Quantifying the Effects of Frequency and Amplitude of Periodic Oxygen-Related Stress on Recombinant Protein Production in Pichia pastoris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Model System
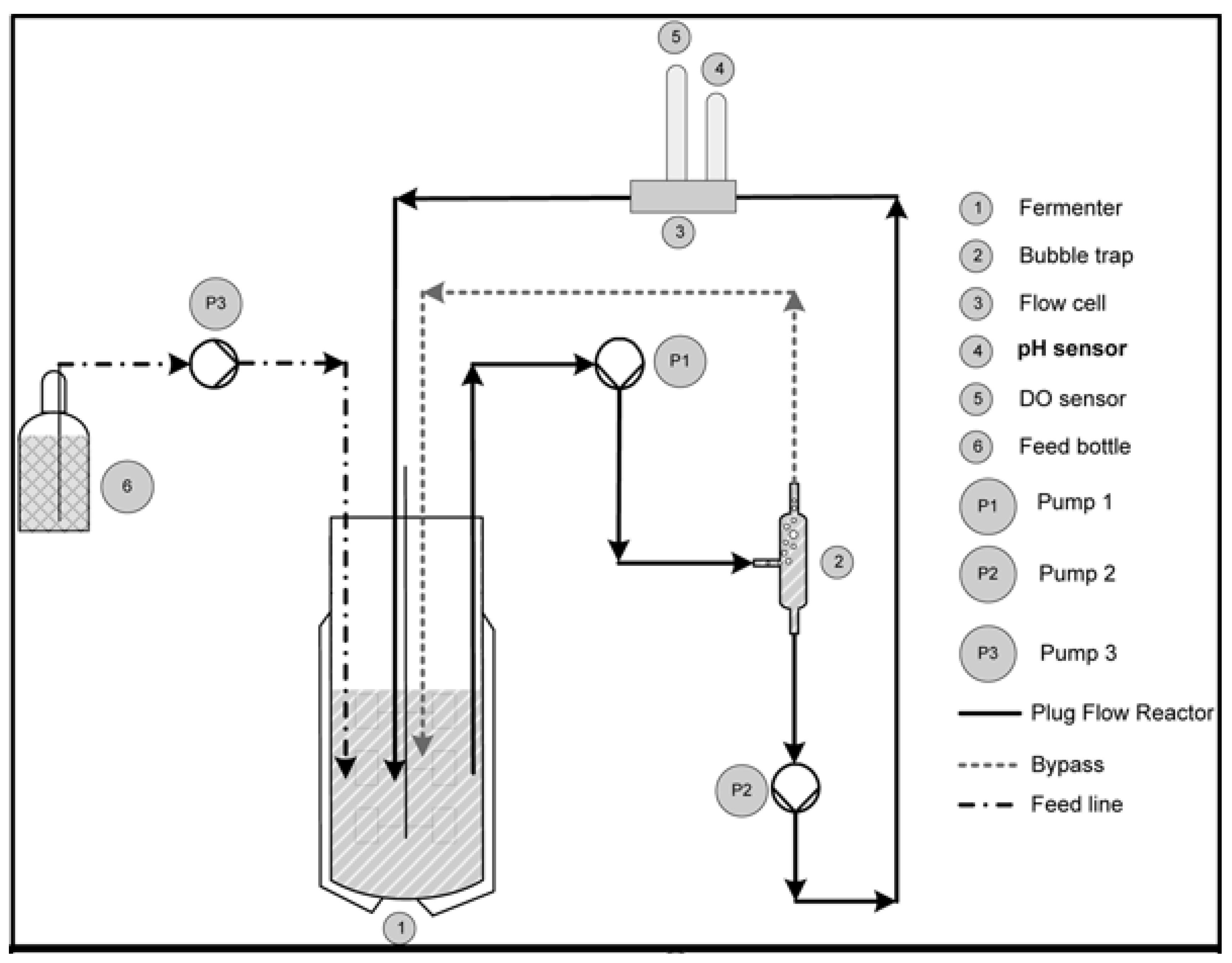
2.2. Experimental Setup and Approach
| (a) | ||
| Exp. No. | Residence time | Dissolved oxygen |
| 1 | Low (3.3 min) | Intermediate (25%) |
| 2 | High (15 min) | Intermediate (25%) |
| 3 | Low (3.3 min) | High (70%) |
| 4 | High (15 min) | High (70%) |
| 5 | Low (3.3 min) | Intermediate (25%) |
| (b) | ||
| 6 | Low (3.3 min) | Low (5%) |
| 7 | 0 min | Low (5%) |
| (c) | ||
| 1 | (3.3 min) | (25%) |
| 5 | (3.3 min) | (25%) |
| 6 | (3.3 min) | (5%) |
| 7 | 0 | (5%) |
| 8 | 0 | (25%) |
| 9 | 0 | (70%) |
| 10 | (3.3 min) | (70%) |
| 11 | (15 min) | (5%) |
| 12 | (15min) | (25%) |
| 13 | (15 min) | (70%) |
2.3. Analytics
2.4. Data Treatment
3. Results and Discussion
3.1. Time Course of Dissolved Oxygen Level
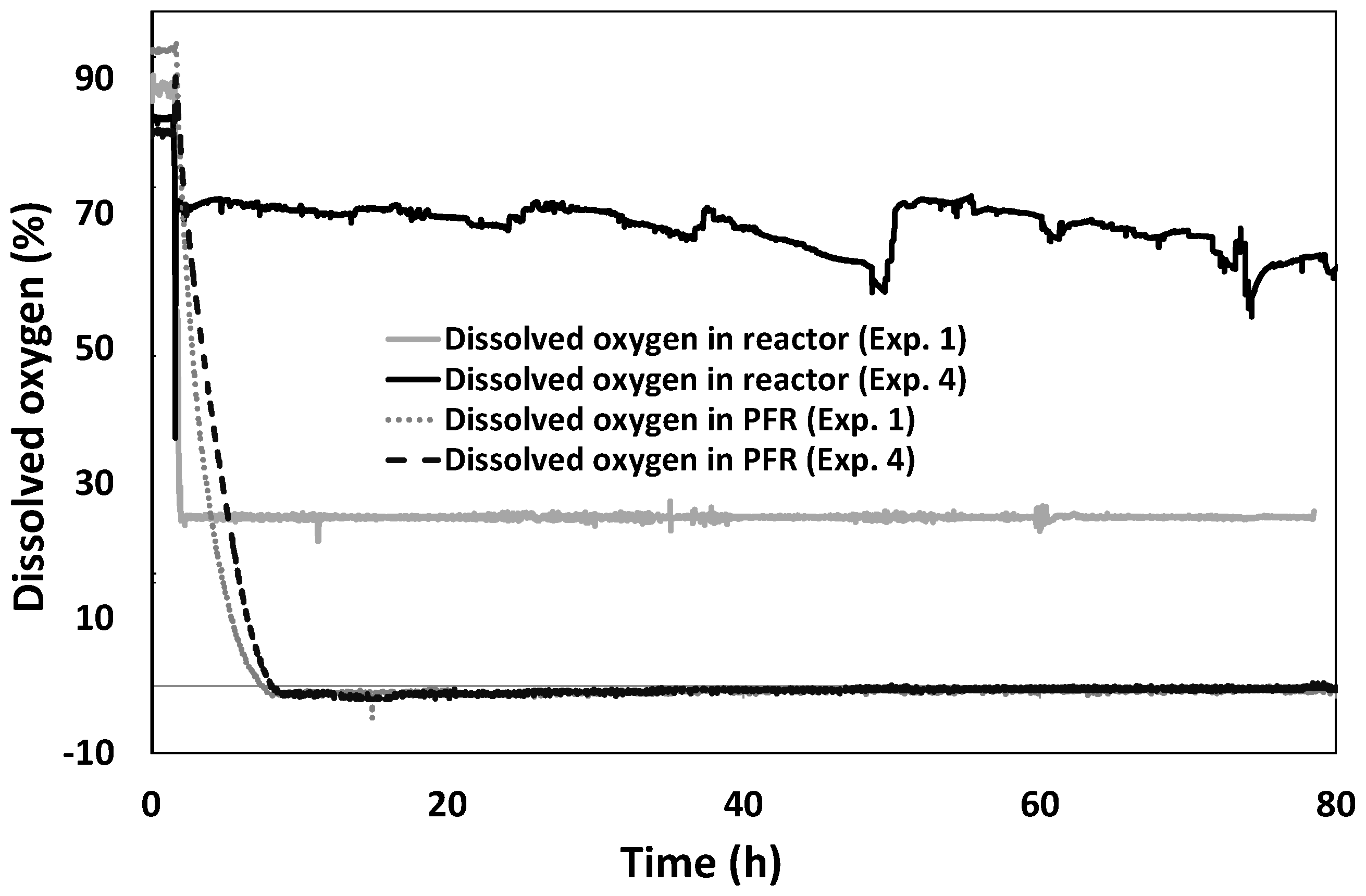
3.2. Results DOE1
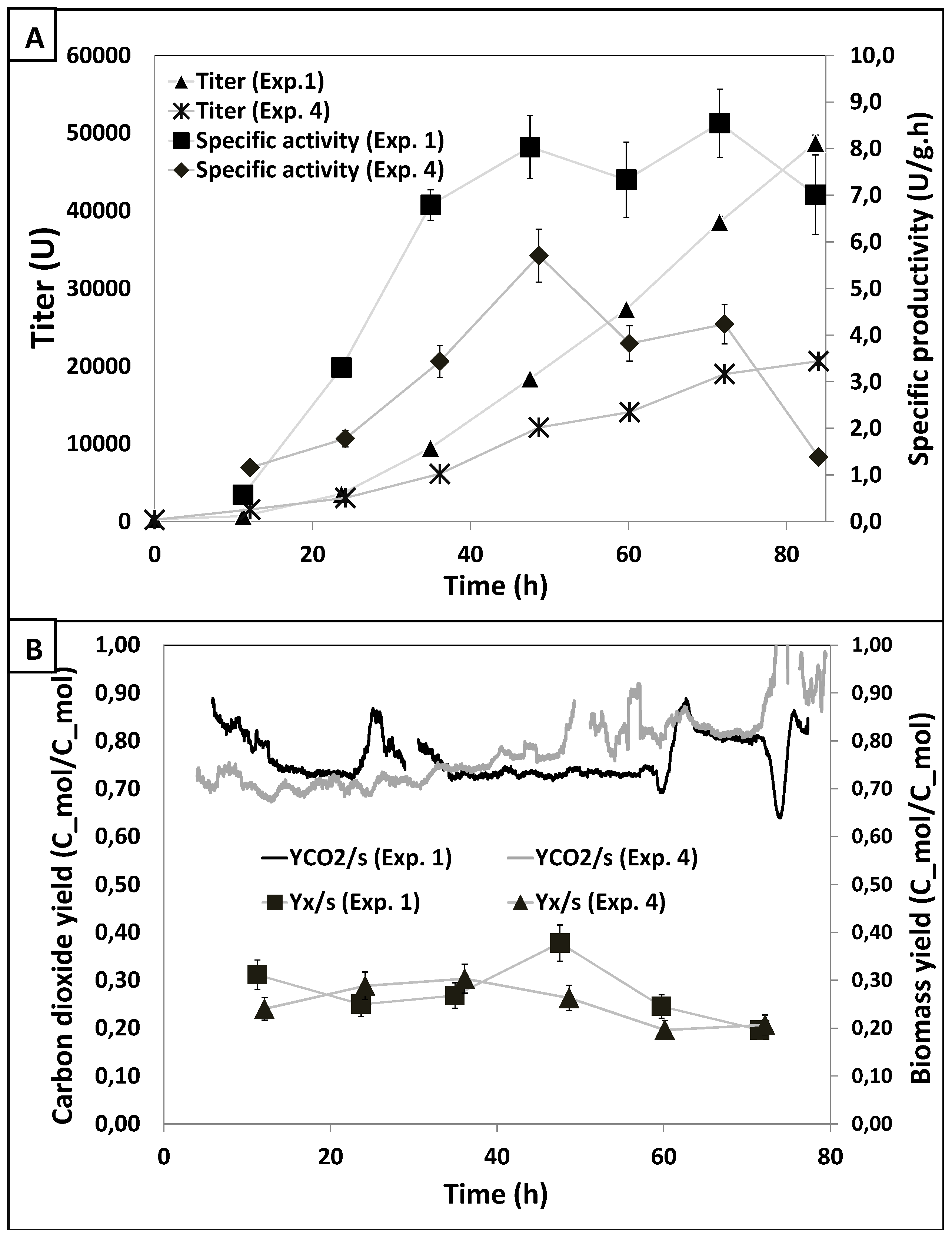
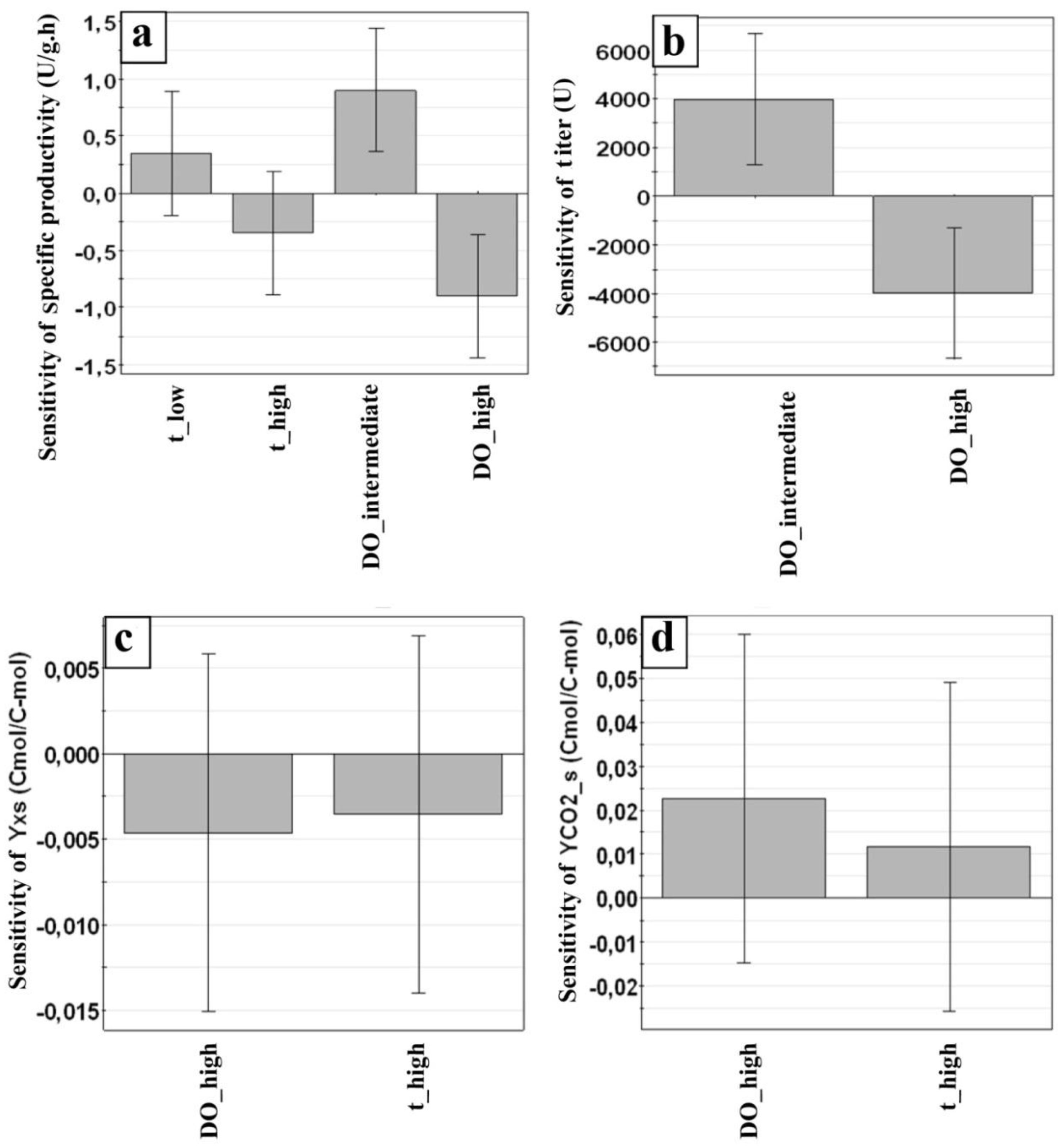
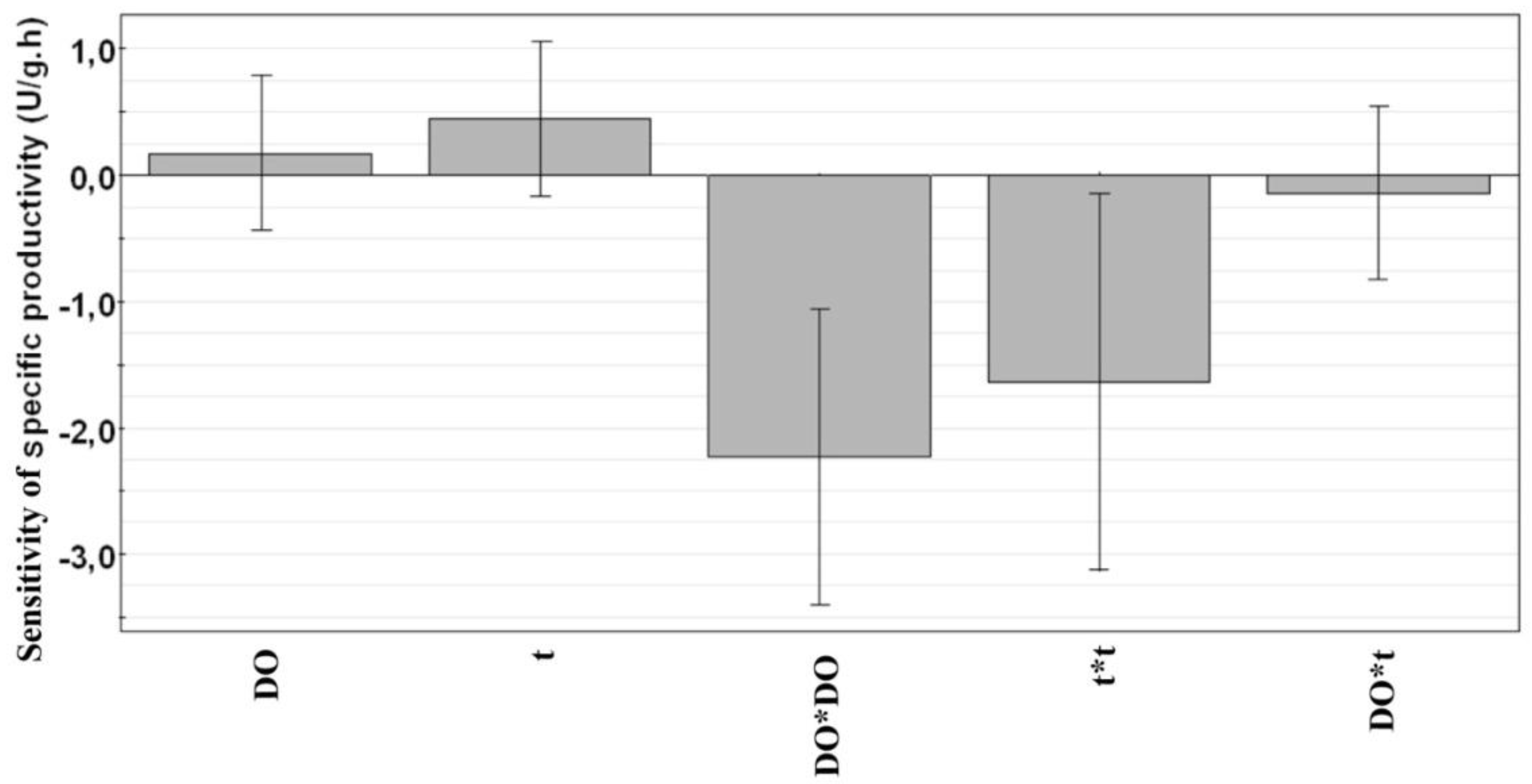
3.3. Along Which Function is Productivity Coupled to Dissolved Oxygen?

4. Conclusions
Supplementary Files
