A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics
Abstract
:1. Introduction
2. Data Sources and Methodology
3. Results and Discussion
3.1. Bibliometric Analysis of Research on the Hemicellulose Valorization (2000–2016)
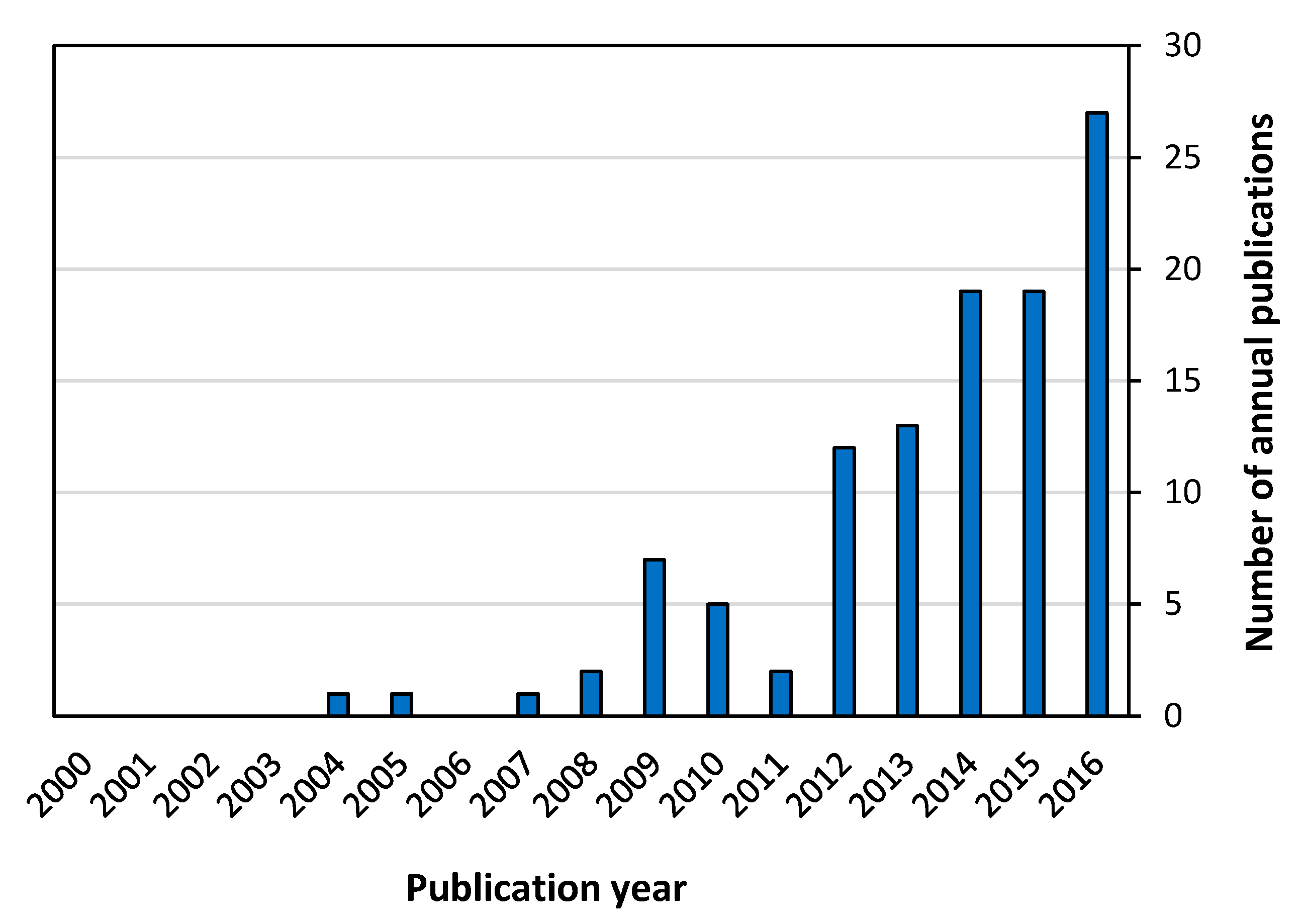
3.1.1. Publication Year, Document Type and Language of Documents
3.1.2. Distribution of Output in Subject Categories and Journals
3.1.3. Publication Distribution of Countries and Institutions
3.1.4. Most Frequently Cited Papers
3.2. Analysis of Author Keywords and Hot Topics of the Research on Hemicellulose Valorization
3.3. Review of the Main Alternatives for Hemicellulose Valorization and Current Trending Topics
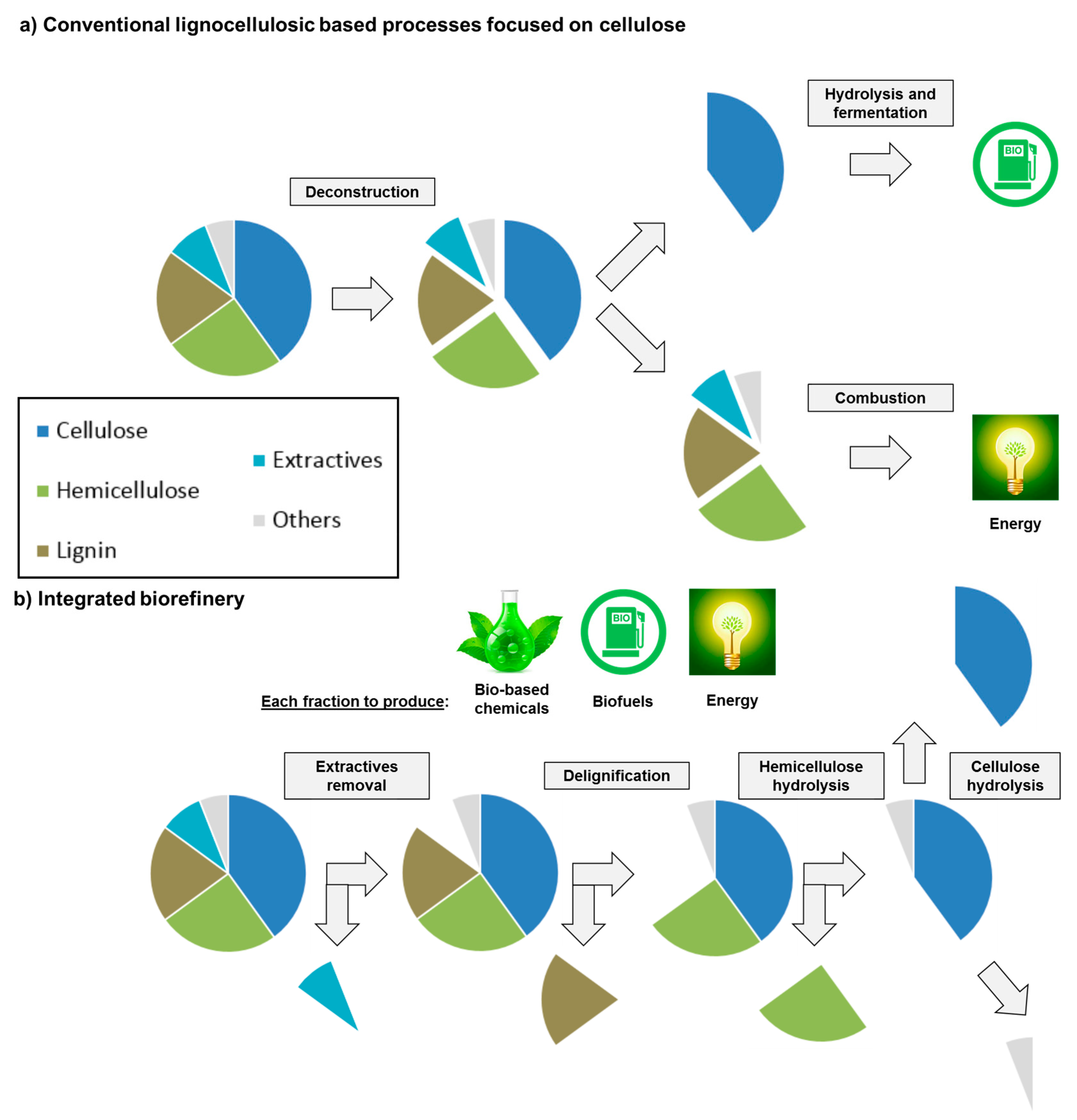
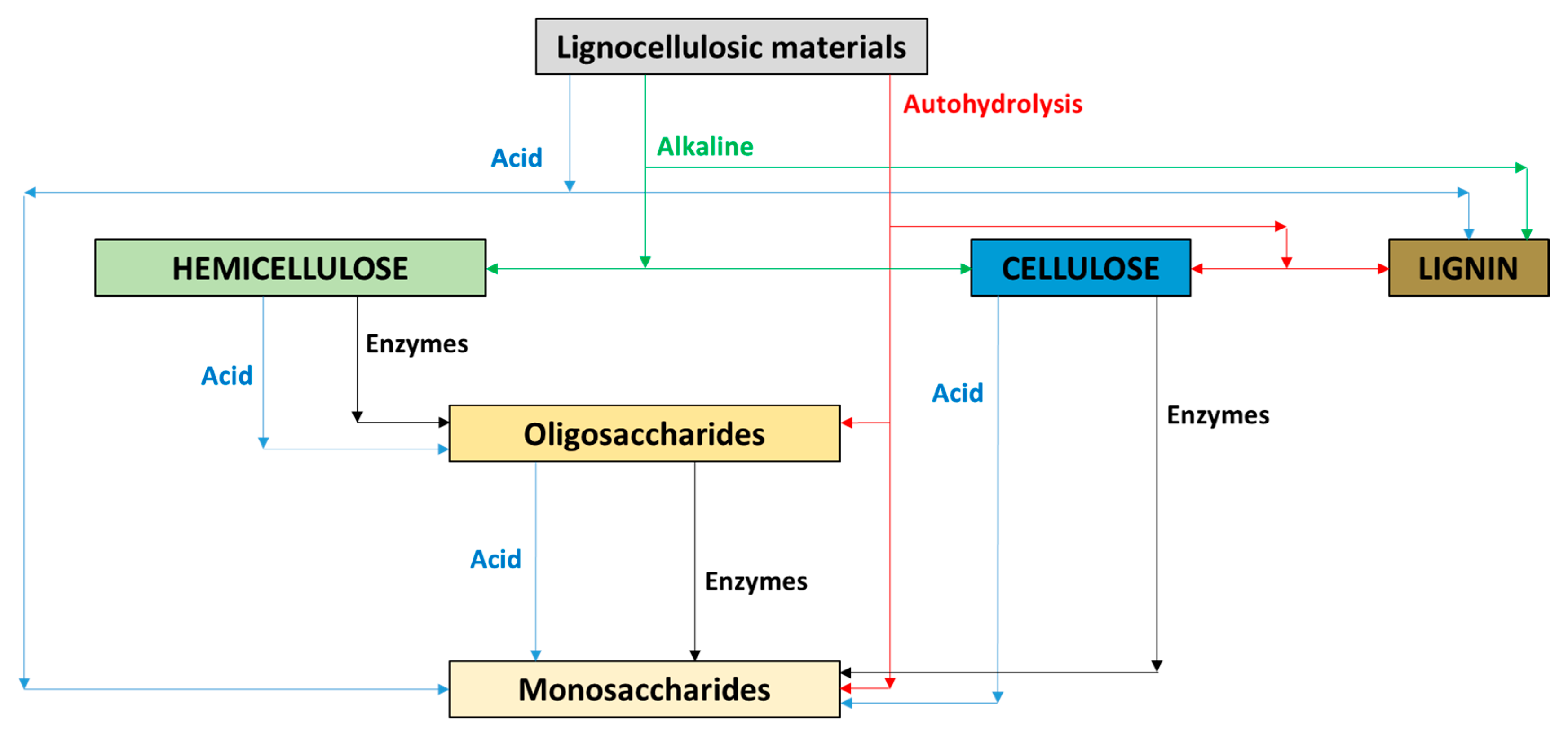
3.3.1. Pretreatment of Lignocellulosic Biomass for Hemicellulose Valorization
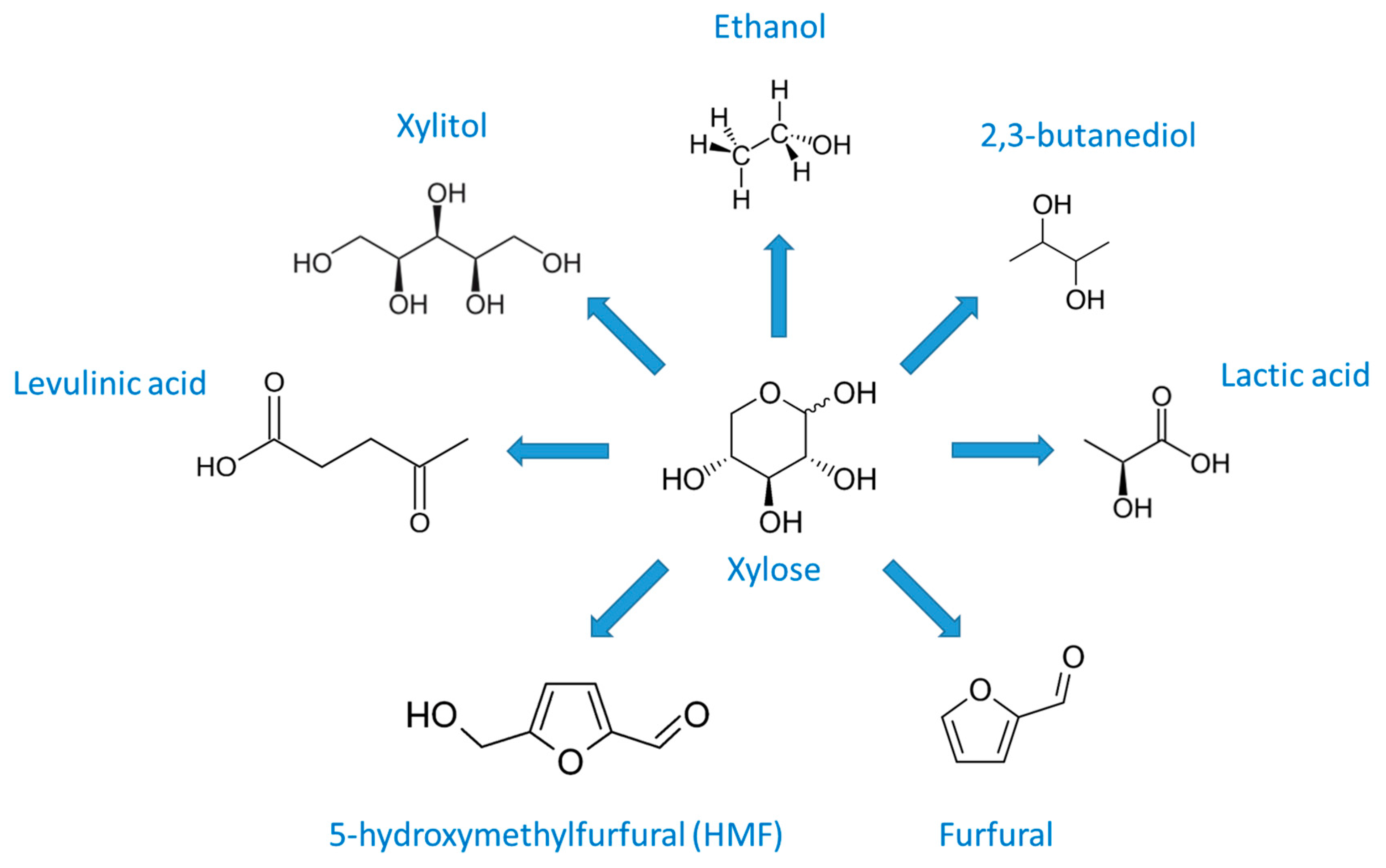
3.3.2. Chemicals from Hemicellulose
Ethanol
Furfural, 5-Hydroxymethylfurfural (HMF) and Levulinic Acid (LeA)
2,3-Butanediol (2,3-BD)
Xylitol
Lactic Acid (LA)
Xylo-Oligosaccharides (XOSs)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Lanteri, L.N. Determinantes de los precios reales del petróleo y su impacto sobre las principales variables macroeconómicas: EU, España, Noruega y Argentina. Econ. Teor. Práct. 2014, 41, 45–70. [Google Scholar] [CrossRef]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial processes and products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Mittal, A.; Katahira, R.; Donohoe, B.S.; Black, B.A.; Pattathil, S.; Stringer, J.M.; Beckham, G.T. Alkaline peroxide delignification of corn stover. ACS Sustain. Chem. Eng. 2017, 5, 6310–6321. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged thermal fractionation for segregation of lignin and cellulose pyrolysis products: An experimental study of residence time and temperature effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Hornafius, K.Y.; Hornafius, J.S. Carbon negative oil: A pathway for CO2 emission reduction goals. Int. J. Greenh. Gas Control 2015, 37, 492–503. [Google Scholar] [CrossRef]
- Leite, J.G.D.B.; Leal, M.R.L.V.; Nogueira, L.A.H.; Cortez, L.A.B.; Dale, B.E.; da Maia, R.C.; Adjorlolo, C. Sugarcane: A way out of energy poverty. Biofuel. Bioprod. Biorefin. 2016, 10, 393–408. [Google Scholar] [CrossRef]
- Plath, M.; Moser, C.; Bailis, R.; Brandt, P.; Hirsch, H.; Klein, A.M.; Walmsley, D.; von Wehrden, H. A novel bioenergy feedstock in Latin America? Cultivation potential of Acrocomia aculeata under current and future climate conditions. Biomass Bioenergy 2016, 91, 186–195. [Google Scholar] [CrossRef]
- Sharma, N.; Bohra, B.; Pragya, N.; Cianella, R.; Dobie, P.; Lehmann, S. Bioenergy from agroforestry can lead to improved food security, climate change, soil quality, and rural development. Food Energy Secur. 2016, 5, 165–183. [Google Scholar] [CrossRef]
- Ko, C.H.; Chaiprapat, S.; Kim, L.H.; Hadi, P.; Hsu, S.C.; Leu, S.Y. Carbon sequestration potential via energy harvesting from agricultural biomass residues in Mekong River basin, Southeast Asia. Renew. Sustain. Energy Rev. 2017, 68, 1051–1062. [Google Scholar] [CrossRef]
- Zaafouri, K.; Ziadi, M.; ben Hassen-Trabelsi, A.; Mekni, S.; Aïssi, B.; Alaya, M.; Hamdi, M. Enzymatic saccharification and liquid state fermentation of hydrothermal pretreated Tunisian Luffa cylindrica (L.) fibers for cellulosic bioethanol production. Renew. Energy 2017, 114, 1209–1213. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Cavani, F.; Albonetti, S.; Basile, F.; Gandini, A. Chemicals and Fuels from Bio-Based Building Blocks; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- French, A.D.; Bertoniere, N.R.; Brown, R.M.; Chanzy, H.; Gray, D.; Hattori, K.; Glasser, W. Cellulose. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Elumalai, S.; Pan, X.J. Chemistry and reactions of forest biomass in biorefining. In Sustainable Production of Fuels, Chemicals and Fibers from Forest Biomass; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Lebo, S.E.; Gargulak, J.D.; McNally, T.J. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Glas, D.; Van Doorslaer, C.; Depuydt, D.; Liebner, F.; Rosenau, T.; Binnemans, K.; De Vos, D.E. Lignin solubility in non-imidazolium ionic liquids. J. Chem. Technol. Biotechnol. 2015, 90, 1821–1826. [Google Scholar] [CrossRef]
- Soyez, K.; Kamm, B.; Kamm, M. The Green Biorefinery. In Proceedings of the 1st International Green Biorefinery Conference, Neuruppin, Germany, 8–9 October 1997. [Google Scholar]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading Lignocellulosic Biomasses: Hydrogenolysis of Platform Derived Molecules Promoted by Heterogeneous Pd-Fe Catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical bibliography or bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Broadus, R.N. Toward a definition of ”bibliometrics”. Scientometrics 1987, 12, 373–379. [Google Scholar] [CrossRef]
- Zyoud, S.H.H.; Fuchs-Hanusch, D.; Zyoud, S.H.; Al-Rawajfeh, A.E.; Shaheen, H.Q. A bibliometric-based evaluation on environmental research in the Arab world. Int. J. Environ. Sci. Technol. 2017, 14, 689–706. [Google Scholar] [CrossRef]
- Van Raan, A.F. For your citations only? Hot topics in bibliometric analysis. Measurement 2005, 3, 50–62. [Google Scholar] [CrossRef]
- Ho, Y.S. Bibliometric analysis of biosorption technology in water treatment research from 1991 to 2004. Int. J. Environ. Pollut. 2008, 34, 1–13. [Google Scholar] [CrossRef]
- Xie, S.D.; Zhang, J.; Ho, Y.S. Assessment of world aerosol research trends by bibliometric analysis. Scientometrics 2008, 77, 113–130. [Google Scholar] [CrossRef]
- Fu, H.Z.; Ho, Y.S.; Sui, Y.M.; Li, Z.S. A bibliometric analysis of solid waste research during the period 1993–2008. Waste Manag. 2010, 30, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Cindrella, L.; Kannan, A.M.; Lin, J.F.; Saminathan, K.; Ho, Y.S.; Lin, C.W.; Wertz, J. Gas diffusion layer for proton exchange membrane fuel cells—A review. J. Power Sources 2009, 194, 146–160. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xie, S.D.; Ho, Y.S. A bibliometric analysis of world volatile organic compounds research trends. Scientometrics 2010, 83, 477–492. [Google Scholar] [CrossRef]
