5 | 0.20 | 0.60 | 3.0 |
| 1.0 | 0.48 | 1.34 | 2.8 | |||
| PSS-01-700 | 0.2 | 700 | 0.5 | <1.02 × 10−5 | <1.02 × 10−5 | - |
| 1.0 | <1.02 × 10−5 | <1.02 × 10−5 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furones, L.; Alique, D. Interlayer Properties of In-Situ Oxidized Porous Stainless Steel for Preparation of Composite Pd Membranes. ChemEngineering 2018, 2, 1. https://doi.org/10.3390/chemengineering2010001
Furones L, Alique D. Interlayer Properties of In-Situ Oxidized Porous Stainless Steel for Preparation of Composite Pd Membranes. ChemEngineering. 2018; 2(1):1. https://doi.org/10.3390/chemengineering2010001
Chicago/Turabian StyleFurones, Laura, and David Alique. 2018. "Interlayer Properties of In-Situ Oxidized Porous Stainless Steel for Preparation of Composite Pd Membranes" ChemEngineering 2, no. 1: 1. https://doi.org/10.3390/chemengineering2010001





