Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
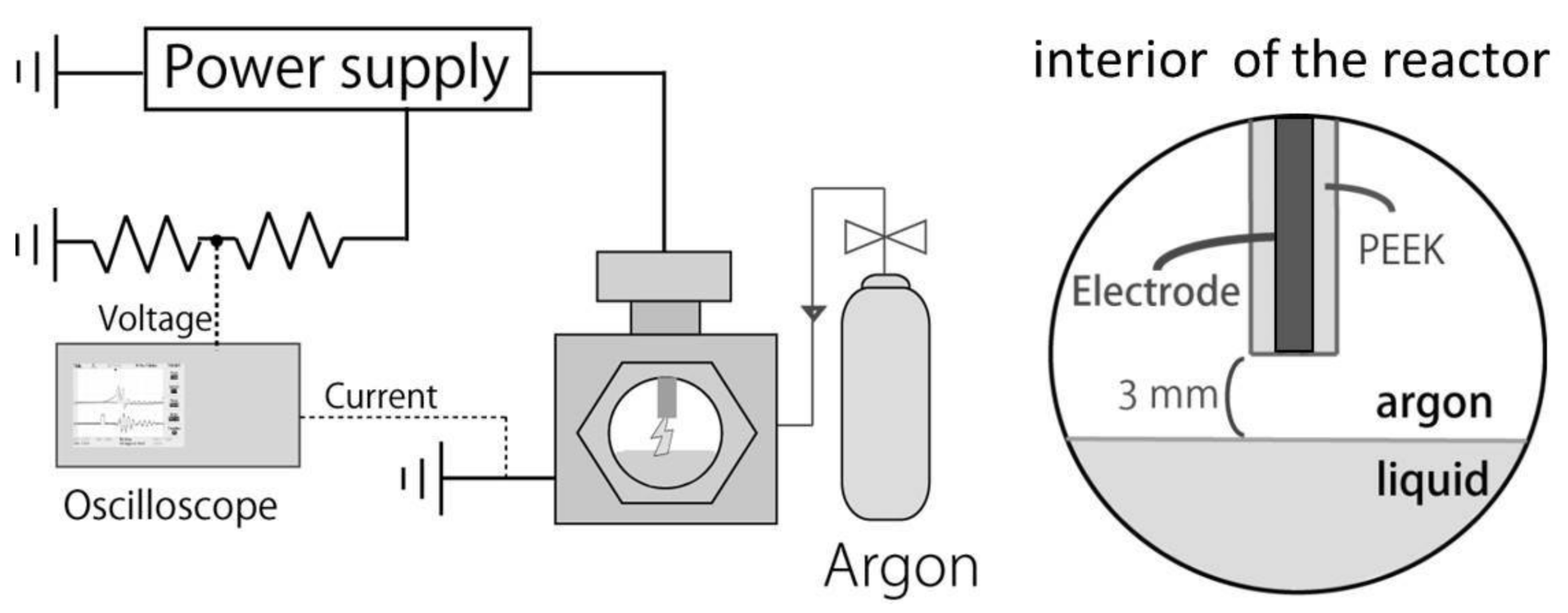
2.2. Experimental Setup and Procedure
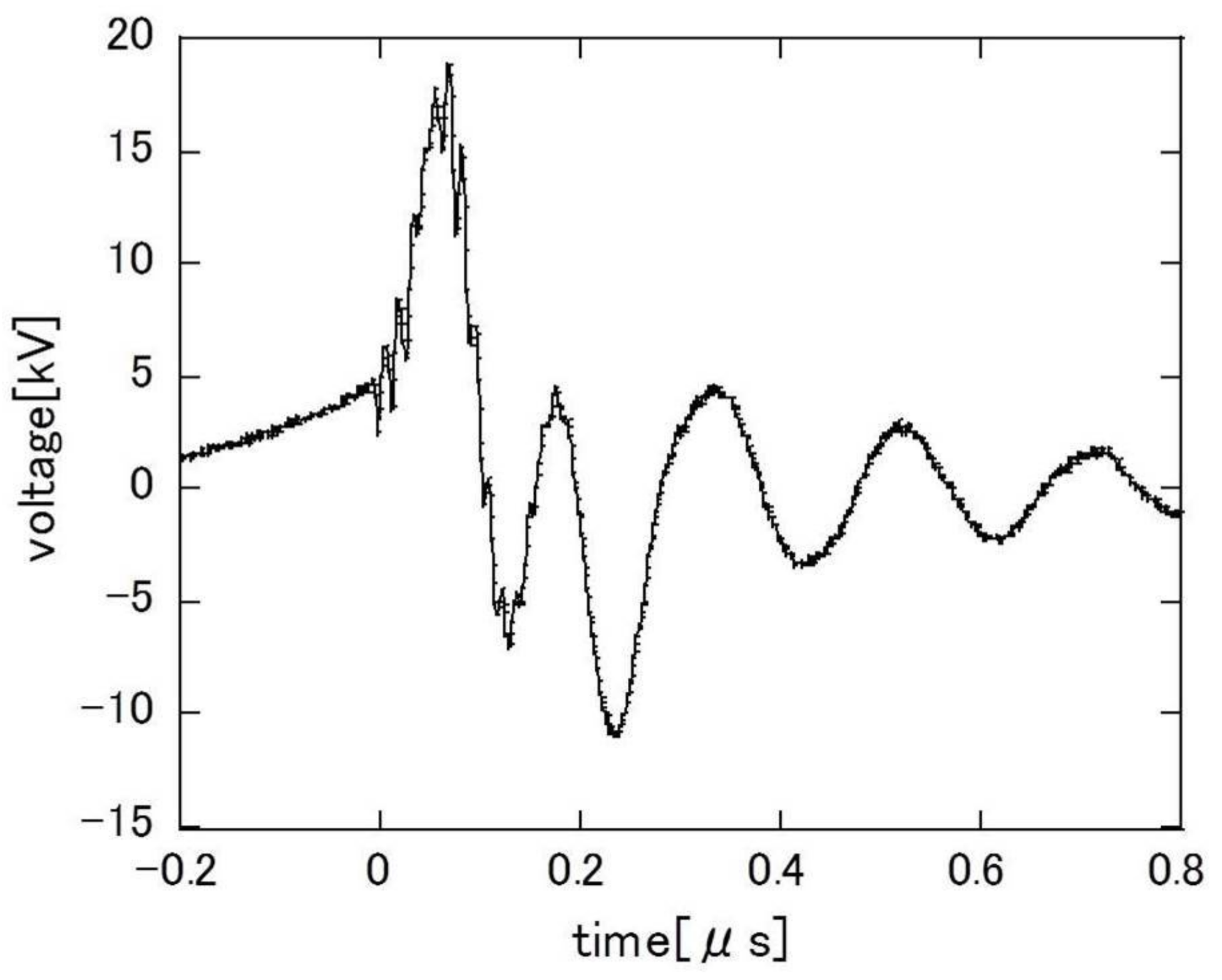
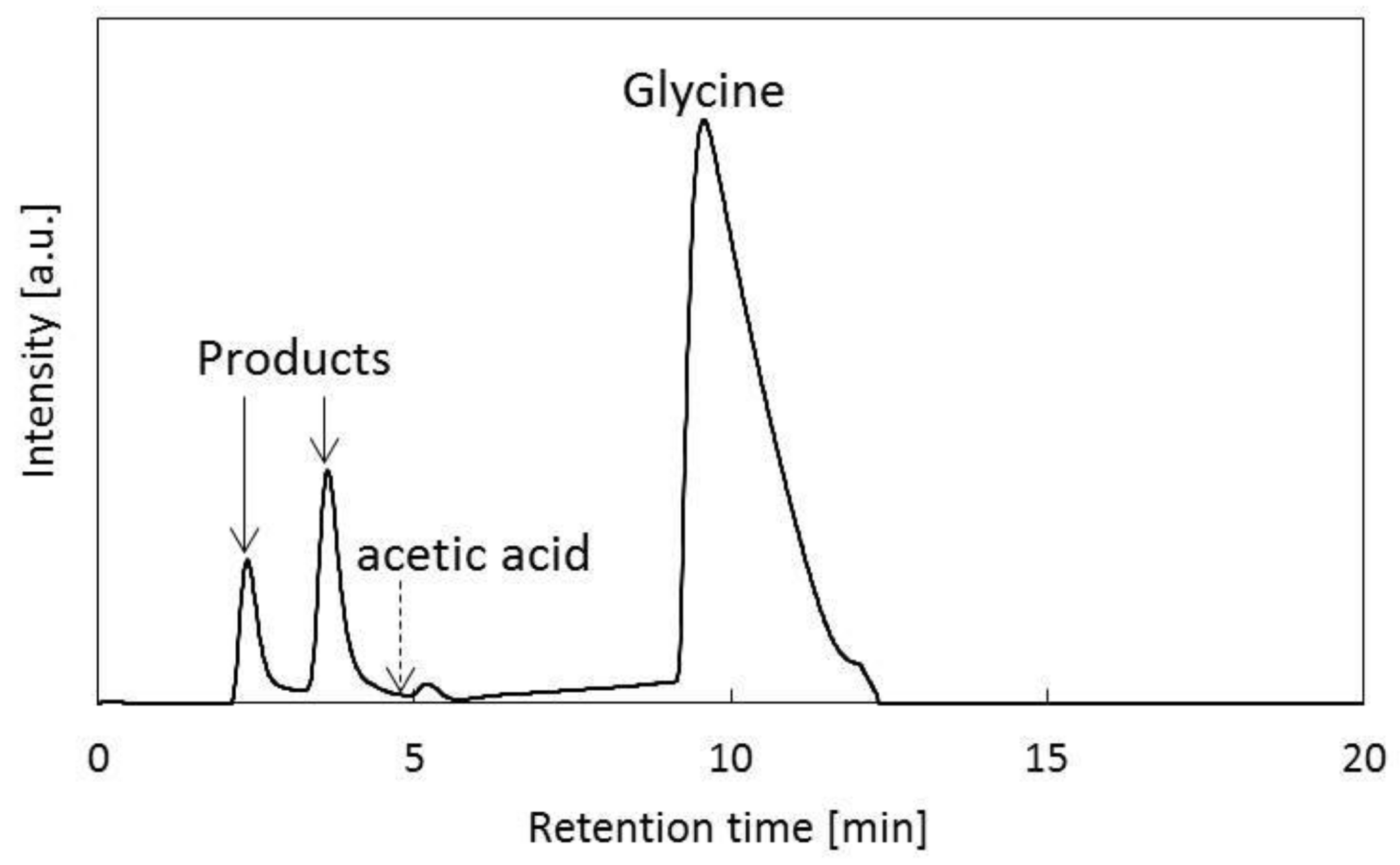
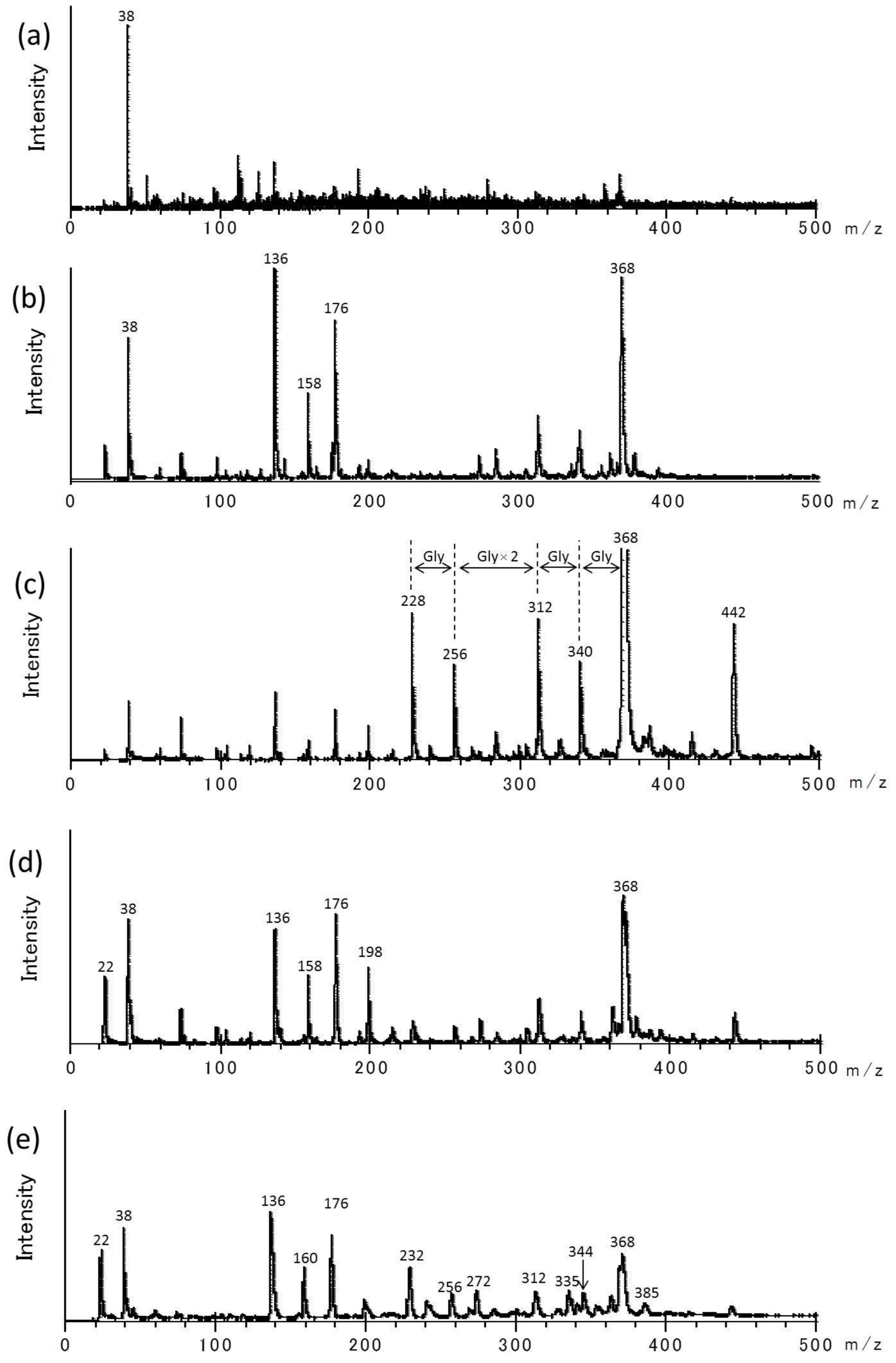
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruggman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Hayashi, Y.; Diono, W.; Machmudah, S.; Kanda, H.; Takada, N.; Sasaki, K.; Goto, M. Removal of Water Pollutants by Pulsed Discharge Plasma and Observation of Its Optical Emission Intensity at Atmospheric Pressure. Jpn. J. Appl. Phys. 2013, 52, 11NE02. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in Water Electro-Sprayed through Transient Spark Discharge in Air and their Bactericidal Effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Shirai, N.; Uchida, S.; Tochikubo, F. Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 046202. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Diono, W.; Machmudah, S.; Nagafuchi, K.; Sasaki, M.; Akiyama, H.; Goto, M. Oxidative Decoloration of Dyes by Pulsed Discharge Plasma over a Water Surface under Argon Atmospheric. Trans. Mater. Res. Soc. Jpn. 2013, 38, 61–67. [Google Scholar] [CrossRef]