- Santos, A.; Ma, W.; Judd, S.J. Membrane bioreactors: Two decades of research and implementation. Desalination 2011, 273, 148–154. [Google Scholar] [CrossRef]
- Sun, J.; Ni, J.; Ho, Y.S. Scientometric analysis of coastal eutrophication research during the period of 1993 to 2008. Environ. Dev. Sustain. 2011, 13, 353–366. [Google Scholar] [CrossRef]
- Tanaka, H.; Ho, Y.S. Global trends and performances of desalination research. Desalin. Water Treat. 2011, 25, 1–12. [Google Scholar] [CrossRef]
- Ho, Y.S. Top-cited articles in chemical engineering in Science Citation Index Expanded: A bibliometric analysis. Chin. J. Chem. Eng. 2012, 20, 478–488. [Google Scholar] [CrossRef]
- Wan, T.J.; Shen, S.M.; Bandyopadhyay, A.; Shu, C.M. Bibliometric analysis of carbon dioxide reduction research trends during 1999–2009. Sep. Purif. Technol. 2012, 94, 87–91. [Google Scholar] [CrossRef]
- Chuang, K.Y.; Wang, M.H.; Ho, Y.S. High-impact papers published in journals listed in the field of chemical engineering. Malays. J. Libr. Inf. Sci. 2013, 18, 47–63. [Google Scholar]
- Cindrella, L.; Fu, H.Z.; Ho, Y.S. Global thrust on fuel cells and their sustainability—An assessment of research trends by bibliometric analysis. Int. J. Sustain. Energy 2014, 33, 125–140. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S. Hot topics and application trends of the anammox biotechnology: A review by bibliometric analysis. SpringerPlus 2014, 3, 220. [Google Scholar] [CrossRef] [PubMed]
- Abejón, R.; Garea, A. A bibliometric analysis of research on arsenic in drinking water during the 1992–2012 period: An outlook to treatment alternatives for arsenic removal. J. Water Process. Eng. 2015, 6, 105–119. [Google Scholar] [CrossRef]
- Chen, H.; Ho, Y.S. Highly cited articles in biomass research: A bibliometric analysis. Renew. Sustain. Energy Rev. 2015, 49, 12–20. [Google Scholar] [CrossRef]
- Koelmel, J.; Prasad, M.N.V.; Pershell, K. Bibliometric analysis of phytotechnologies for remediation: Global scenario of research and applications. Int. J. Phytoremediat. 2015, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Yang, L.; Xi, S. Study on trends and performance of landfill research from 1999 to 2013 by using bibliometric analysis. Environ. Prog. Sustain. Energy 2015, 34, 1349–1355. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, T.; Wang, Q.; Xu, B.; Wang, L. A bibliometric review of research trends on bioelectrochemical systems. Curr. Sci. 2015, 109, 2204–2211. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, J.; Wang, Q.; Nie, C.; Smale, N.; Shi, Z.; Wang, X. A bibliometric analysis of industrial wastewater research: Current trends and future prospects. Scientometrics 2015, 105, 863–882. [Google Scholar] [CrossRef]
- Can-Güven, E.; Gedik, K. Global research activities on dioxins and dioxin-like compounds. Int. J. Environ. Pollut. 2016, 60, 12–33. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals and the Environment (PiE): Evolution and impact of the published literature revealed by bibliometric analysis. Sci. Total Environ. 2016, 562, 391–426. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Faria, L.G.D.; Jurowetzki, R. Unveiling scientific communities about sustainability and innovation. A bibliometric journey around sustainable terms. J. Clean. Prod. 2016, 127, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Ojha, A.; Borthakur, A.; Singh, R.; Lahiry, D.; Tiwary, D.; Mishra, P.K. Emerging trends in photodegradation of petrochemical wastes: A review. Environ. Sci. Pollut. Res. 2016, 23, 22340–22364. [Google Scholar] [CrossRef] [PubMed]
- Thomé, A.M.T.; Scavarda, A.; Ceryno, P.S.; Remmen, A. Sustainable new product development: A longitudinal review. Clean Technol. Environ. Policy 2016, 18, 2195–2208. [Google Scholar] [CrossRef]
- Wambu, E.W.; Ho, Y.S. A bibliometric analysis of drinking water research in Africa. Water SA 2016, 42, 612–620. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, T.; Wang, Q.; Wang, C.; Wang, L. Global trends of electrodialysis research during 1991–2014: A bibliometric analysis. J. Chem. Soc. Pak. 2016, 38, 775–788. [Google Scholar]
- Zhang, M.; Gao, Z.; Zheng, T.; Ma, Y.; Wang, Q.; Gao, M.; Sun, X. A bibliometric analysis of biodiesel research during 1991–2015. J. Mater. Cycles Waste Manag. 2016. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Garea, A. A bibliometric analysis of research on supported ionic liquid membranes during the 1995–2015 period: Study of the main applications and trending topics. Membranes 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Jiang, W.; Yang, Y.; Yang, Y.; Man, X. State of the art on food waste research: A bibliometrics study from 1997 to 2014. J. Clean. Prod. 2017, 140, 840–846. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, W.; Liu, Z.; Chiu, A.S.F.; Han, W.; Liu, Z.; Zhong, S.; Qian, Y.; You, W.; Cui, X. A bibliometric review: Energy consumption and greenhouse gas emissions in the residential sector. J. Clean. Prod. 2017, 159, 301–316. [Google Scholar] [CrossRef]
- Judd, S.J. Membrane technology costs and me. Water Res. 2017, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y. Simulation-based approaches for design of smart energy system: A review applying bibliometric analysis. J. Chem. Eng. Jpn. 2017, 50, 385–396. [Google Scholar] [CrossRef]
- Kolle, S.R.; Shankarappa, T.H.; Arun, M.; Manjunatha Reddy, T.B. Characteristics and trends in global lead removal research: A science citation index expanded-based analysis. Desalin. Water Treat. 2017, 80, 164–173. [Google Scholar] [CrossRef]
- Li, J.; Reniers, G.; Cozzani, V.; Khan, F. A bibliometric analysis of peer-reviewed publications on domino effects in the process industry. J. Loss Prev. Process Ind. 2017, 49, 103–110. [Google Scholar] [CrossRef]
- Li, X.; Nan, R. A bibliometric analysis of eutrophication literatures: An expanding and shifting focus. Environ. Sci. Pollut. Res. 2017, 24, 17103–17115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Xie, S. Bibliometric analysis: Global research trends in biogenic volatile organic compounds during 1991–2014. Environ. Earth Sci. 2017, 76, 11. [Google Scholar] [CrossRef]