Supplementary File 1Conflicts of Interest
References
- Potvin, G.; Ahmad, A.; Zhang, Z. Bioprocess engineering aspects of heterologous protein production in pichia pastoris: A review. Biochem. Eng. J. 2012, 64, 91–105. [Google Scholar] [CrossRef]
- Li, P.; Anumanthan, A.; Gao, X.G.; Ilangovan, K.; Suzara, V.V.; Düzgüneş, N.; Renugopalakrishnan, V. Expression of recombinant proteins in pichia pastoris. Appl. Biochem. Biotech. 2007, 142, 105–124. [Google Scholar] [CrossRef]
- Cregg, J.; Cereghino, J.; Shi, J.; Higgins, D. Recombinant protein expression in pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef]
- Dietzsch, C.; Spadiut, O.; Herwig, C. A fast approach to determine a fed batch feeding profile for recombinant pichia pastoris strains. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef]
- Trentmann, O.; Khatri, N.K.; Hoffmann, F. Reduced oxygen supply increases process stability and product yield with recombinant pichia pastoris. Biotechnol. Progr. 2004, 20, 1766–1775. [Google Scholar] [CrossRef]
- Hellwig, S.; Emde, F.; Raven, N.P.G.; Henke, M.; van der Logt, P.; Fischer, R. Analysis of single-chain antibody production in pichia pastoris using on-line methanol control in fed-batch and mixed-feed fermentations. Biotechnol. Bioeng. 