- Ni, G.; Zhao, G.; Jiang, Y.; Li, J.; Meng, Y.; Wang, X. Steam Plasma Jet Treatment of Phenol in Aqueous Solution at Atmospheric Pressure. Plasma Process. Polym. 2013, 10, 353–363. [Google Scholar] [CrossRef]
- Thagard, S.M.; Takashima, K.; Mizuno, A. Chemistry of the positive and negative electrical discharges formed in liquid water and above a gas-liquid surface. Plasma Chem. Plasma Process. 2009, 29, 455–473. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water purification by electrical discharges. Plasma Sour. Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Stoffels, E.; Flikweert, A.J.; Stoffels, W.W.; Kroesen, G.M.W. Plasma needle: A non-destructive atmospheric plasma source for fine surface treatment of (bio)materials. Plasma Sour. Sci. Technol. 2002, 11, 383–388. [Google Scholar] [CrossRef]
- Sato, M. Environmental and biotechnological applications of high-voltage pulsed discharges in water. Plasma Sour. Sci. Technol. 2008, 17, 024021. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood Coagulation and Living Tissue Sterilization by Floating-Electrode Dielectric Barrier Discharge in Air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Fridman, A.; Clifford, J.A.; Friedman, G. DNA damage in mammalian cells by non-thermal atmospheric pressure microsecond pulsed dielectric barrier discharge plasma is not mediated by ozone. Plasma Process. Polym. 2012, 9, 726–732. [Google Scholar] [CrossRef]
- Ke, Z.; Huang, Q.; Su, X.; Jiang, J.; Wang, X.; Yu, Z. A paradigm study for assessment of phenylalanine’s damage under arc-discharge irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 1618–1625. [Google Scholar] [CrossRef]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikata, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Ke, Z.; Yu, Z.; Huang, Q. Assessment of Damage of Glutathione by Glow Discharge Plasma at the Gas-Solution Interface through Raman Spectroscopy. Plasma Process. Polym. 2013, 10, 181–188. [Google Scholar] [CrossRef]
- Miller, S.L. The mechanism of synthesis of amino acids by electric discharges. Biochim. Biophys. Acta 1957, 23, 480–489. [Google Scholar] [CrossRef]