- Magrí, A.; Giovannini, F.; Connan, R.; Bridoux, G.; Béline, F. Nutrient management from biogas digester effluents: A bibliometric-based analysis of publications and patents. Int. J. Environ. Sci. Technol. 2017, 14, 1739–1756. [Google Scholar] [CrossRef]
- Mishra, D.; Gunasekaran, A.; Papadopoulos, T.; Hazen, B. Green supply chain performance measures: A review and bibliometric analysis. Sustain. Prod. Consum. 2017, 10, 85–99. [Google Scholar] [CrossRef]
- Zare, F.; Elsawah, S.; Iwanaga, T.; Jakeman, A.J.; Pierce, S.A. Integrated water assessment and modelling: A bibliometric analysis of trends in the water resource sector. J. Hydrol. 2017, 552, 765–778. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, G.; Crittenden, J.; Liu, X.; Du, H. Groundwater remediation from the past to the future: A bibliometric analysis. Water Res. 2017, 119, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Fu, H.Z.; Ho, Y.S. Research trends and hotspots related to ammonia oxidation based on bibliometric analysis. Environ. Sci. Pollut. Res. 2017, 24, 20409–20421. [Google Scholar] [CrossRef] [PubMed]
- Scopus. Scopus Content Coverage Guide, 02.16 version; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- De Schrijver, M.; De Leenheer, L.; Mathieu, C.; Vanderbeke, E.; Iserentant, D. Valorisation of lignocellulose waste material. Meded. Fak. Landbouwwet. R. U. Gent 1988, 2, 1799–1807. [Google Scholar]
- Yachioui, M.E.; Halloui, N.E.; Villa, R. Enhancement of Jerusalem artichoke tubercules by enzymatic hydrolysis. J. Food Eng. 1994, 23, 1–19. [Google Scholar]
- De Lopez, S.; Tissot, M.; Delmas, M. Integrated cereal straw valorization by an alkaline pre-extraction of hemicellulose prior to soda-anthraquinone pulping, case study of barley straw. Biomass Bioenergy 1996, 10, 201–211. [Google Scholar] [CrossRef]
- Hu, J.; Ma, Y.; Zhang, L.; Gan, F.; Ho, Y.S. A historical review and bibliometric analysis of research on lead in drinking water field from 1991 to 2007. Sci. Total Environ. 2010, 408, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Jie, W. A bibliometric study of the trend in articles related to eutrophication published in Science Citation Index. Scientometrics 2011, 89, 919–927. [Google Scholar] [CrossRef]
- Mao, G.; Liu, X.; Du, H.; Zuo, J.; Wang, L. Way forward for alternative energy research: A bibliometric analysis during 1994–2013. Renew. Sustain. Energy Rev. 2015, 48, 276–286. [Google Scholar] [CrossRef]
- Durmusoglu, A. A pre-assessment of past research on the topic of environmental-friendly electronics. J. Clean. Prod. 2016, 129, 305–314. [Google Scholar] [CrossRef]
- Ward, T.A.; Rezadad, M.; Fearday, C.J.; Viyapuri, R. A review of biomimetic air vehicle research: 1984–2014. Int. J. Micro Air Veh. 2015, 7, 375–394. [Google Scholar] [CrossRef]
- De Castro e Silva Neto, D.; Cruz, C.O.; Rodrigues, F.; Silva, P. Bibliometric analysis of PPP and PFI literature: Overview of 25 years of research. J. Const. Eng. Manag. 2016, 142, 06016002. [Google Scholar] [CrossRef]
- Sweileh, W.M. Bibliometric analysis of medicine-related publications on refugees, asylum-seekers, and internally displaced people: 2000–2015. BMC Int. Health Hum. Right. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M.; Al-Jabi, S.W.; AbuTaha, A.S.; Zyoud, S.H.; Anayah, F.M.A.; Sawalha, A.F. Bibliometric analysis of worldwide scientific literature in mobile-health: 2006–2016. BMC Med. Inform. Decis. Mak. 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.P.M.; Da Costa Lopes, A.M.; Roseiro, L.B.; Bogel-Lukasik, R. Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids. RSC Adv. 2013, 3, 16040–16050. [Google Scholar] [CrossRef]
- Moshkelani, M.; Marinova, M.; Perrier, M.; Paris, J. The forest biorefinery and its implementation in the pulp and paper industry: Energy overview. Appl. Therm. Eng. 2013, 50, 1427–1436. [Google Scholar] [CrossRef]
- Boussarsar, H.; Rogé, B.; Mathlouthi, M. Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour. Technol. 2009, 100, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.V.T.; Carvalho, M.G.V.S.; Baptista, C.M.S.G.; Rocha, J.M.S.; Soares, B.I.G.; Sousa, G.D.A. Valorisation of hardwood hemicelluloses in the kraft pulping process by using an integrated biorefinery concept. Food Bioprod. Process. 2009, 87, 197–207. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Chatel, G.; Rogers, R.D. Review: Oxidation of lignin using ionic liquids-an innovative strategy to produce renewable chemicals. ACS Sustain. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Dereli, T.; Durmusoglu, A.; Delibaş, D.; Avlanmaz, N. An analysis of the papers published in Total Quality Management & Business Excellence from 1995 through 2008. Total Q. Manag. 2011, 22, 373–386. [Google Scholar]
- Mazar, A.; Jemaa, N.; Wafa, A.; Dajani, W.; Marinova, M.; Perrier, M. Furfural production from a pre-hydrolysate generated using aspen and maple chips. Biomass Bioenergy 2017, 104, 8–16. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Moodley, P.; Kana, E.B.G. Comparison of a two-stage and a combined single stage salt-acid based lignocellulosic pretreatment for enhancing enzymatic saccharification. Ind. Crops Prod. 2017, 108, 219–224. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.; Zhu, X. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Pyrolysis kinetics of forestry residues from the Portuguese Central Inland Region. Chem. Eng. Res. Des. 2013, 91, 2682–2690. [Google Scholar] [CrossRef]
- Moliner, C.; Bosio, B.; Arato, E.; Ribes-Greus, A. Comparative study for the energy valorisation of rice straw. Chem. Eng. Trans. 2014, 37, 241–246. [Google Scholar]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [PubMed]
- De Wild, P.J.; den Uil, H.; Reith, J.H.; Kiel, J.H.A.; Heeres, H.J. Biomass valorisation by staged degasification. A new pyrolysis-based thermochemical conversion option to produce value-added chemicals from lignocellulosic biomass. J. Anal. Appl. Pyrolysis 2009, 85, 124–133. [Google Scholar] [CrossRef]