2001, 74, 344–352. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, S.J.; Jung, K.H.; Katoh, S.; Lee, E.K. High dissolved oxygen tension enhances heterologous protein expression by recombinant pichia pastoris. Process Biochem. 2003, 38, 1147–1154. [Google Scholar] [CrossRef]
- Baumann, K.; Maurer, M.; Dragosits, M.; Cos, O.; Ferrer, P.; Mattanovich, D. Hypoxic fed-batch cultivation of pichia pastoris increases specific and volumetric productivity of recombinant proteins. Biotechnol. Bioeng. 2008, 100, 177–183. [Google Scholar] [CrossRef]
- Baumann, K.; Carnicer, M.; Dragosits, M.; Graf, A.; Stadlmann, J.; Jouhten, P.; Maaheimo, H.; Gasser, B.; Albiol, J.; Mattanovich, D.; et al. A multi-level study of recombinant pichia pastoris in different oxygen conditions. BMC Syst. Biol. 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Jazini, M.H.; Herwig, C. Two-compartment processing as a tool to boost recombinant protein production. Eng. Life Sci. 2013. [Google Scholar] [CrossRef]
- Lorantfy, B.; Jazini, M.; Herwig, C. Investigation of the physiological response to oxygen limited process conditions of Pichia pastoris Mut(+) strain using a two-compartment scale-down system. J. Biosci. Bioeng. 2013, 116, 371–379. [Google Scholar] [CrossRef]