- Miller, S.L. Organic Compound Synthes on the Primitive Eart. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Baba, J.L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.R.; Schumann, P.J.; Lippincott, E.R. Origin of terrestrial polypeptides: A theory based on data from discharge-tube experiments. Space Life Sci. 1973, 4, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R. Prebiotic cytosine synthesis: A critical analysis and implications for the origin of life. Proc. Natl. Acad. Sci. USA 1999, 96, 4396–4401. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tsuchiya, M. Abiotic formation of amino acids and imidazole by proton irradiation of simulated primitive earth atmospheres. Orig. Life Evol. Biosph. 1990, 20, 99–109. [Google Scholar] [CrossRef]
- Takahashi, J.; Hosokawa, T.; Saito, T.; Utsumi, Y. Abiotic synthesis of amino acids by X-ray irradiation of simple inorganic gases. Appl. Phys. Lett. 1999, 74, 877. [Google Scholar] [CrossRef]
- Sagan, C.; Khare, B.N. Long-Wavelength Ultraviolet Photoproduction of Amino Acids on the Primitive Earth. Science 1971, 173, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jikuya, M.; Yoshida, S.; Namihira, T.; Katsuki, S.; Akiyama, H. Positive- and negative-pulsed streamer discharges generated by a 100-ns pulsed-power in atmospheric air. IEEE Trans. Plasma Sci. 2007, 35, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, K.; Okada, S.; Wang, D.; Namihira, T.; Katsuki, S.; Akiyama, H. Effect of Polarity and Rise Time of Applied Pulsed Voltage on Streamer Discharge Phenomena. Acta Phys. Pol. A 2009, 115, 1050. [Google Scholar] [CrossRef]
- Peritis, K.N.; Chaimbault, P.; Elfakir, C.; Dreux, M. Ion-pair reversed-phase liquid chromatography for determination of polar underivatized amino acids using perfluorinated carboxylic acids as ion pairing agent. J. Chromatogr. A 1999, 833, 147–155. [Google Scholar] [CrossRef]
- Petritis, K.; Elfakir, C.; Dreux, M. A comparative study of commercial liquid chromatographic detectors for the analysis of underivatized amino acids. J. Chromatogr. A 2002, 961, 9–21. [Google Scholar] [CrossRef]