- Chuetor, S.; Luque, R.; Barron, C.; Solhy, A.; Rouau, X.; Barakat, A. Innovative combined dry fractionation technologies for rice straw valorization to biofuels. Green Chem. 2015, 17, 926–936. [Google Scholar] [CrossRef]
- Basset, C.; Kedidi, S.; Barakat, A. Chemical- and solvent-free mechanophysical fractionation of biomass induced by tribo-electrostatic charging: Separation of proteins and lignin. ACS Sustain. Chem. Eng. 2016, 4, 4166–4173. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hagerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microbiol. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Deloule, V.; Chirat, C.; Boisset, C.; Toussaint, B.; Chroboczek, J. Production of hemicellulose oligomers from softwood chips using autohydrolysis followed by an enzymatic post-hydrolysis. Holzforschung 2017, 71, 575–581. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Araya, F.; Troncoso, E.; Mendonça, R.T.; Freer, J. Condensed lignin structures and re-localization achieved at high severities in autohydrolysis of Eucalyptus globulus wood and their relationship with cellulose accessibility. Biotechnol. Bioeng. 2015, 112, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Romaní, A.; Domingues, L.; Garrote, G. Evaluation of strategies for second generation bioethanol production from fast growing biomass Paulownia within a biorefinery scheme. Appl. Energy 2017, 187, 777–789. [Google Scholar] [CrossRef] [Green Version]
- Vargas, F.; Domínguez, E.; Vila, C.; Rodríguez, A.; Garrote, G. Agricultural residue valorization using a hydrothermal process for second generation bioethanol and oligosaccharides production. Bioresour. Technol. 2015, 191, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Egües, I.; Alriols, M.G.; Herseczki, Z.; Marton, G.; Labidi, J. Hemicelluloses obtaining from rapeseed cake residue generated in the biodiesel production process. J. Ind. Eng. Chem. 2010, 16, 293–298. [Google Scholar] [CrossRef]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of suitability of vine shoots for hemicellulosic oligosaccharides production through aqueous processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Ares-Peón, I.A.; Romaní, A.; Garrote, G.; Parajó, J.C. Invasive biomass valorization: Environmentally friendly processes for obtaining second generation bioethanol and saccharides from Ulex europaeus. J. Chem. Technol. Biotechnol. 2013, 88, 999–1006. [Google Scholar] [CrossRef]
- Santucci, B.S.; Maziero, P.; Rabelo, S.C.; Curvelo, A.A.S.; Pimenta, M.T.B. Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermal pretreatment: A kinetic assessment. Bioenergy Res. 2015, 8, 1778–1787. [Google Scholar] [CrossRef]
- Yáñez, R.; Garrote, G.; Díaz, M.J. Valorisation of a leguminous specie, Sesbania grandiflora, by means of hydrothermal fractionation. Bioresour. Technol. 2009, 100, 6514–6523. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.C.; Dionísio, A.M.; Torrado, I.; Carvalheiro, F.; Castilho, P.C.; Duarte, L.C. Autohydrolysis of Annona cherimola Mill. Seeds: Optimization, modeling and products characterization. Biochem. Eng. J. 2015, 104, 2–9. [Google Scholar] [CrossRef]
- Romaní, A.; Tomaz, P.D.; Garrote, G.; Teixeira, J.A.; Domingues, L. Combined alkali and hydrothermal pretreatments for oat straw valorization within a biorefinery concept. Bioresour. Technol. 2016, 220, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; Moncalvo, A.; De Faveri, D.M.; Silva, A. Valorisation of stalks from different grape cultivars for sugars recovery. Chem. Eng. Trans. 2014, 37, 745–750. [Google Scholar]
- Vithanage, L.N.G.; Barbosa, A.M.; Kankanamge, G.R.N.; Rakshit, S.K.; Dekker, R.F.H. Valorization of hemicelluloses: Production of bioxylitol from poplar wood prehydrolyzates by Candida guilliermondii FTI 20037. Bioenergy Res. 2016, 9, 181–197. [Google Scholar] [CrossRef]
- Morone, A.; Chakrabarti, T.; Pandey, R.A. Assessment of alkaline peroxide-assisted wet air oxidation pretreatment for rice straw and its effect on enzymatic hydrolysis. Cellulose 2017. [Google Scholar] [CrossRef]
- Kassaye, S.; Pant, K.K.; Jain, S. Hydrolysis of cellulosic bamboo biomass into reducing sugars via a combined alkaline solution and ionic liquid pretreament steps. Renew. Energy 2017, 104, 177–184. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Bioref. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Constant, S.; Barakat, A.; Robitzer, M.; Di Renzo, F.; Dumas, C.; Quignard, F. Composition, texture and methane potential of cellulosic residues from Lewis acids organosolv pulping of wheat straw. Bioresour. Technol. 2016, 216, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Pistone, L.; Ottolina, G.; Xu, P.; Riva, S. Hemp hurds biorefining: A path to green l-(+)-lactic acid production. Bioresour. Technol. 2015, 191, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schwiderski, M.; Kruse, A.; Grandl, R.; Dockendorf, D. Comparison of the influence of a Lewis acid AlCl3 and a Brønsted acid HCl on the organosolv pulping of beech wood. Green Chem. 2014, 16, 1569–1578. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Bastola, K.P.; Madl, R.L.; Vadlani, P.V. Novel biomass pretreatment using alkaline organic solvents: A green approach for biomass fractionation and 2,3-butanediol production. Bioenergy Res. 2016, 9, 643–655. [Google Scholar] [CrossRef]
- Raita, M.; Denchokepraguy, N.; Champreda, V.; Laosiripojana, N. Effects of alkaline catalysts on acetone-based organosolv pretreatment of rice straw. 3 Biotech 2017, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem. 2016, 9, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Fabicovicova, K.; Lucas, M.; Claus, P. From barley straw to valuable polyols: A sustainable process using ethanol/water mixtures and hydrogenolysis over ruthenium-tungsten catalyst. ChemSusChem 2016, 9, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total utilization of Miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Huang, X.; Gonzalez, O.M.M.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Reductive fractionation of woody biomass into lignin monomers and cellulose by tandem metal triflate and Pd/C catalysis. Green Chem. 2017, 19, 175–187. [Google Scholar] [CrossRef]