- Amanullah, A.; McFarlane, C.M.; Emery, A.N.; Nienow, A.W. Scale-down model to simulate spatial ph variations in large-scale bioreactors. Biotechnol. Bioeng. 2001, 73, 390–399. [Google Scholar] [CrossRef]
- Bylund, F.; Castan, A.; Mikkola, R.; Veide, A.; Larsson, G. Influence of scale-up on the quality of recombinant human growth hormone. Biotechnol. Bioeng. 2000, 69, 119–128. [Google Scholar] [CrossRef]
- Lara, A.R.; Leal, L.; Flores, N.; Gosset, G.; Bolívar, F.; Ramírez, O.T. Transcriptional and metabolic response of recombinant Escherichia coli to spatial dissolved oxygen tension gradients simulated in a scale-down system. Biotechnol. Bioeng. 2006, 93, 372–385. [Google Scholar] [CrossRef]
- Lara, A.R.; Vazquez-Limón, C.; Gosset, G.; Bolívar, F.; López-Munguía, A.; Ramírez, O.T. Engineering Escherichia coli to improve culture performance and reduce formation of by-products during recombinant protein production under transient intermittent anaerobic conditions. Biotechnol. Bioeng. 2006, 94, 1164–1175. [Google Scholar] [CrossRef]
- Sandoval-Basurto, E.A.; Gosset, G.; Bolívar, F.; Ramírez, O.T. Culture of Escherichia coli under dissolved oxygen gradients simulated in a two-compartment scale-down system: Metabolic response and production of recombinant protein. Biotechnol. Bioeng. 2005, 89, 453–463. [Google Scholar] [CrossRef]