- Demeure, K.; Gabelica, V.; De Pauw, E.A. New advances in the understanding of the in-source decay fragmentation of peptides in MALDI-TOF-MS. J. Am. Soc. Mass Spectrom. 2010, 21, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Morand, K.; Talbo, G.; Mann, M. Oxidation of peptides during electrospray ionization. Rapid Commun. Mass Spectrom. 1993, 7, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Yokoyama, T.; Nakashima, S. Hydration-dehydration interactions between glycine and anhydrous salts: Implications for a chemical evolution of life. Geochim. Cosmochim. Acta 2011, 75, 6285–6299. [Google Scholar] [CrossRef]
- Cox, J.S.; Seward, T.M. The reaction kinetics of alanine and glycine under hydrothermal conditions. Geochim. Cosmochim. Acta 2007, 71, 2264–2284. [Google Scholar] [CrossRef]
- Flores, J.J.; Ponnamperuma, C. Polymerization of amino acids under primitive earth conditions. J. Mol. Evol. 1972, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otake, T.; Taniguchi, T.; Furukawa, Y.; Kawamura, F.; Nakazawa, H.; Kakegawa, T. Stability of amino acids and their oligomerization under high-pressure conditions: Implications for prebiotic chemistry. Astrobiology 2011, 11, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Sahni, M.; Locke, B.R. The effects of reaction conditions on liquid-phase hydroxyl radical production in gas–liquid pulsed-electrical-discharge reactors. Plasma Process. Polym. 2006, 3, 668–681. [Google Scholar] [CrossRef]
- Chen, Q.; Shirai, H. Diagnostics of atmospheric pressure microplasma with a liquid electrode. Eur. Phys. J. D 2012, 66, 161. [Google Scholar] [CrossRef]
- Shirai, N.; Nakazawa, M.; Ibuka, S.; Ishii, S. Atmospheric DC Glow Microplasmas Using Miniature Gas Flow and Electrolyte Cathode. Jpn. J. Appl. Phys. 2009, 48, 036002. [Google Scholar] [CrossRef]
- Sakata, K.; Kitadai, N.; Yokoyama, T. Effects of pH and temperature on dimerization rate of glycine: Evaluation of favorable environmental conditions for chemical evolution of life. Geochim. Cosmochim. Acta 2010, 74, 6841–6851. [Google Scholar] [CrossRef]
- Zamaraev, K.I.; Romanikov, V.N.; Salganik, R.I.; Wlassoff, W.A.; Khramtsov, V.V. Modeling of the prebiotic synthesis of oligopeptides: Silicate catalysts help to overcome the critical stage. Orig. Life Evol. Biosph. 1997, 27, 325–337. [Google Scholar] [CrossRef] [PubMed]