- Renders, T.; Van Den Bosch, S.; Vangeel, T.; Ennaert, T.; Koelewijn, S.F.; Van Den Bossche, G.; Courtin, C.M.; Schutyser, W.; Sels, B.F. Synergetic effects of alcohol/water mixing on the catalytic reductive fractionation of poplar wood. ACS Sustain. Chem. Eng. 2016, 4, 6894–6904. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Viell, J.; Inouye, H.; Szekely, N.K.; Frielinghaus, H.; Marks, C.; Wang, Y.; Anders, N.; Spiess, A.C.; Makowski, L. Multi-scale processes of beech wood disintegration and pretreatment with 1-ethyl-3-methylimidazolium acetate/water mixtures. Biotechnol. Biofuels 2016, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Z.; Ren, H.; Duan, E. Synthesis and properties of non-aromatic ionic liquids and their role in cellulose dissolution. BioResources 2017, 12, 5407–5416. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic liquid as a green solvent for lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Le-That, T.; Mikkola, J.P. Carbohydrate free lignin: A dissolution-recovery cycle of sodium lignosulfonate in a switchable ionic liquid system. ACS Sustain. Chem. Eng. 2016, 4, 7032–7040. [Google Scholar] [CrossRef]
- Akiba, T.; Tsurumaki, A.; Ohno, H. Induction of lignin solubility for a series of polar ionic liquids by the addition of a small amount of water. Green Chem. 2017, 19, 2260–2265. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Alias, Y. Lignin extraction from coconut shell using aprotic ionic liquids. BioResources 2017, 12, 5749–5774. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Kim, M.; Blanch, H.W.; Prausnitz, J.M. Solubility and rate of dissolution for Miscanthus in hydrophilic ionic liquids. Fluid Phase Equilib. 2011, 309, 89–96. [Google Scholar] [CrossRef]
- Viell, J.; Marquardt, W. Disintegration and dissolution kinetics of wood chips in ionic liquids. Holzforschung 2011, 65, 519–525. [Google Scholar] [CrossRef]
- Yamada, H.; Miyafuji, H.; Ohno, H.; Yamada, T. Rapid and complete dissolution of softwood biomass in tetra-n-butylphosphonium hydroxide with hydrogen peroxide. BioResources 2017, 12, 4515–4526. [Google Scholar] [CrossRef]
- Fort, D.A.; Remsing, R.C.; Swatloski, R.P.; Moyna, P.; Moyna, G.; Rogers, R.D. Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem. 2007, 9, 63–69. [Google Scholar] [CrossRef]
- Froschauer, C.; Hummel, M.; Iakovlev, M.; Roselli, A.; Schottenberger, H.; Sixta, H. Separation of hemicellulose and cellulose from wood pulp by means of ionic liquid/cosolvent systems. Biomacromolecules 2013, 14, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Stepan, A.M.; Monshizadeh, A.; Hummel, M.; Roselli, A.; Sixta, H. Cellulose fractionation with IONCELL-P. Carbohydr. Polym. 2016, 150, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.G.P.; Kollau, L.J.B.M.; Bruinhorst, A.V.D.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T.; et al. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. ChemSusChem 2017, 10, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Feng, G.J.; Ye, M.; Huang, C.M.; Zhang, Y. Significantly enhanced enzymatic hydrolysis of rice straw via a high performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Hromádkova, Z.; Kostalova, Z.; Sasinkova, V. Chemical valorization of agricultural by-products: Isolation and characterization of xylan-based antioxidants from almond shell biomass. BioResources 2008, 3, 60–70. [Google Scholar]
- Derriche, R.; Berrahmoune, K.S. Valorisation of olive oil cake by extraction of hemicelluloses. J. Food Eng. 2007, 78, 1149–1154. [Google Scholar] [CrossRef]
- Kostalova, Z.; Hromadkova, Z.; Berit, S.P.; Ebringerova, A. Bioactive hemicelluloses alkali-extracted from Fallopia sachalinensis leaves. Carbohydr. Res. 2014, 398, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chaa, L.; Joly, N.; Lequart, V.; Faugeron, C.; Mollet, J.C.; Martin, P.; Morvan, H. Isolation, characterization and valorization of hemicelluloses from Aristida pungens leaves as biomaterial. Carbohydr. Polym. 2008, 74, 597–602. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhang, X.; Pan, J. Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Gatenholm, P.; Tenkanen, M. Hemicelluloses: Science and Technology; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Soni, H.; Kango, N. Hemicellulases in lignocellulose biotechnology: Recent patents. Recent Pat. Biotechnol. 2013, 7, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Banka, A.L.; Guralp, S.A.; Gulari, E. Secretory expression and characterization of two hemicellulases, xylanase, and β-xylosidase, isolated from Bacillus subtilis M015. Appl. Biochem. Biotechnol. 2014, 174, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, R.; Damásio, A.R.L.; Gonçalves, T.A.; Machado, C.B.; Paixao, D.A.A.; Wolf, L.D.; Mandelli, F.; Rocha, G.J.M.; Ruller, R.; Squina, F.M. Development of hemicellulolytic enzyme mixtures for plant biomass deconstruction on target biotechnological applications. Appl. Microbiol. Biotechnol. 2014, 98, 8513–8525. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.N.; Menkhaus, T.J. Continuous enzymatic hydrolysis of lignocellulosic biomass with simultaneous detoxification and enzyme recovery. Appl. Biochem. Biotechnol. 2014, 173, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Winger, A.M.; Heazlewood, J.L.; Chan, L.J.G.; Petzold, C.J.; Permaul, K.; Singh, S. Secretome analysis of the thermophilic xylanase hyper-producer Thermomyces lanuginosus SSBP cultivated on corn cobs. J. Ind. Microbiol. Biotechnol. 2014, 41, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.S.; Bhattacharya, A.; Pletschke, B.I. Synergism of fungal and bacterial cellulases and hemicellulases: A novel perspective for enhanced bio-ethanol production. Biotechnol. Lett. 2015, 37, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Cobucci-Ponzano, B.; Strazzulli, A.; Iacono, R.; Masturzo, G.; Giglio, R.; Rossi, M.; Moracci, M. Novel thermophilic hemicellulases for the conversion of lignocellulose for second generation biorefineries. Enzyme Microb. Technol. 2015, 78, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Diogo, J.A.; Hoffmam, Z.B.; Zanphorlin, L.M.; Cota, J.; Machado, C.B.; Wolf, L.D.; Squina, F.; Damásio, A.R.L.; Murakami, M.T.; Ruller, R. Development of a chimeric hemicellulase to enhance the xylose production and thermotolerance. Enzyme Microb. Technol. 2015, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Qiao, W.; Mi, S.; Jia, X.; Su, H.; Han, Y. Characterization of hemicellulase and cellulase from the extremely thermophilic bacterium Caldicellulosiruptor owensensis and their potential application for bioconversion of lignocellulosic biomass without pretreatment. Biotechnol. Biofuels 2015, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, R.; Gonçalves, T.A.; Damásio, A.R.L.; Brenelli, L.B.; Wolf, L.D.; Paixão, D.A.A.; Rocha, G.J.M.; Squina, F.M. Effect of hemicellulolytic enzymes to improve sugarcane bagasse saccharification and xylooligosaccharides production. J. Mol. Catal. B Enzym. 2016, 131, 36–46. [Google Scholar] [CrossRef]