- Lara, A.; Galindo, E.; Ramirez, O.; Palomares, L. Living with heterogeneities in bioreactors. Mol. Biotechnol. 2006, 34, 355–381. [Google Scholar] [CrossRef]
- Herwig, C.; Marison, I.; von Stockar, U. On-line stoichiometry and identification of metabolic state under dynamic process conditions. Biotechnol. Bioeng. 2001, 75, 345–354. [Google Scholar] [CrossRef]
- Jahic, M.; Wallberg, F.; Bollok, M.; Garcia, P.; Enfors, S.-O. Temperature limited fed-batch technique for control of proteolysis in pichia pastoris bioreactor cultures. Microb. Cell Fact. 2003, 2. [Google Scholar] [CrossRef] [Green Version]
- Hohenblum, H.; Borth, N.; Mattanovich, D. Assessing viability and cell-associated product of recombinant protein producing pichia pastoris with flow cytometry. J. Biotechnol. 2003, 102, 281–290. [Google Scholar] [CrossRef]
- Khatri, N.K.; Hoffmann, F. Impact of methanol concentration on secreted protein production in oxygen-limited cultures of recombinant pichia pastoris. Biotechnol. Bioeng. 2006, 93, 871–879. [Google Scholar] [CrossRef]
- Mattanovich, D.; Gasser, B.; Hohenblum, H.; Sauer, M. Stress in recombinant protein producing yeasts. J. Biotechnol. 2004, 113, 121–135. [Google Scholar] [CrossRef]
- Arsene, F.; Tomoyasu, T.; Bukau, B. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 2000, 55, 3–9. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.-L.; Xue, C.; Xiong, X.-H.; Yao, X.-Q.; Li, X.-Y.; Chen, H.-P.; Liu, Z.-M. Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of saccharomyces cerevisiae chaperone proteins. Biotechnol. Progr. 2006, 22, 1090–1095. [Google Scholar] [CrossRef]
Appendix
| Exp. No. | Residence time (min) | Dissolved oxygen (%) | Specific productivity (U/g·h) | Titer(U) | Y_CO2/s (C-mol/C-mol) | Y_x/s (C-mol/C-mol) |
|---|---|---|---|---|---|---|
| 1 | Low (3.3) | Intermediate (25) | 4.95 ± 0.24 | 16,198.2 ± 95 | 0.58 ± 0.021 | 0.30 ± 0.012 |
| 2 | High (15) | Intermediate (25) | 3.98 ± 0.19 | 20,665.1 ± 110 | 0.63 ± 0.022 | 0.29 ± 0.011 |
| 3 | Low (3.3) | High (70) | 2.87 ± 0.14 | 10,915.4 ± 50 | 0.65 ± 0.022 | 0.28 ± 0.011 |
| 4 | High (15) | High (70) | 2.19 ± 0.10 | 10,048.3 ± 48 | 0.64 ± 0.03 | 0.28 ± 0.011 |
| 5 | Low (3.3) | Intermediate (25) | 4.41 ± 0.22 | 18,482.8 ± 85 | 0.59 ± 0.021 | 0.29 ± 0.011 |
| Exp. No | Residence time (min) | Dissolved oxygen (%) | Specific productivity (U/g·h) |