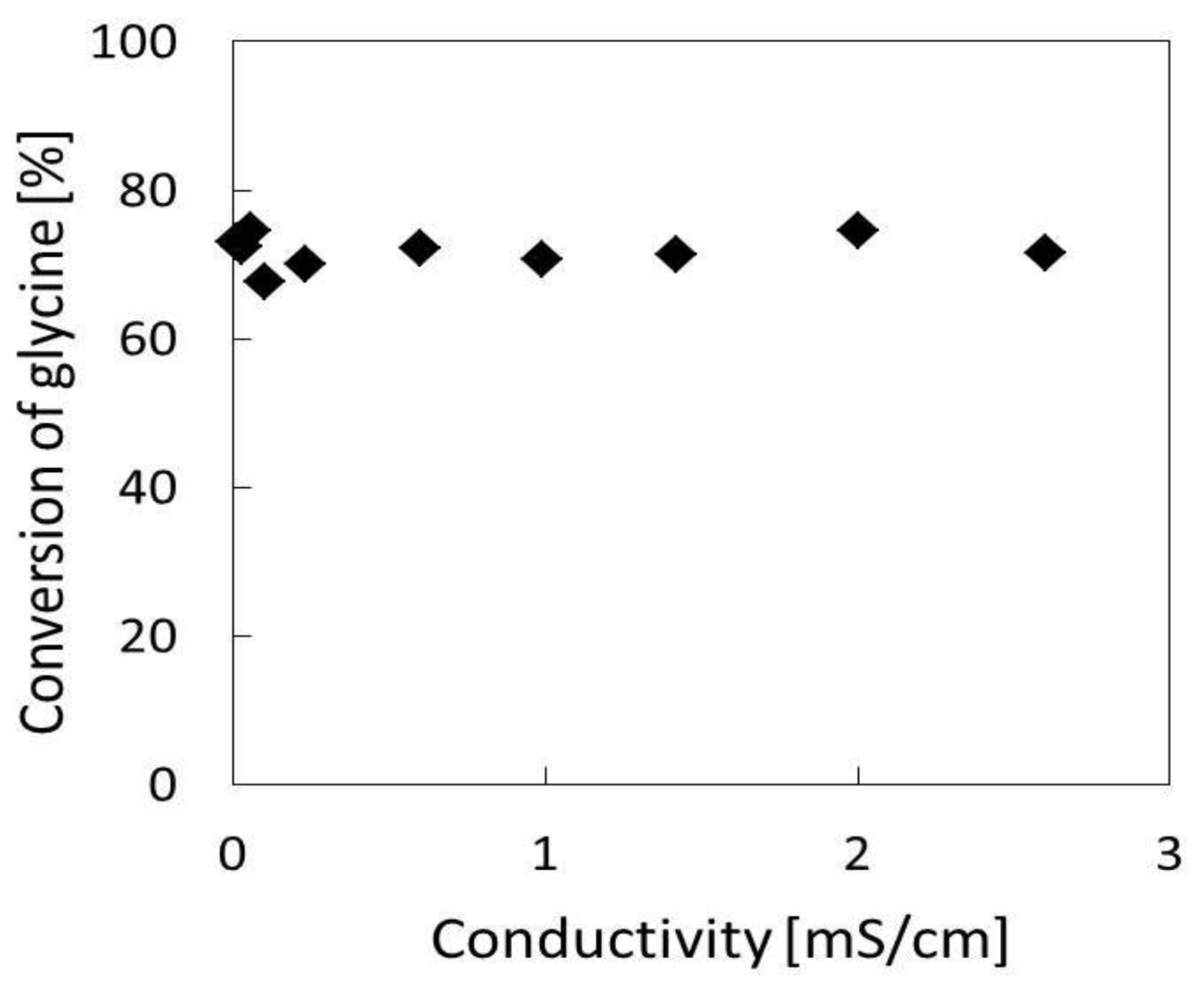
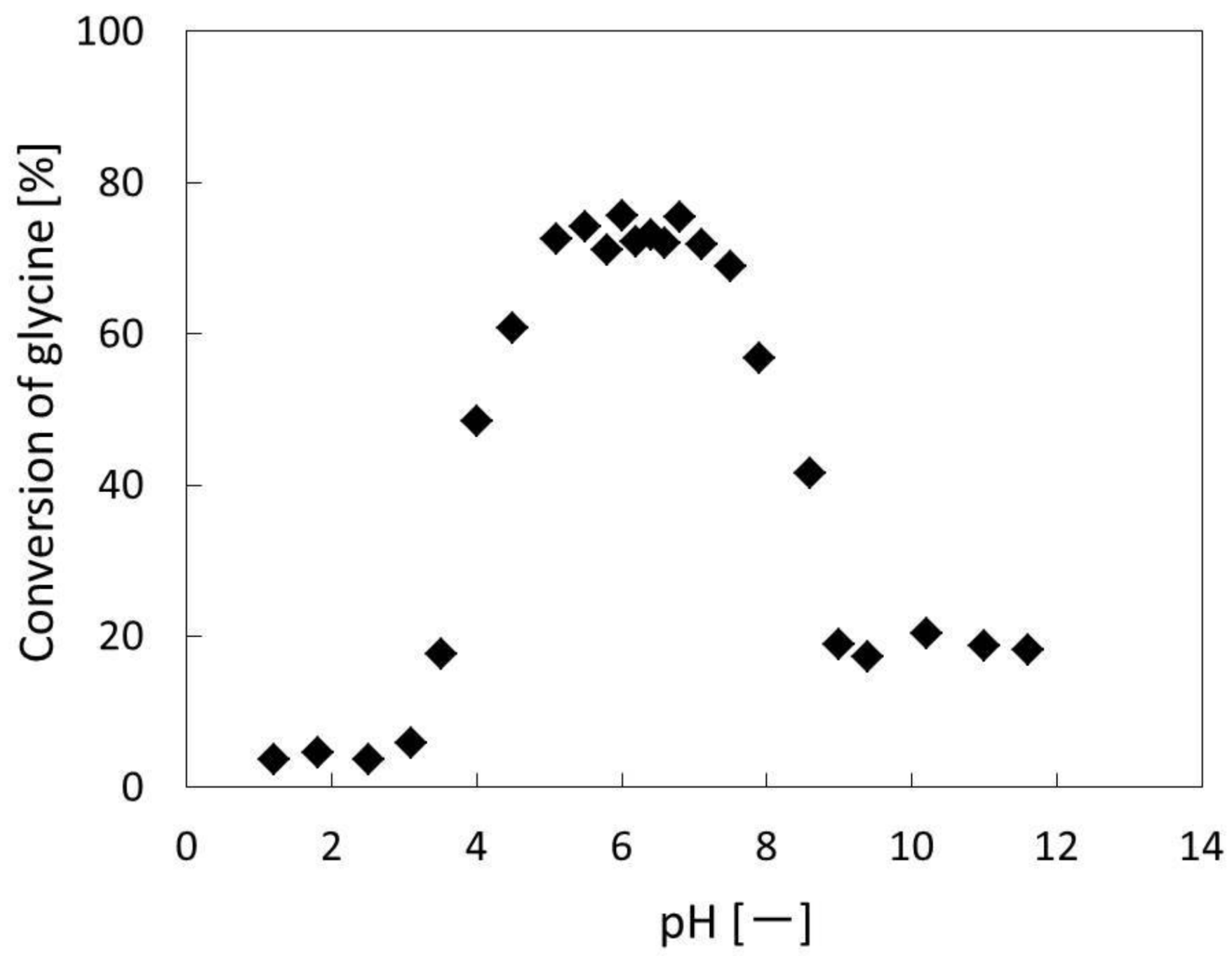
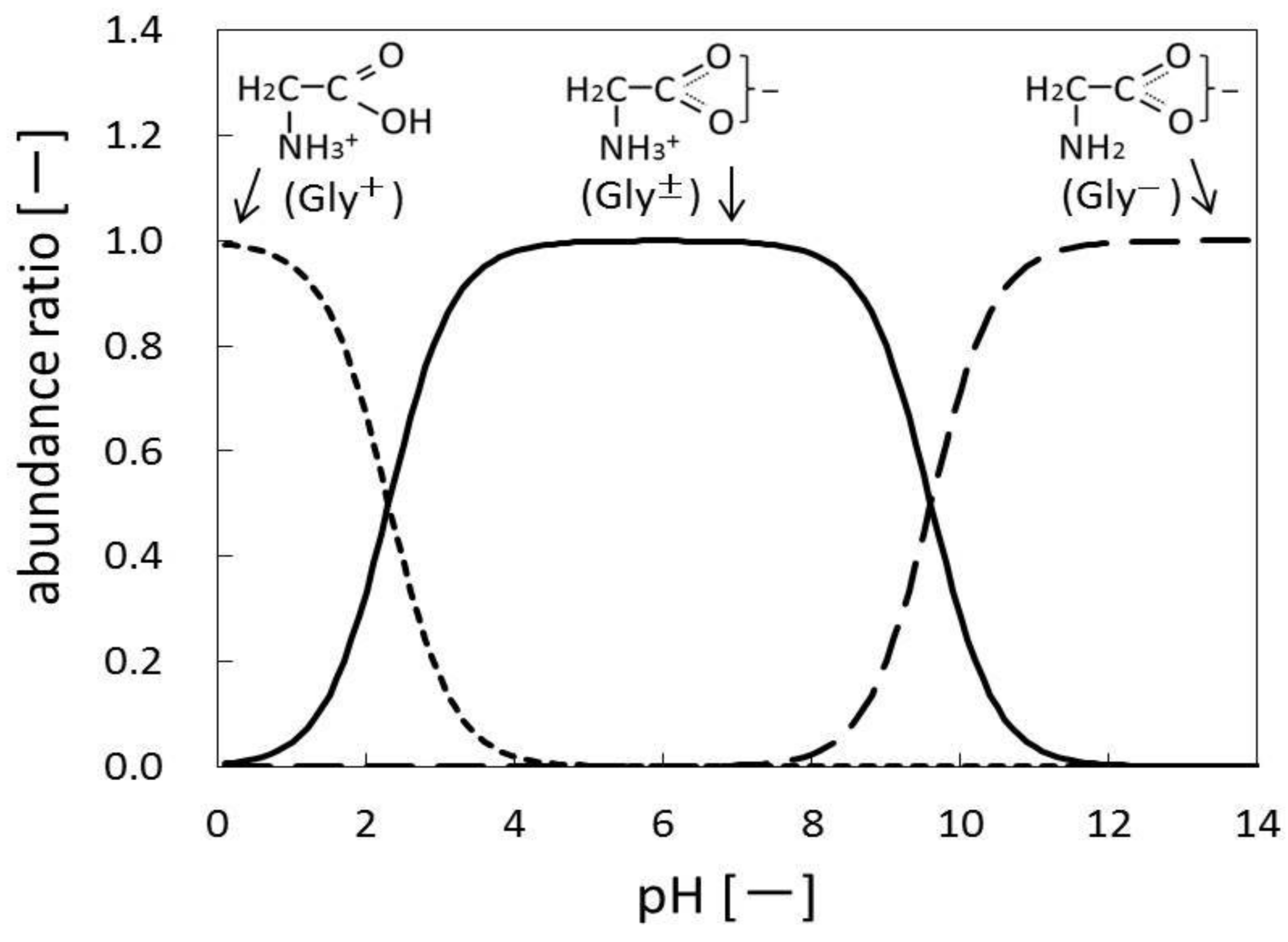
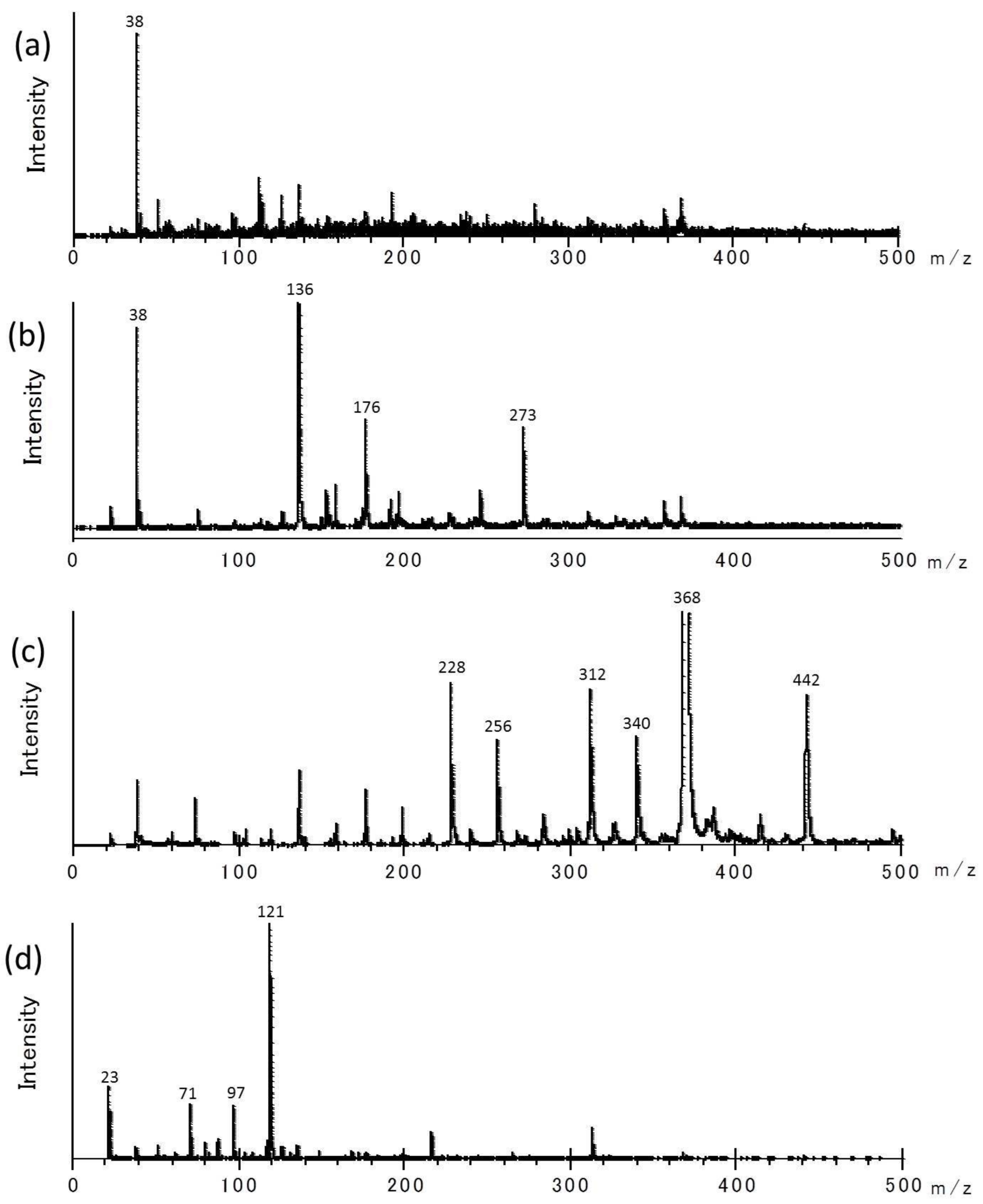
| Experiments | Treatment Time | Conversion of Glycine |
|---|---|---|
| Discharge plasma (18.6 kV) | 250 s | 73.1% |
| Heating (80 °C) | 24 h | 0.0% |
| UV irradiation | 24 h | 0.4% |
| OH radical (Fenton reaction) | 10 min | 3.4% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Diono, W.; Takada, N.; Kanda, H.; Goto, M. Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure. ChemEngineering 2018, 2, 17. https://doi.org/10.3390/chemengineering2020017
Hayashi Y, Diono W, Takada N, Kanda H, Goto M. Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure. ChemEngineering. 2018; 2(2):17. https://doi.org/10.3390/chemengineering2020017
Chicago/Turabian StyleHayashi, Yui, Wahyu Diono, Noriharu Takada, Hideki Kanda, and Motonobu Goto. 2018. "Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure" ChemEngineering 2, no. 2: 17. https://doi.org/10.3390/chemengineering2020017