- Michelin, M.; Ximenes, E.; Polizeli, M.L.T.M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Montiel, C.; Hernández-Meléndez, O.; Vivaldo-Lima, E.; Hernández-Luna, M.; Bárzana, E. Enhanced bioethanol production from blue agave bagasse in a combined extrusion-saccharification process. Bioenergy Res. 2016, 9, 1005–1014. [Google Scholar] [CrossRef]
- Rakotoarivonina, H.; Revol, P.V.; Aubry, N.; Rémond, C. The use of thermostable bacterial hemicellulases improves the conversion of lignocellulosic biomass to valuable molecules. Appl. Microbiol. Biotechnol. 2016, 100, 7577–7590. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.N.; Arrosbide, M.F.; Franzoni, P.; Cassella, N. Integrated forest biorefineries: Green liquor extraction in eucalyptus wood prior to kraft pulping. Biomass Convers. Biorefin. 2016, 6, 465–474. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Lukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Topakas, E.; Moukouli, M.; Christakopoulos, P.; Olsson, L. Studying the ability of Fusarium oxysporum and recombinant Saccharomyces cerevisiae to efficiently cooperate in decomposition and ethanolic fermentation of wheat straw. Biomass Bioenergy 2011, 35, 3727–3732. [Google Scholar] [CrossRef]
- Saleh, M.; Cuevas, M.; García, J.F.; Sánchez, S. Valorization of olive stones for xylitol and ethanol production from dilute acid pretreatment via enzymatic hydrolysis and fermentation by Pachysolen tannophilus. Biochem. Eng. J. 2014, 90, 286–293. [Google Scholar] [CrossRef]
- Guigou, M.; Cebreiros, F.; Ferrari, M.D.; Cabrera, M.N.; Lareo, C. Bioethanol production from Eucalyptus grandis hemicellulose hydrolysate recovered before kraft pulping using an integrated forest bioerfinery concept. Biomass Conv. Bioref. 2017, 7, 191–197. [Google Scholar] [CrossRef]
- Cadete, R.M.; Melo-Cheab, M.A.; Dussán, K.J.; Rodrigues, R.C.L.B.; Da Silva, S.S.; Gomes, F.C.O.; Rosa, C.A.; Rosa, C.A. Production of bioethanol in sugarcane bagasse hemicellulosic hydrolysate by Scheffersomyces parashehatae, Scheffersomyces illinoinensis, and Spathaspora arborariae isolated from Brazilian ecosystems. J. Appl. Microbiol. 2017, 123, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.C.; Soares, L.B.; Biazi, L.E.; Nascimiento, V.M.; Costa, A.C.; Rocha, G.J.M.; Ienczak, J.L. Fermentation strategy for second generation ethanol production from sugarcane bagasse hydrolyzate by Spathaspora passalidarum and Scheffersomyces stipites. Biotechnol. Bioeng. 2017, 114, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Li, Z.; Han, B.; Karim, M.N. Saccharification and fermentation of waste sweet potato for bioethanol production. J. Food Process Eng. 2013, 36, 739–747. [Google Scholar] [CrossRef]
- Hector, R.E.; Dien, B.S.; Cotta, M.A.; Mertens, J.A. Growth and fermentation of d-xylose by Saccharomyces cerevisiae expressing a novel d-xylose isomerase originating from the bacterium Prevotella ruminicola TC2-24. Biotechnol. Biofuels 2013, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Katahira, S.; Muramoto, N.; Moriya, S.; Nagura, R.; Tada, N.; Yasutani, N.; Ohkuma, M.; Onishi, T.; Tokuhiro, K. Screening and evolution of a novel protist xylose isomerase from the termite Reticulitermes speratus for efficient xylose fermentation in Saccharomyces cerevisiae. Biotechnol. Biofuels 2017, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Mert, M.J.; Rose, S.H.; la Grange, D.C.; Bamba, T.; Hasunuma, T.; Kondo, A.; van Zyl, W.H. Quantitative metabolomics of a xylose-utilizing Saccharomyces cerevisiae strain expressing the Bacteroides thetaiotaomicron xylose isomerase on glucose and xylose. J. Ind. Microbiol. Biotechnol. 2017, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zahed, O.; Jouzani, G.S.; Abbasalizadeh, S.; Khodaiyan, F.; Tabatabaei, M. Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol. 2016, 61, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.B.; Marzo, C.; Caro, I.; de Ory, I.; Blandino, A. Valorization of exhausted sugar beet cossettes by successive hydrolysis and two fermentations for the production of bio-products. Bioresour. Technol. 2017, 225, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kottke, R.H. Furan derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Dussan, K.; Girisuta, B.; Lopes, M.; Leahy, J.J.; Hayes, M.H.B. Conversion of hemicellulose sugars catalyzed by formic acid: Kinetics of the dehydration of d-xylose, l-arabinose, and d-glucose. ChemSusChem 2015, 8, 1411–1428. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Modified versions of sulfated zirconia as catalysts for the conversion of xylose to furfural. Catal. Letters 2007, 114, 151–160. [Google Scholar] [CrossRef]