|---|---|---|---|
| 1 | 3.3 | 25 | 4.95 ± 0.24 |
| 5 | 3.3 | 25 | 4.41 ± 0.22 |
| 6 | 3.3 | 5 | 2.25 ± 0.22 |
| 7 | 0 | 5 | 2.80 ± 0.28 |
| 8 | 0 | 25 | 4.60 ± 0.30 |
| 9 | 0 | 70 | 2.60 ± 0.31 |
| 10 | 3.3 | 70 | 2.20 ± 0.21 |
| 11 | 15 | 5 | 2.18 ± 0.20 |
| 12 | 15 | 25 | 2.33 ± 0.22 |
| 13 | 15 | 70 | 2.08 ± 0.19 |
Description of the Two-Compartment System
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jazini, M.; Cekici, G.; Herwig, C. Quantifying the Effects of Frequency and Amplitude of Periodic Oxygen-Related Stress on Recombinant Protein Production in Pichia pastoris. Bioengineering 2014, 1, 47-61. https://doi.org/10.3390/bioengineering1010047
Jazini M, Cekici G, Herwig C. Quantifying the Effects of Frequency and Amplitude of Periodic Oxygen-Related Stress on Recombinant Protein Production in Pichia pastoris. Bioengineering. 2014; 1(1):47-61. https://doi.org/10.3390/bioengineering1010047
Chicago/Turabian StyleJazini, Mohammadhadi, Gülbahar Cekici, and Christoph Herwig. 2014. "Quantifying the Effects of Frequency and Amplitude of Periodic Oxygen-Related Stress on Recombinant Protein Production in Pichia pastoris" Bioengineering 1, no. 1: 47-61. https://doi.org/10.3390/bioengineering1010047