- Karinen, R.; Vilonen, K.; Niemelä, M. Biorefining: Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Exceptionally high yields of furfural from assorted raw biomass over solid acids. RSC Adv. 2014, 4, 26215. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Dhepe, P.L. Effects of cations, anions and H+ concentration of acidic ionic liquids in the valorization of polysaccharides into furfural. New J. Chem. 2017, 41, 6137–6144. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.M.; Neves, P.; Fernandes, A.; Lima, S.; Silva, A.F.; Ribeiro, M.F.; Silva, C.M.; Pillinger, M.; Valente, A.A. Bulk and composite catalysts combining BEA topology and mesoporosity for the valorisation of furfural. Catal. Sci. Technol. 2016, 6, 7812–7829. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Torres, A.I.; Tsapatsis, M.; Daoutidis, P. Biomass to chemicals: Design of an extractive-reaction process for the production of 5-hydroxymethylfurfural. Comput. Chem. Eng. 2012, 42, 130–137. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Jia, S.; He, X.; Xu, Z. Valorization of an underused sugar derived from hemicellulose: Efficient synthesis of 5-hydroxymethylfurfural from mannose with aluminum salt catalyst in dimethyl sulfoxide/water mixed solvent. RSC Adv. 2017, 7, 39221–39227. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z. One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural. Catal. Sci. Technol. 2016, 6, 3694–3712. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Zhen, M.; Liu, L.; Chen, Y.; Yang, Q.; Huang, F.; Zhang, S.; Ju, M. An ionic liquid–organic solvent biphasic system for efficient production of 5-hydroxymethylfurfural from carbohydrates at high concentrations. RSC Adv. 2017, 7, 47288–47296. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-łukasik, E.; Bogel-łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B Environ. 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.F.; Wilson, K. Recent advances in the production of γ-valerolactone from biomass-derived feedstocks via heterogeneous catalytic transfer hydrogenation. J. Chem. Technol. Biotechnol. 2017, 92, 1125–1135. [Google Scholar] [CrossRef]
- Chang, C.; Cen, P.; Ma, X. Levulinic acid production from wheat straw. Bioresour. Technol. 2007, 98, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, S.; Wang, X.; Jiang, Z. Lysine functional heteropolyacid nanospheres as bifunctional acid-base catalysts for cascade conversion of glucose to levulinic acid. Fuel 2016, 164, 262–266. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient Conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2017, 181, 208–214. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Gullón, B.; Rivas, S.; Feijoo, G.; Moreira, M.T. Environmental performance of biomass refining into high-added value compounds. J. Clean. Prod. 2016, 120, 170–180. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dai, J.Y.; Xiu, Z.L. A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2,3-butanediol by Klebsiella pneumoniae. Bioresour. Technol. 2010, 101, 8342–8347. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, S.; Zanaroli, G.; Fava, F. 2,3-butanediol production from biowastes with Bacillus licheniformis: A preliminary study. Chem. Eng. Trans. 2016, 49, 379–384. [Google Scholar]
- Xin, F.; Basu, A.; Weng, M.C.; Yang, K.L.; He, J. Production of 2,3-butanediol from sucrose by a Klebsiella species. Bioenergy Res. 2016, 9, 15–22. [Google Scholar] [CrossRef]
- Kallbach, M.; Horn, S.; Kuenz, A.; Prüße, U. Screening of novel bacteria for the 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.W.; Lee, Y.G.; Park, Y.C.; Seo, J.H. Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.T. Metabolic engineering strategies for acetoin and 2,3-butanediol production: Advances and prospects. Crit. Rev. Biotechnol. 2017, 37, 990–1005. [Google Scholar] [CrossRef] [PubMed]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.M.; Jedrzejczak-Krzepkowska, M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Kubik, C.; Schütt, F.; Turkiewicz, M. Effects of genetic modifications and fermentation conditions on 2,3-butanediol production by alkaliphilic Bacillus subtilis. Appl. Microbiol. Biotechnol. 2016, 100, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.E. Sugar alcohols. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lee, J.; Xu, Y.; Huber, G.W. High-throughput screening of monometallic catalysts for aqueous-phase hydrogenation of biomass-derived oxygenates. Appl. Catal. B Environ. 2013, 140–141, 98–107. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Duque, A.; Arve, K.; Sifontes, V.; Aho, A.; Eränen, K.; Salmi, T. Catalytic hydrogenation of sugars. In Biomass Sugars for Non-Fuel Applications; The Royal Society of Chemistry: Cambrigde, UK, 2016. [Google Scholar]
- Rangaswamy, S.; Agblevor, F.A. Screening of facultative anaerobic bacteria utilizing d-xylose for xylitol production. Appl. Microbiol. Biotechnol. 2003, 60, 88–93. [Google Scholar]
- Dasgupta, D.; Bandhu, S.; Adhikari, D.K.; Ghosh, D. Challenges and prospects of xylitol production with whole cell bio-catalysis: A review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Pappu, S.M.J.; Gummadi, S.N. Artificial neural network and regression coupled genetic algorithm to optimize parameters for enhanced xylitol production by Debaryomyces nepalensis in bioreactor. Biochem. Eng. J. 2017, 120, 136–145. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Salgado, J.M.; Torrado, A.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Culture parameters affecting xylitol production by Debaryomyces hansenii immobilized in alginate beads. Process Biochem. 2013, 48, 387–397. [Google Scholar] [CrossRef]
- Aghcheh, R.K.; Bonakdarpour, B.; Ashtiani, F.Z. The influence of sugar cane bagasse type and its particle size on xylose production and xylose-to-xylitol bioconversion with the yeast Debaryomyces Hansenii. Appl. Biochem. Biotechnol. 2016, 180, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.M.; Rodríguez, N.; Cortés, S.; Domínguez, J.M. Coupling two sizes of CSTR-type bioreactors for sequential lactic acid and xylitol production from hemicellulosic hydrolysates of vineshoot trimmings. New Biotechnol. 2012, 29, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Muthuvelayudham, R.; Rajesh Kannan, R.; Viruthagiri, T. Stastical optimization of process variables for corncob hemicellulose hydrolysate to xylitol by Debaryomyces hansenii var hanseii. Int. J. ChemTech Res. 2013, 5, 186–196. [Google Scholar]
- Pappu, J.S.M.; Gummadi, S.N. Multi response optimization for enhanced xylitol production by Debaryomyces nepalensis in bioreactor. 3 Biotech 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an emerging prebiotic: Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, C. Extracting xylooligosaccharides in wheat bran by screening and cellulase assisted enzymatic hydrolysis. Int. J. Biol. Macromol. 2016, 92, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Deshmukh, R.A.; Menon, S.; Das, S. Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour. Technol. 2017, 245, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, T.S.; Retter, A.; Hobbs, P.J.; Budde, J.; Heiermann, M.; Plöchl, M.; Ravella, S.R. Production of xylooligosaccharides from renewable agricultural lignocellulose biomass. Biofuels 2015, 6, 147–155. [Google Scholar] [CrossRef]
- Azelee, N.I.W.; Jahim, J.M.; Ismail, A.F.; Fuzi, S.F.Z.M.; Rahman, R.A.; Md Illias, R. High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Ind. Crops Prod. 2016, 81, 11–19. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B.; Hirdyani, H.; Gupta, P. Enzymatic Production of Xylooligosaccharides from Brown Coconut Husk Treated with Sodium Hydroxide. Waste Biomass Valoriz. 2017. [Google Scholar] [CrossRef]
- Kumar, V.; Satyanarayana, T. Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour. Technol. 2015, 179, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; González, A.; Ballesteros, M.; Negro, M.J.; Manzanares, P.; Sáez, F. Valorization of Wheat Straw Hemicellulose to Obtain High Value Added Compounds. In Proceedings of the 24th EUBCE European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016; pp. 1207–1210. [Google Scholar]
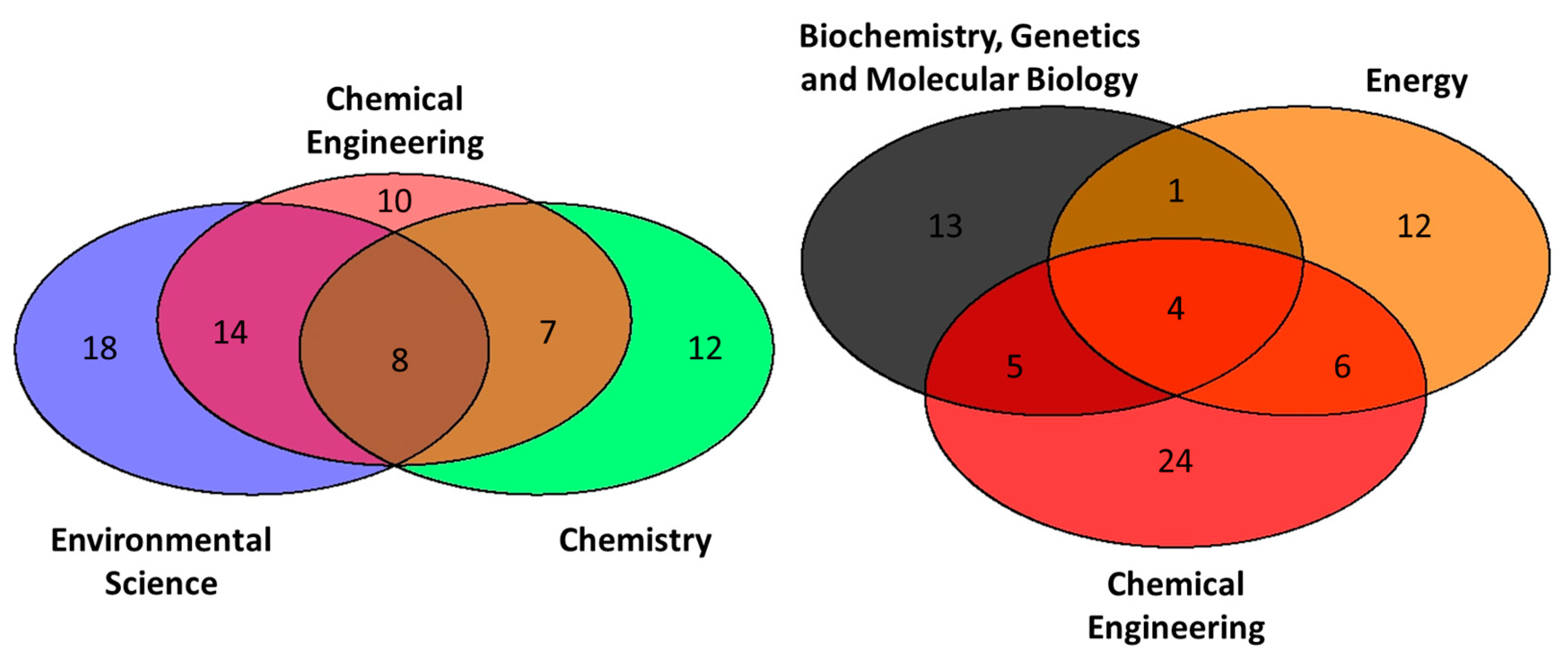

| Ranking | Subject Categories | Documents | Percentage (%) |
|---|---|---|---|
| 1 | Environmental Science | 40 | 36.7 |
| 2 | Chemical Engineering | 39 | 35.8 |
| 3 | Chemistry | 27 | 24.8 |
| 4 | Agricultural and Biological Sciences | 24 | 22.0 |
| 5 | Biochemistry, Genetics and Molecular Biology | 23 | 21.1 |
| 5 | Energy | 23 | 21.1 |
| 7 | Materials Science | 13 | 11.9 |
| 8 | Engineering | 10 | 9.2 |
| Ranking | Journal | IF (WoS) | SJR (Scopus) | Documents | Percentage (%) |
|---|---|---|---|---|---|
| 1 | Bioresource Technology | 5.651 | 2.191 | 8 | 7.3 |
| 1 | Industrial Crops and Products | 3.181 | 1.059 | 8 | 7.3 |
| 3 | Journal of Chemical Technology and Biotechnology | 3.135 | 0.843 | 4 | 3.7 |
| 4 | ACS Sustainable Chemistry and Engineering | 5.951 | 1.523 | 3 | 2.8 |
| 4 | Applied Microbiology and Biotechnology | 3.420 | 1.177 | 3 | 2.8 |
| 4 | Green Chemistry | 9.125 | 2.564 | 3 | 2.8 |
| Ranking | Country | Documents | Percentage (%) |
|---|---|---|---|
| 1 | Spain | 23 | 21.1 |
| 2 | France | 19 | 17.4 |
| 3 | Portugal | 12 | 11.0 |
| 4 | United States | 8 | 7.3 |
| 5 | Belgium | 7 | 6.4 |
| 6 | Italy | 6 | 5.5 |
| 7 | Germany | 5 | 4.6 |
| 7 | The Netherlands | 5 | 4.6 |
| Ranking | Institutions | Documents | Percentage (%) |
|---|---|---|---|
| 1 | University of Vigo (SPAIN) | 8 | 7.3 |
| 2 | Centre National de la Recherche Scientifique (FRANCE) | 4 | 3.7 |
| 2 | RWTH Aachen University (GERMANY) | 4 | 3.7 |
| 2 | University of Reims Champagne-Ardenne (FRANCE) | 4 | 3.7 |
| 2 | KU Leuven (BELGIUM) | 4 | 3.7 |
| Ranking | Articles | Times Cited |
|---|---|---|
| 1 | Title: Valorization of biomass: Deriving more value from waste Author(s): Tuck, C.O., Pérez, E., Horváth, I.T., Sheldon, R.A., Poliakoff, M. Source: Science Published: 2012 | 442 |
| 2 | Title: Formic-acid-induced depolymerization of oxidized lignin to aromatics Author(s): Rahimi, A., Ulbrich, A., Coon, J.J., Stahl, S.S. Source: Nature Published: 2014 | 193 |
| 3 | Title: Adipic acid production from lignin Author(s): Vardon, D.R., Franden, M.A., Johnson, C.W., Karp, E.M., et al. Source: Energy and Environmental Science Published: 2015 | 96 |
| 4 | Title: Review: Oxidation of lignin using ionic liquids-an innovative strategy to produce renewable chemicals Author(s): Chatel, G., Rogers, R.D. Source: ACS Sustainable Chemistry And Engineering Published: 2014 | 85 |
| 5 | Title: Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering Author(s): Delidovich, I., Leonhard, K., Palkovits, R. Source: Energy and Environmental Science Published: 2014 | 79 |
| 6 | Title: Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose Author(s): Boussarsar, H., Rogé, B., Mathlouthi, M. Source: Bioresource Technology Published: 2009 | 76 |
| 7 | Title: The forest biorefinery and its implementation in the pulp and paper industry: Energy overview Author(s): Moshkelani, M., Marinova, M., Perrier, M., Paris, J. Source: Applied Thermal Engineering Published: 2013 | 56 |
| 8 | Title: Lignin-degrading enzymes Author(s): Pollegioni, L., Tonin, F., Rosini, E. Source: FEBS Journal Published: 2015 | 55 |
| 9 | Title: Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids Author(s): Magalhães Da Silva, S.P., Da Costa Lopes, A.M., Roseiro, L.B., et al. Source: RSC Advances Published: 2013 | 51 |
| 10 | Title: Valorisation of hardwood hemicelluloses in the kraft pulping process by using an integrated biorefinery concept Author(s): Mendes, C.V.T., Carvalho, M.G.V.S., Baptista, C.M.S.G., et al. Source: Food and Bioproducts Processing Published: 2009 | 48 |
| Organism | Natural Sugar Utilization Pathways | Main Products | O2 Required | Tolerance | PH Range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Mannose | Galactose | Xylose | Arabinose | Ethanol | Others | Alcohol | Acids | Hydrolysate | |||
| S. cerevisiae | + | + | - | - | - | + | - | - | ++ | ++ | ++ | Acidic |
| E. coli | + | + | - | + | + | - | + | - | - | - | - | Neutral |
| Z. mobilis | + | - | - | - | - | + | - | - | + | - | - | Neutral |
| P. stipitis | + | + | + | + | + | + | - | + | - | - | - | Acidic |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abejón, R. A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics. ChemEngineering 2018, 2, 7. https://doi.org/10.3390/chemengineering2010007
Abejón R. A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics. ChemEngineering. 2018; 2(1):7. https://doi.org/10.3390/chemengineering2010007
Chicago/Turabian StyleAbejón, Ricardo. 2018. "A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics" ChemEngineering 2, no. 1: 7. https://doi.org/10.3390/chemengineering2010007





